Detection of African Swine Fever Virus in Ornithodoros Tick Species Associated with Indigenous and Extralimital Warthog Populations in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Collection of Ticks
2.2. Nucleic Acid Extraction and Gene Amplification
2.3. Isolation of Virus
2.4. Identification of Tick Blood Meal Donor Species
2.5. ASFV Antibody Tests on Alternative Vertebrate Hosts of Ornithodoros Ticks
3. Results
3.1. Collection and Identification of Ornithodoros Ticks
3.2. Detection and p72 Phylogeny of ASFV Nucleic Acid in Ticks
3.3. Isolation of Virus
3.4. Molecular Identification of Blood Meal Hosts
3.5. ASFV Antibody Tests on Hyaena and Tortoise Sera
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plowright, W.; Parker, J.; Peirce, M.A. African swine fever virus in ticks (Ornithodoros moubata, Murray) collected from animal burrows in Tanzania. Nature 1969, 221, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Pini, A. African swine fever: Some observations and considerations. S. Afr. J. Anim. Sci. 1977, 73, 133–134. [Google Scholar]
- Thomson, G.R.; Gainaru, M.; Lewis, A.; Biggs, H.; Nevill, E.; van der Pypekamp, H.; Gerber, L.; Esterhuysen, J.; Bengis, R.; Bezuidenhout, D.; et al. The relationship between ASF virus, the warthog and Ornithodoros species in southern Africa. In African Swine Fever; Wilkinson, P.J., Ed.; EUR. 8466 EN; Commission of the European Communities: Brussels, Belgium, 1983; pp. 85–100. [Google Scholar]
- Steyn, D.G. Preliminary Report on a South African Virus Disease Amongst Pigs; 13th and 14th Reports of the Director of Veterinary Education and Research; Government Printer and Stationery Office: Pretoria, South Africa, 1928; pp. 415–428.
- De Kock, G.; Robinson, E.M.; Keppel, J.J.G. Swine Fever in South Africa. Onderstepoort J. Vet. Res. 1940, 14, 31–93. [Google Scholar]
- DALRRD (Department of Agriculture, Land Reform and Rural Development). Disease Database. 1993–2019. Available online: https://www.dalrrd.gov.za/Branches/Agricultural-Production-Health-Food-Safety/Animal-Health/Epidemiology/diseasedatabase (accessed on 31 August 2020).
- DALRRD (Department of Agriculture, Land Reform and Rural Development). African Swine Fever Outbreak and Surveillance Update Report. 2020. Available online: http://nahf.co.za/wp-content/uploads/ASF-update-2020-05-19.pdf (accessed on 24 April 2022).
- DALRRD (Department of Agriculture, Land Reform and Rural Development). African Swine Fever Outbreak Reported in the Western Cape for the First Time. 2021. Available online: https://www.drdlr.gov.za/sites/Internet/Latest%20News/Pages/African-swine-fever-outbreak-reported-in-the-Western-Cape-for-the-first-time.aspx (accessed on 23 December 2021).
- WAHIS. Immediate Notifications and Follow-Ups. 2005–2020. Available online: http://www.oie.int/wahis2/public/wahid.php/Diseaseinformation/Immsummary (accessed on 21 December 2020).
