Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Serological Assay
2.3. Multiplex Avidity Assay
2.4. Statistical Analysis
3. Results
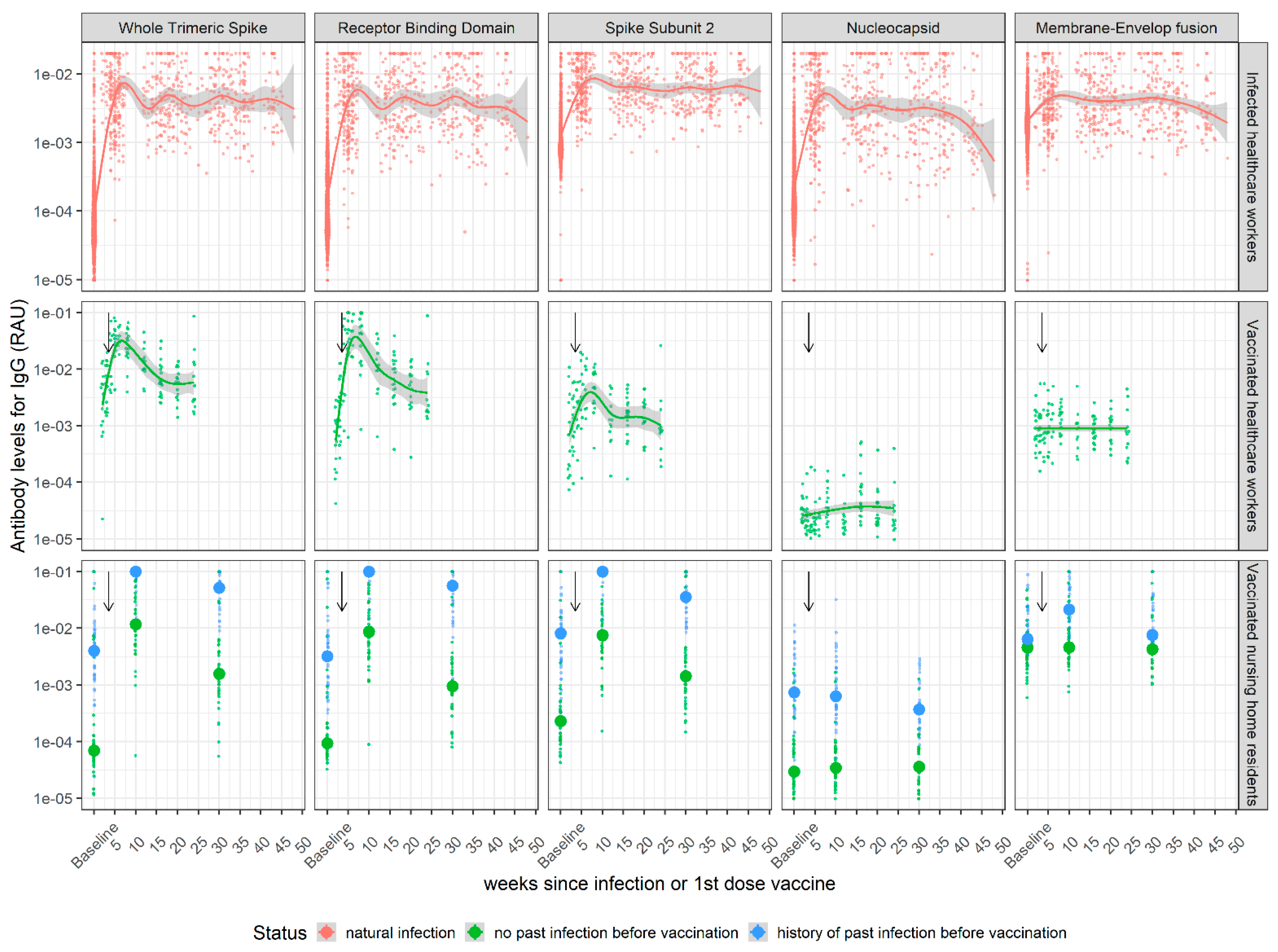
3.1. Kinetics of SARS-CoV-2 Antibody Levels
3.2. Kinetics of SARS-CoV-2 IgG Antibody Avidity
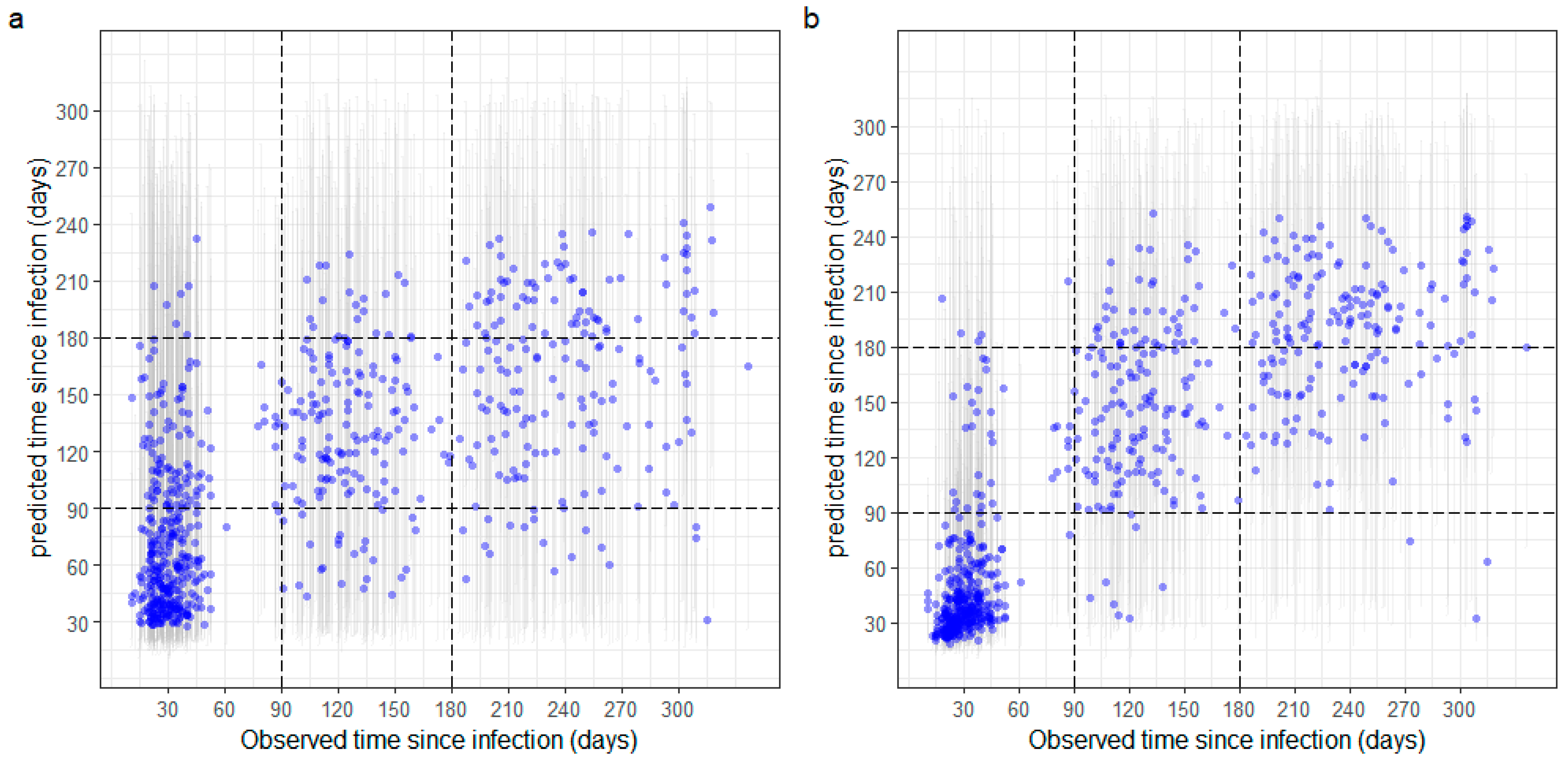
3.3. Estimation of Time since Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.-J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of Antibody Responses up to 13 Months after SARS-CoV-2 Infection and Risk of Reinfection. EBio Med. 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Pelleau, S.; Woudenberg, T.; Rosado, J.; Donnadieu, F.; Garcia, L.; Obadia, T.; Gardais, S.; Elgharbawy, Y.; Velay, A.; Gonzalez, M.; et al. Kinetics of the Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Response and Serological Estimation of Time Since Infection. J. Infect. Dis. 2021, 224, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Slifka, M.K.; Antia, R.; Whitmire, J.K.; Ahmed, R. Humoral Immunity Due to Long-Lived Plasma Cells. Immunity 1998, 8, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Quan, Y.; Xin, Z.-T.; Wrammert, J.; Ma, M.-J.; Lv, H.; Wang, T.-B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-up Study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tas, J.M.J.; Mesin, L.; Pasqual, G.; Targ, S.; Jacobsen, J.T.; Mano, Y.M.; Chen, C.S.; Weill, J.-C.; Reynaud, C.-A.; Browne, E.P.; et al. Visualizing Antibody Affinity Maturation in Germinal Centers. Science 2016, 351, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlomchik, M.J.; Weisel, F. Germinal Center Selection and the Development of Memory B and Plasma Cells. Immunol. Rev. 2012, 247, 52–63. [Google Scholar] [CrossRef]
- Bergeri, I.; Whelan, M.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global Epidemiology of SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis of Standardized Population-Based Seroprevalence Studies, Jan 2020-Dec 2021. medRxiv 2022. [Google Scholar] [CrossRef]