- Bastos, A.D.; Penrith, M.L.; Cruciere, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.G.R.T.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African swine fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef] [Green Version]
- Van Rensburg, L.J.; Van Heerden, J.; Penrith, M.L.; Heath, L.E.; Rametse, T.; Etter, E.M.C. Investigation of African swine fever outbreaks in pigs outside the controlled areas of South Africa, 2012–2017. J. S. Afr. Veter. Assoc. 2020, 91, 1–9. [Google Scholar] [CrossRef]
- Amar, S.; De Boni, L.; de Voux, A.; Heath, L.; Geertsma, P. An outbreak of African swine fever in small-scale pigs, Gauteng, South Africa, July 2020. Int. J. Infect. Dis. 2021, 110, S44–S49. [Google Scholar] [CrossRef]
- Penrith, M.L.; Kivaria, F.M. One hundred years of African swine fever in Africa: Where have we been, where are we now, where are we going? Transbound. Emerg. Dis. 2022, 2640–2642, advance online publication. [Google Scholar] [CrossRef]
- Penzhorn, B.L. A summary of the re-introduction of ungulates into South African National Parks (to 31 December 1970). Koedoe 1971, 14, 145–159. [Google Scholar] [CrossRef]
- Carruthers, J.C. Wilding the farm or farming the wild”? The evolution of scientific game ranching in South Africa from the 1960s to the present. Trans. R. Soc. S. Afr. 2008, 63, 160–181. [Google Scholar]
- Swanepoel, M. Distribution, Utilization and Management of the Extra-Limital Common Warthog (Phacochoerus africanus) in South Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]
- Swanepoel, M.; Schulze, E.; Cumming, D. A Conservation Assessment of Phacochoerus africanus. The Red List of Mammals of South Africa, Swaziland and Lesotho; South African National Biodiversity Institute and Endangered Wildlife Trust: Pretoria, South Africa, 2016. [Google Scholar]
- Magadla, N.R.; Gummow, B.; Vosloo, W.; Heath, L. The African swine fever control zone in South Africa and its current relevance. Onderstepoort J. Vet. Res. 2016, 83, 1–7. [Google Scholar] [CrossRef]
- Craig, A.F.; Schade-Weskott, M.L.; Harris, H.J.; Heath, L.; Kriel, G.J.; de Klerk-Lorist, L.M.; van Schalkwyk, L.; Buss, P.; Trujillo, J.D.; Crafford, J.E.; et al. Extension of Sylvatic Circulation of African Swine Fever Virus in Extralimital Warthogs in South Africa. Front. Vet. Sci. 2021, 8, 746129. [Google Scholar] [CrossRef]
- Bakkes, D.K.; De Klerk, D.; Latif, A.A.; Mans, B.J. Integrative taxonomy of Afrotropical Ornithodoros (Ornithodoros) (Acari: Ixodida: Argasidae). Ticks Tick Borne Dis. 2018, 9, 1006–1037. [Google Scholar] [CrossRef]
- Dohoo, I.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research; University of Prince Edward Island: Charlottetown, PE, Canada, 2003. [Google Scholar]
- Larsen, R.; Holmern, T.; Prager, S.D.; Maliti, H.; Røskaft, E. Using the extended quarter degree grid cell system to unify mapping and sharing of biodiversity data. Afr. J. Ecol. 2009, 47, 382–392. [Google Scholar] [CrossRef]
- Jori, F.; Vial, L.; Penrith, M.-L.; Pérez-Sánchez, R.; Etter, E.; Albina, E.; Michaud, V.; Roger, F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 2013, 173, 212–227. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Scoles, G.A.; Burrage, T.G.; Sur, J. African swine fever virus replication in the midgut epithelium is required for infection of Ornithodoros ticks. J. Virol. 1999, 73, 8587–8598. [Google Scholar] [CrossRef] [Green Version]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical diagnosis of African swine fever in contact-exposed swine by a real-time PCR assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Sunwoo, S.Y.; Pérez-Núñez, D.; Morozov, I.; Sánchez, E.G.; Gaudreault, N.N.; Trujillo, J.D.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-protein vaccination strategy does not protect from challenge with African swine fever virus Armenia 2007 strain. Vaccines 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 356, 1547–1549. [Google Scholar] [CrossRef]
- Mushagalusa, C.A.; Etter, E.; Penrith, M.-L. Review of African swine fever outbreaks history in South Africa: From 1926 to 2018. Onderstepoort J. Vet. Res. 2013, 88, a1919. [Google Scholar] [CrossRef]
- Bastos, A.D.S.; Penrith, M.L.; Macome, F.; Pinto, F.; Thomson, G.R. Co-circulation of two genetically distinct viruses in an outbreak of African swine fever in Mozambique: No evidence for individual co-infection. Veter. Microbiol. 2014, 103, 169–182. [Google Scholar] [CrossRef]
- Bastos, A.D.; Arnot, L.F.; Jacquier, M.D.; Maree, S. A host species-informative internal control for molecular assessment of African swine fever virus infection rates in the African sylvatic cycle Ornithodoros vector. Med. Vet. Entomol. 2009, 23, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vizcaíno, J.M.; Heath, D.L. African Swine Fever (Infection with African Swine Fever Virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; Office International des Epizooties: Paris, France, 2019; Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.01_ASF.pdf (accessed on 12 February 2021).