- Ainsworth, M.; Andersson, M.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Beveridge, A.; Bibi, S.; Blackwell, L.; Borak, M.; et al. Performance Characteristics of Five Immunoassays for SARS-CoV-2: A Head-to-Head Benchmark Comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal Analysis of Antibody Dynamics in COVID-19 Convalescents Reveals Neutralizing Responses up to 16 Months after Infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef]
- Drouot, L.; Hantz, S.; Jouen, F.; Velay, A.; Lamia, B.; Veber, B.; Sibilia, J.; Lotellier, M.; Candon, S.; Alain, S.; et al. Evaluation of Humoral Immunity to SARS-CoV-2: Diagnostic Value of a New Multiplex Addressable Laser Bead Immunoassay. Front. Microbiol. 2020, 11, 603931. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C.; Larson, E.; Babasyan, S.; Rollins, A.; Joshi, L.R.; Laverack, M.; Parrilla, L.; Plocharczyk, E.; Diel, D.G.; Wagner, B. Development of a Quantitative COVID-19 Multiplex Assay and Its Use for Serological Surveillance in a Low SARS-CoV-2 Incidence Community. PLoS ONE 2022, 17, e0262868. [Google Scholar] [CrossRef] [PubMed]
- Brochot, E.; Souplet, V.; Follet, P.; Ponthieu, P.; Olivier, C.; Even, G.; Audebert, C.; Malbec, R. A Multiplex Serological Assay for the Characterization of IgG Immune Response to SARS-CoV-2. PLoS ONE 2022, 17, e0262311. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Liu, A.; Gibbs, E.; Tanunliong, G.; Marquez, A.C.; Gantt, S.; Frykman, H.; Krajden, M.; Morshed, M.; Prystajecky, N.A.; et al. A Novel Multiplex Electrochemiluminescent Immunoassay for Detection and Quantification of Anti-SARS-CoV-2 IgG and Anti-Seasonal Endemic Human Coronavirus IgG. J. Clin. Virol. 2022, 146, 105050. [Google Scholar] [CrossRef]
- Chawla, A.; Murphy, G.; Donnelly, C.; Booth, C.L.; Johnson, M.; Parry, J.V.; Phillips, A.; Geretti, A.M. Human Immunodeficiency Virus (HIV) Antibody Avidity Testing To Identify Recent Infection in Newly Diagnosed HIV Type 1 (HIV-1)-Seropositive Persons Infected with Diverse HIV-1 Subtypes. J. Clin. Microbiol. 2007, 45, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Prince, H.E.; Lapé-Nixon, M. Role of Cytomegalovirus (CMV) IgG Avidity Testing in Diagnosing Primary CMV Infection during Pregnancy. Clin. Vaccine Immunol. 2014, 21, 1377–1384. [Google Scholar] [CrossRef] [Green Version]
- Agbede, O.O.; Adeyemi, O.O.; Olatinwo, A.W.O. Significance of IgG-Avidity in Antenatal Rubella Diagnosis. J. Fam. Reprod. Health 2013, 7, 131–137. [Google Scholar]
- Mercader, S.; Garcia, P.; Bellini, W.J. Measles Virus IgG Avidity Assay for Use in Classification of Measles Vaccine Failure in Measles Elimination Settings. Clin. Vaccine Immunol. 2012, 19, 1810–1817. [Google Scholar] [CrossRef] [Green Version]
- Sowers, S.B.; Rota, J.S.; Hickman, C.J.; Mercader, S.; Redd, S.; McNall, R.J.; Williams, N.; McGrew, M.; Walls, M.L.; Rota, P.A.; et al. High Concentrations of Measles Neutralizing Antibodies and High-Avidity Measles IgG Accurately Identify Measles Reinfection Cases. Clin. Vaccine Immunol. 2016, 23, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Grzelak, L.; Velay, A.; Madec, Y.; Gallais, F.; Staropoli, I.; Schmidt-Mutter, C.; Wendling, M.-J.; Meyer, N.; Planchais, C.; Rey, D.; et al. Sex Differences in the Evolution of Neutralizing Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2. J. Infect. Dis. 2021, 224, 983–988. [Google Scholar] [CrossRef]
- Dyer, A.H.; Noonan, C.; McElheron, M.; Batten, I.; Reddy, C.; Connolly, E.; Pierpoint, R.; Murray, C.; Leonard, A.; Higgins, C.; et al. Previous SARS-CoV-2 Infection, Age, and Frailty Are Associated With 6-Month Vaccine-Induced Anti-Spike Antibody Titer in Nursing Home Residents. J. Am. Med. Dir. Assoc. 2022, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. Comput. Sci. 2007, 2, 18–22. [Google Scholar]
- Koerber, N.; Priller, A.; Yazici, S.; Bauer, T.; Cheng, C.-C.; Mijočević, H.; Wintersteller, H.; Jeske, S.; Vogel, E.; Feuerherd, M.; et al. Dynamics of Spike-and Nucleocapsid Specific Immunity during Long-Term Follow-up and Vaccination of SARS-CoV-2 Convalescents. Nat. Commun. 2022, 13, 153. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Lu, R.; Zhang, Y.; Du, M.; Xing, M.; Wu, Z.; Kong, X.; Zhu, Y.; Zhou, X.; et al. Longitudinal Immune Profiling Reveals Dominant Epitopes Mediating Long-Term Humoral Immunity in COVID-19-Convalescent Individuals. J. Allergy Clin. Immunol. 