- Lah, E.F.C.; Yaakop, S.; Ahamad, M.; Nor, S.M. Molecular identification of blood meal sources of ticks (Acari, Ixodidae) using cytochrome b gene as a genetic marker. ZooKeys 2015, 478, 27. [Google Scholar]
- Van Rensburg, L.J.; Etter, E.; Heath, L.; Penrith, M.L.; Van Heerden, J. Understanding African swine fever outbreaks in domestic pigs in a sylvatic endemic area: The case of the South African controlled area between 1977–2017. Transbound. Emerg. Dis. 2020, 67, 2753–2769. [Google Scholar] [CrossRef]
- Horak, I.G.; Mckay, I.J.; Henen, B.T.; Heyne, H.; Hofmeyr, M.D.; De Villiers, A.L. Parasites of domestic and wild animals in South Africa. XLVII. Ticks of tortoises and other reptiles. Onderstepoort J. Vet. Res. 2006, 73, 215–227. [Google Scholar] [CrossRef]
- Arnot, L.F.; Du Toit, J.T.; Bastos, A.D. Molecular monitoring of African swine fever virus using surveys targeted at adult Ornithodoros ticks: A re-evaluation of Mkuze game reserve, South Africa. Onderstepoort J. Vet. Res. 2009, 76, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Boshoff, C.I.; Bastos, A.D.S.; Dube, M.M.; Heath, L. First molecular assessment of the African swine fever virus status of Ornithodoros ticks from Swaziland. Onderstepoort J. Vet. Res. 2014, 81, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mans, B.J.; Featherston, J.; Kvas, M.; Pillay, K.A.; de Klerk, D.G.; Pienaar, R.; de Castro, M.H.; Schwan, T.G.; Lopez, J.E.; Teel, P.; et al. Argasid and ixodid systematics: Implications for soft tick evolution and systematics, with a new argasid species list. Ticks Tick Borne Dis. 2019, 10, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mangold, A.J.; Nava, S.; Venzal, J.M.; Labruna, M.; Guglielmone, A.A. A review of the systematics of the tick family Argasidae (Ixodida). Acarologia 2010, 50, 317–333. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Burger, T.D.; Shao, R.; Labruna, M.B.; Barker, S.C. Molecular phylogeny of soft ticks (Ixodida: Argasidae) inferred from mitochondrial genome and nuclear rRNA sequences. Ticks Tick Borne Dis. 2014, 5, 195–207. [Google Scholar] [CrossRef]
- Hafez, M.; El-Ziady, S.; Hefnawy, T. Biochemical and physiological studies of certain ticks (Ixodoidea). Cuticular permeability of Hyalomma (H.) dromedarii Koch (Ixodidae) and Ornithodoros (O.) savignyi (Audouin)(Argasidae). J. Parasitol. 1970, 56, 154–168. [Google Scholar] [CrossRef]
- Vial, L. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite 2009, 16, 191–202. [Google Scholar] [CrossRef]
- Kaufman, R.W. Integument and ecdysis. In Biology of Ticks, 2nd ed.; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 99–121. [Google Scholar]
- Gray, J.S.; Estrada-Peña, A.; Vial, L. Ecology of nidicolous ticks. In Biology of Ticks; ProQuest Ebook Central; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2013; Volume 2, pp. 39–60. [Google Scholar]
- Plowright, W.; Perry, C.T.; Peirce, M.A.; Parker, J. Experimental infection of the argasid tick, Ornithodoros moubata porcinus, with African swine fever virus. Arch. Gesamte Virusforsch. 1970, 31, 33–50. [Google Scholar] [CrossRef]
- Plowright, W.; Perry, C.T.; Peirce, M.A. Transovarial infection with African swine fever virus in the argasid tick, Ornithodoros moubata porcinus, Walton. Res. Veter. Sci. 1970, 11, 582–584. [Google Scholar] [CrossRef]
- Plowright, W.; Perry, C.T.; Greig, A. Sexual Transmission of African Swine Fever virus in the tick, Ornithodoros moubata porcinus, Walton. Res. Veter. Sci. 1974, 17, 106–113. [Google Scholar] [CrossRef]
- Groocock, C.M.; Hess, W.R.; Gladney, W.J. Experimental transmission of African swine fever virus by Ornithodoros coriaceus, an argasid tick indigenous to the United States. Am. J. Vet. Res. 1980, 41, 591–594. [Google Scholar]