2022, 149, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Morita, Y.; Nakano, Y.; Yokoyama, R.; Shimura, T.; Qian, C.; Xia, F.; He, F.; Zheng, L.; Ohmiya, H.; et al. Response Kinetics of Different Classes of Antibodies to SARS-CoV2 Infection in the Japanese Population: The IgA and IgG Titers Increased Earlier than the IgM Titers. Int. Immunopharmacol. 2022, 103, 108491. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune Correlates Analysis of the MRNA-1273 COVID-19 Vaccine Efficacy Clinical Trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Bauer, G. The Potential Significance of High Avidity Immunoglobulin G (IgG) for Protective Immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021, 106, 61–64. [Google Scholar] [CrossRef]
- Dobaño, C.; Sanz, H.; Sorgho, H.; Dosoo, D.; Mpina, M.; Ubillos, I.; Aguilar, R.; Ford, T.; Díez-Padrisa, N.; Williams, N.A.; et al. Concentration and Avidity of Antibodies to Different Circumsporozoite Epitopes Correlate with RTS,S/AS01E Malaria Vaccine Efficacy. Nat. Commun. 2019, 10, 2174. [Google Scholar] [CrossRef]
- Usinger, W.R.; Lucas, A.H. Avidity as a Determinant of the Protective Efficacy of Human Antibodies to Pneumococcal Capsular Polysaccharides. Infect. Immun. 1999, 67, 2366–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauzin, A.; Gendron-Lepage, G.; Nayrac, M.; Anand, S.P.; Bourassa, C.; Medjahed, H.; Goyette, G.; Dubé, M.; Bazin, R.; Kaufmann, D.E.; et al. Evolution of Anti-RBD IgG Avidity Following SARS-CoV-2 Infection. Viruses 2022, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Glück, V.; Tydykov, L.; Mader, A.-L.; Warda, A.-S.; Bertok, M.; Weidlich, T.; Gottwald, C.; Köstler, J.; Salzberger, B.; Wagner, R.; et al. Humoral Immunity in Dually Vaccinated SARS-CoV-2-Naïve Individuals and in Booster-Vaccinated COVID-19-Convalescent Subjects. Infection 2022. [Google Scholar] [CrossRef] [PubMed]