- Mellor, P.S.; Wilkinson, P.J. Experimental transmission of African swine fever by Ornithodoros savignyi (Audouin). Res. Vet. Sci. 1985, 39, 353–356. [Google Scholar] [CrossRef]
- Hess, W.R.; Endris, R.G.; Haslett, T.M.; Monahan, M.J.; McCoy, J.P. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Veter. Parasitol. 1987, 26, 145–155. [Google Scholar] [CrossRef]
- Hess, W.R.; Endris, R.G.; Lousa, A.; Caiado, J.M. Clearance of African swine fever virus from infected tick (Acari) colonies. J. Med. Entomol. 1989, 26, 314–317. [Google Scholar] [CrossRef]
- Endris, R.G.; Haslett, T.M.; Hess, W.R. Experimental transmission of African swine fever virus by the tick Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae). J. Med. Entomol. 1991, 28, 854–858. [Google Scholar] [CrossRef]
- Endris, R.G.; Haslett, T.M.; Hess, W.R. African swine fever virus infection in the soft tick, Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae). J. Med. Entomol. 1992, 29, 990–994. [Google Scholar] [CrossRef]
- Endris, R.G.; Hess, W.R. Experimental transmission of African swine fever virus by the soft tick Ornithodoros (Pavlovskyella) marocanus (Acari: Ixodoidea: Argasidae). J. Med. Entomol. 1992, 29, 652–656. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Burrage, T.G.; Scoles, G.A.; Fish, D.; Rock, D.L. African swine fever virus infection in the argasid host, Ornithodoros porcinus. J. Virol. 1998, 72, 1711–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.C.; Hutchings, G.H.; Mukarati, N.; Wilkinson, P.J. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 1998, 62, 1–15. [Google Scholar] [CrossRef]
- Rennie, L.; Wilkinson, P.J.; Mellor, P.S. Effects of infection of the tick Ornithodoros moubata with African swine fever virus. Med. Vet. Entomol. 2000, 14, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Rennie, L.; Wilkinson, P.J.; Mellor, P.S. Transovarial transmission of African swine fever virus in the argasid tick Ornithodoros moubata. Med. Vet. Entomol. 2001, 15, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrage, T.G.; Lu, Z.; Neilan, J.G.; Rock, D.L.; Zsak, L. African swine fever virus multigene family 360 genes affect virus replication and generalization of infection in Ornithodoros porcinus ticks. J. Virol. 2004, 78, 2445–2453. [Google Scholar] [CrossRef] [Green Version]
- Dixon, L.K.; Abrams, C.C.; Bowick, G.; Goatley, L.C.; Kay-Jackson, P.C.; Chapman, D.; Liverani, E.; Nix, R.; Silk, R.; Zhang, F. African swine fever virus proteins involved in evading host defence systems. Veter. Immunol. Immunopathol. 2004, 100, 117–134. [Google Scholar] [CrossRef]
- Basto, A.P.; Nix, R.J.; Boinas, F.; Mendes, S.; Silva, M.J.; Cartaxeiro, C.; Portugal, R.S.; Leitao, A.; Dixon, L.K.; Martins, C. Kinetics of African swine fever virus infection in Ornithodoros erraticus ticks. J. Gen. Virol. 2006, 87, 1863–1871. [Google Scholar] [CrossRef]
- Rowlands, R.J.; Duarte, M.M.; Boinas, F.; Hutchings, G.; Dixon, L.K. The CD2v protein enhances African swine fever virus replication in the tick vector, Ornithodoros erraticus. Virology 2009, 393, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Diaz, A.V.; Netherton, C.L.; Dixon, L.K.; Wilson, A.J. African swine fever virus strain Georgia 2007/1 in Ornithodoros erraticus ticks. Emerg. Infect. Dis. 2012, 18, 1026. [Google Scholar] [CrossRef]
- Burrage, T.G. African swine fever virus infection in Ornithodoros ticks. Virus Res. 2013, 173, 131–139. [Google Scholar] [CrossRef]
- Ribeiro, R.; Otte, J.; Madeira, S.; Hutchings, G.H.; Boinas, F. Experimental infection of Ornithodoros erraticus sensu stricto with two Portuguese African swine fever virus strains. Study of factors involved in the dynamics of infection in ticks. PLoS ONE 2015, 10, e0137718. [Google Scholar] [CrossRef]
- Bernard, J. Caractérisation de la compétence vectorielle des tiques Ornithodores pour le virus de la peste porcine africaine et étude de deux déterminants: La relation souche virale-vecteur et l’influence de la salive de tiques sur l’infection chez le porc domestique. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2015. [Google Scholar]
- Netherton, C.L.; Connell, S.; Benfield, C.T.; Dixon, L.K. The genetics of life and death: Virus-host interactions underpinning resistance to African swine fever, a viral hemorrhagic disease. Front. Genet. 2019, 10, 402. [Google Scholar] [CrossRef] [Green Version]
- Pereira de Oliveira, R.; Hutet, E.; Paboeuf, F.; Duhayon, M.; Boinas, F.; Perez de Leon, A.; Filatov, S.; Vial, L.; Le Potier, M.F. Comparative vector competence of the Afrotropical soft tick Ornithodoros moubata and Palearctic species, O. erraticus and O. verrucosus, for African swine fever virus strains circulating in Eurasia. PLoS ONE 2019, 14, e0225657. [Google Scholar] [CrossRef] [Green Version]
- Pereira De Oliveira, R.; Hutet, E.; Lancelot, R.; Paboeuf, F.; Duhayon, M.; Boinas, F.; Pérez de León, A.A.; Filatov, S.; Le Potier, M.F.; Vial, L. Differential vector competence of Ornithodoros soft ticks for African swine fever virus: What if it involves more than just crossing organic barriers in ticks? Parasite Vectors 2020, 9, 618. [Google Scholar] [CrossRef]
- Armstrong, B.A.; Kneubehl, A.R.; Mitchell, R.D., III; Krishnavajhala, A.; Teel, P.D.; Pérez de León, A.A.; Lopez, J.E. Differential expression of putative Ornithodoros turicata defensins mediated by tick feeding. Front. Cell. Infect. Microbiol. 2020, 10, 152. [Google Scholar] [CrossRef]
- Forth, J.H.; Forth, L.F.; Lycett, S.; Bell-Sakyi, L.; Keil, G.M.; Blome, S.; Calvignac-Spencer, S.; Wissgott, A.; Krause, J.; Höper, D.; et al. African swine fever virus-like integrated elements in a soft tick genome—An ancient virus vector arms race? bioRxiv 2020. [Google Scholar]
- Forth, J.H.; Forth, L.F.; Lycett, S.; Bell-Sakyi, L.; Keil, G.M.; Blome, S.; Calvignac-Spencer, S.; Wissgott, A.; Krause, J.; Höper, D.; et al. Identification of African swine fever virus-like elements in the soft tick genome provides insights into the virus’ evolution. BMC Biol. 2020, 18, 136. [Google Scholar] [CrossRef]
- El-Hennawy, H.K. The first landmark in the route of Egyptian Arachnology:” Explication Sommaire des Planches d’Arachnides de l’Égypte et de la Syrie”(1825). Serket 2000, 6, 115–128. [Google Scholar]
- Ordman, D. The occurence of relapsing fever and the geographical distribution of Ornithodorus Moubata in South Africa: With an account of investigations carried out in the Northern and Eastern Transvaal. S. Afr. Med. J. 1941, 15, 383–388. [Google Scholar]
- Koch, C.L. Systematische ubersicht uber die Ordnung der Zecken. Arch. Naturgeschichte 1844, 10, 217–239. [Google Scholar] [CrossRef]
- Petney, T.N.; Maiwald, M. Tick nomenclature. Lancet 1996, 348, 1251. [Google Scholar] [CrossRef]
- Nuttall, G.H.F.; Warburton, C.; Cooper, W.F.; Robinson, L.E. Ticks, a monograph of the Ixodoidea; Cambridge University Press: Cambridge, UK, 1911. [Google Scholar]
- Theiler, G.; Hoogstraal, H. The identity of Ornithodoros savignyi (Audouin, 1827) and O. pavimentosus Neumann, 1901 (ixodoidea, argasidae). J. Parasitol. 1955, 41, 245–247. [Google Scholar] [CrossRef]
- Ross, P.H.; Milne, A.D. Tick fever. Br. Med. J. 1904, 2, 1453. [Google Scholar] [CrossRef]