- Koehler, M.; Ray, A.; Moreira, R.A.; Juniku, B.; Poma, A.B.; Alsteens, D. Molecular Insights into Receptor Binding Energetics and Neutralization of SARS-CoV-2 Variants. Nat. Commun. 2021, 12, 6977. [Google Scholar] [CrossRef] [PubMed]


| Natural Infection | Vaccination | |||||
|---|---|---|---|---|---|---|
| Strasbourg HCWs | Paris HCWs; Infected | Paris HCWs; Uninfected | Orléans HCWs | Dublin CHR; No Past Infection | Dublin CHR; Past Infection | |
| Participants | 174 | 32 | 752 | 16 | 47 | 39 |
| Samples | 522 | 64 | 752 | 120 | 126 | 106 |
| Female | 139 | 19 | 543 | 5 | 33 | 23 |
| Male | 35 | 13 | 209 | 11 | 14 | 16 |
| Age | 43 (25–73) | 37 (24–63) | 41 (19–72) | 59 (35–74) | 83 (53–98) | 83 (55–100) |
| Maximum days post-symptom onset | 219 (161–284) | 304 (285–336) | NA | NA | NA | 265 (224–298) * |
| Days post-vaccination | NA | NA | NA | 154 (151–168) | 206 (201–210) | 206 (201–210) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, L.; Woudenberg, T.; Rosado, J.; Dyer, A.H.; Donnadieu, F.; Planas, D.; Bruel, T.; Schwartz, O.; Prazuck, T.; Velay, A.; et al. Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination. Viruses 2022, 14, 1491. https://doi.org/10.3390/v14071491
Garcia L, Woudenberg T, Rosado J, Dyer AH, Donnadieu F, Planas D, Bruel T, Schwartz O, Prazuck T, Velay A, et al. Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination. Viruses. 2022; 14(7):1491. https://doi.org/10.3390/v14071491
Chicago/Turabian StyleGarcia, Laura, Tom Woudenberg, Jason Rosado, Adam H. Dyer, Françoise Donnadieu, Delphine Planas, Timothée Bruel, Olivier Schwartz, Thierry Prazuck, Aurélie Velay, and et al. 2022. "Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination" Viruses 14, no. 7: 1491. https://doi.org/10.3390/v14071491
APA StyleGarcia, L., Woudenberg, T., Rosado, J., Dyer, A. H., Donnadieu, F., Planas, D., Bruel, T., Schwartz, O., Prazuck, T., Velay, A., Fafi-Kremer, S., Batten, I., Reddy, C., Connolly, E., McElheron, M., Kennelly, S. P., Bourke, N. M., White, M. T., & Pelleau, S. (2022). Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination. Viruses, 14(7), 1491. https://doi.org/10.3390/v14071491