- Dutton, J.E.; Todd, J.L. The nature of human tick-fever in the eastern part of the Congo Free State with notes on the distribution and bionomics of the tick. Liverpool Sch. Trop. Med. Mem. 1905, 17, 1–18. [Google Scholar]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing fevers: Neglected tick-borne diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 2: Borrelia Relapsing Fever Group and Unclassified Borrelia. Biology 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Jakab, Á.; Kahlig, P.; Kuenzli, E.; Neumayr, A. Tick borne relapsing fever-a systematic review and analysis of the literature. PLoS Negl. Trop. Dis. 2022, 16, e0010212. [Google Scholar] [CrossRef] [PubMed]
- Merriman, G. The geographical distribution of Ornithodorus moubata (Murray, 1877). Parasitology 1911, 4, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Ordman, D. Relapsing fever in South Africa with a record of its occurrence in Europeans. S. Afr. Med. J. 1955, 29, 518–521. [Google Scholar]
- Phipps, J. Ornithodoros moubata Murray in Tanganyika. East Afr. Med. J. 1950, 27, 475–482. [Google Scholar]
- Leeson, H. The recorded Distribution of Ornithodoros moubata (Murray) (Acarina). Bull. Entomol. Res. 1952, 43, 407–411. [Google Scholar] [CrossRef]
- Leeson, H.S. Some notes on the recorded distribution of old world species of Ornithodoros (Acarina). Bull. Entomol. Res. 1953, 44, 517–526. [Google Scholar] [CrossRef]
- Walton, G.A. Observations on biological variation in Ornithodoros moubata (Murr.)(Argasidae) in East Africa. Bull. Entomol. Res. 1957, 48, 669–710. [Google Scholar] [CrossRef]
- Sánchez Botija, C. Reservorios del virus de la Paste Porcina Africana. Investigation del virus de la PPA en las arthropodos mediante la prueba de la hemadsocion. Bull. Off. Int. Epizootiol. 1963, 60, 895–899. [Google Scholar]
- Wellman, F.C. Preliminary note on some bodies found in ticksOrn–ithodoros moubata (Murray)–Fed on blood-containing embryos of Filaria perstans (Manson). Br. Med. J. 1907, 2, 142. [Google Scholar] [CrossRef]
- Lloyd, L.I. On the Association of Warthog and the Nkufu Tick (Ornithodorus moubata). Ann. Trop. Med. Parasitol. 1915, 9, 559–560. [Google Scholar] [CrossRef]
- Schwetz, J. Notes protozoologiques. Les hématozoaires des grenouilles et des crapauds de Stanleyville (Congo Belge). Ann. Parasitol. Hum. Comp. 1930, 8, 122–134. [Google Scholar] [CrossRef]
- Schwetz, J. A mild epidemic among natives in Stanleyville (Belgian Congo). Bull. Soc. Pathol. Exot. 1933, 26, 1176–1181. [Google Scholar]
- Jack, R.W. Report of the Director of Agriculture, Southern Rhodesia; Department of Agriculture: Salisbury, Southern Rhodesia, 1930; p. 65.
- Bedford, G.A.H. The external parasites of poultry with measures for their control. J. Dep. Agric. 1924, 9, 123–140. [Google Scholar]
- Bedford, G.A.H. South African ticks: Part I. Onderstepoort J. Veter. Sci. Anim. Ind. 1934, 2, 49–99. [Google Scholar]
- Bedford, G.A.H. A synoptic check-list and host-list of the ectoparasites found on South African mammalia, aves and reptilia. Onderstepoort J. Veter. Sci. 1936, 7, 69–110. [Google Scholar]
- Wilson, S.G. Cattle ticks and their control by dipping in Nyasaland. Nyasal. Agric. Q. J. 1943, 3, 15. [Google Scholar]
- Chorley, T.W. An unusual occurrence of Ornithodoros moubata (Arachnida). In Proceedings of the Royal Entomological Society of London; Series A, General Entomology; Blackwell Publishing Ltd.: Oxford, UK, 1943; Volume 18, p. 27. [Google Scholar]
- Heisch, R.B.; Grainger, W.E. On the occurrence of Ornithodoros moubata Murray in burrows. Ann. Trop. Med. Parasitol. 1950, 44, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Walton, G.A. Ornithodorus moubata in wart-hog and porcupine burrows in Tanganyika territory. Trans. R. Soc. Trop. Med. Hyg. 1953, 47, 410–411. [Google Scholar] [CrossRef]
- Dias, J.A.T.S. Lista das carracas de Mocambique e respectivos hospedeiros. II. Anais dos Serviços Veterinários e Industria Animal Moçambique 1951, 4, 121–164. [Google Scholar]
- Walton, G.A. The Ornithodoros moubata Superspecies Problem in Relation to Human Relapsing Fever Epidemiology; Symposia of the Zoological Society of London: London, UK, 1962; pp. 83–153. [Google Scholar]
- Walton, G.A. The Ornithodorus “moubata” group of ticks in Africa. Control problems and implications. J. Med. Entomol. 1964, 1, 53–64. [Google Scholar] [CrossRef]
- Walton, G.A. A Biological Variant of Ornithodoros moubata Murray (Ixodoidea: Argasidae) from South Africa. Proc. Royal Entomol. Soc. Lond. 1959, 34, 63–72. [Google Scholar] [CrossRef]
- Walton, G.A. The reaction of some variants of Ornithodoros moubata Murray (Argasidae, Ixodoidea) to desiccation. Parasitology 1960, 50, 81–88. [Google Scholar] [CrossRef]
- Walton, G.A. Notification of the replacing of the single species concept of “Ornithodorus moubata” by a group of new species and the creation of a neotype for the nomen dubium moubata Murray. Trans. R. Soc. Trop. Med. Hyg. 1877, 56, 91–92. [Google Scholar] [CrossRef]
- Van der Merwe, S. Some remarks on the ‘tampans’ of the Ornithodoros moubata complex in Southern Africa. Zool. Anz 1968, 181, 280–289. [Google Scholar]
- Walton, G.A. A taxonomic review of the Ornithodoros moubata (Murray) 1877 (sensu Walton, 1962) species group in Africa. Recent Adv. Acarol. 1979, 1, 491–500. [Google Scholar]
- Walker, J.B. Ticks and human disease in tropical Africa, Proceedings of the International Symposium on Medicine in a Tropical Environment, South Africa, 1976; Gear, J.H.S., Ed.; British Medical Association: London, UK, 1976; pp. 276–290.
- Cutler, S.J. Relapsing fever—A forgotten disease revealed. J. Appl. Microbiol. 2010, 108, 1115–1122. [Google Scholar] [CrossRef]
- Cutler, S.J.; Ruzic-Sabljic, E.; Potkonjak, A. Emerging borreliae -expanding beyond Lyme borreliosis. Mol. Cell. Probes 2017, 31, 22–27. [Google Scholar] [CrossRef]
- Black, W.C.; Piesman, J. Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034–10038. [Google Scholar] [CrossRef] [Green Version]
- Jori, F.; Bastos, A.D. Role of wild suids in the epidemiology of African swine fever. EcoHealth 2009, 6, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Hofmeyr, M.D.H.; Boycott, R.C.; Baard, E.H.W. Chapter 7. Testudinae. In Atlas and Red List of the Reptiles of South Africa, Lesotho, and Swaziland; Bates, M.F., Branch, W.R., Bauer, A.M., Burger, M., Marais, J., Alexander, G.J., de Villliers, M.S., Eds.; Suricata 1; South African National Biodiversity Institute: Pretoria, South Africa, 2014; pp. 70–85. [Google Scholar]
- Hofmeyr, M.D.; Branch, W.R. The padloper’s tortuous path (Chelonia: Testudinidae): Two genera, not one. Afr. J. Herpetol. 2018, 67, 99–112. [Google Scholar] [CrossRef]
- Zhao, Z.; Oosthuizen, J.; Heideman, N. How many species does the Psammobates tentorius (tent tortoise) species complex (Reptilia, Testudinidae) comprise? A taxonomic solution potentially applicable to species complexes. J. Zool. Syst. Evol. Res. 2021, 59, 2189–2211. [Google Scholar] [CrossRef]
- Thomson, G.R. The epidemiology of African swine fever: The role of free-living hosts in Africa. Onderstepoort J. Vet. Res.. 1985, 52, 201–209. [Google Scholar] [PubMed]
- Borland, E.M.; Kading, R.C. Modernizing the Toolkit for Arthropod Bloodmeal Identification. Insects 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martin, V.; Manzano-Roman, R.; SIles-Lucas, M.; Oleaga, A.; Pérez-Sánchez, R. Cloning, characterization and diagnostic performance of the salivary lipocalin protein TSGP1 from Ornithodoros moubata. Veter. Parasitol. 2011, 178, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Plowright, W. African swine fever. In Infectious Diseases in Livestock with Special Reference to Southern Africa; Coetzer, J.A.W., Thomson, G.R., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 1994. [Google Scholar]
- Craig, A.F.; Heath, L.; Crafford, J.E.; Richt, J.A.; Swanepoel, R. Updated distribution and host records for the argasid tick Ornithodoros (Pavlovskyella) zumpti: A potential vector of African swine fever virus in South Africa. Onderstepoort J. Veter. Res. 2021, 88, a1960. [Google Scholar] [CrossRef] [PubMed]
- Vial, L.; Wieland, B.; Jori, F.; Etter, E.; Dixon, L.; Roger, F. African swine fever virus DNA in soft ticks, Senegal. Emerg. Infect. Dis. 2007, 13, 1928–1931. [Google Scholar] [CrossRef]
- Gallardo, C.; Mwaengo, D.M.; Macharia, J.M.; Arias, M.; Taracha, E.A.; Soler, A.; Okoth, E.; Martín, E.; Kasiti, J.; Bishop, R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54, p72, and pB602L (CVR) genes. Virus Genes 2009, 38, 85–95. [Google Scholar] [CrossRef]
- Maciejewski, M.; Kerley, G.I.H. Understanding tourists’ preference for mammal species in private protected areas: Is there a case for extralimital species for ecotourism? PLoS ONE 2014, 9, e88192. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg. Infect. Dis. 2015, 21, 312–315. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Tulman, E.R.; Delhon, G.; Diel, D.G.; Salnikov, N.; Kutish, G.F.; Kolbasov, D.; Rock, D.L. African swine fever virus CD2v and C-type lectin gene loci mediate serological specificity. J. Gen. Virol. 2015, 96, 866–873. [Google Scholar] [CrossRef]
- McIntosh, B.M. The propagation of African swine fever virus in the embryonated hen’s egg. J. S. Afr. Vet. Med. Assoc. 1952, 23, 217–220. [Google Scholar]
- Neitz, W.O. African Swine Fever. In Emerging Diseases of Animals; FAO Agricultural Studies: Rome, Italy, 1963. [Google Scholar]
- Molini, U.; Mushonga, B.; Settypalli, T.B.K.; Dundon, W.G.; Khaiseb, S.; Jago, M.; Cattoli, G.; Lamien, C.E. Molecular characterization of African swine fever virus from outbreaks in Namibia in 2018. Transbound. Emerg. Dis. 2020, 67, 1008–1014. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Stoian, A.M.; Rowland, R.R.; Dritz, S.S.; Petrovan, V.; Constance, L.A.; Gebhardt, J.T.; Olcha, M.; Jones, C.K.; Woodworth, J.C. Infectious dose of African swine fever virus when consumed naturally in liquid or feed. Emerg. Infect. Dis. 2019, 25, 891. [Google Scholar] [CrossRef]
- Montgomery, R.E. On a form of swine fever occurring in British East Africa Kenya Colony. J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef] [Green Version]
- Thomson, G.R.; Gainaru, M.D.; Van Dellen, A.F. Experimental infection of warthog Phacochoerus aethiopicus with African swine fever virus. Onderstepoort J. Vet. Res. 1980, 47, 19–22. [Google Scholar]
- Van Rensburg, L.J.; Penrith, M.L.; Van Heerden, J.; Heath, L.; Eric, M.E. Investigation into eradication of African swine fever in domestic pigs from a previous outbreak (2016/17) area of South Africa. Res. Veter. Sci. 2020, 133, 42–47. [Google Scholar] [CrossRef]
- Fasina, F.O.; Mokoele, J.M.; Spencer, B.T.; Van Leengoed, L.A.; Bevis, Y.; Booysen, I. Spatio-temporal patterns and movement analysis of pigs from smallholder farms and implications for African swine fever spread, Limpopo province, South Africa. Onderstepoort J. Vet. Res. 2015, 82, 11. [Google Scholar] [CrossRef] [Green Version]
- Etter, E.M.; Mushagaluza Ciza, A.; Mapendere, C.; Ferguson, W.; Jori, F.; Penrith, M. Understanding ASF dynamic in South Africa: From spatio-temporal analysis at national level to fine special network analysis. Front. Veter. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Vercammen, P.; Mason, D.R. The Warthogs Phacochoerus Africanus and P. aethiopicus: Status and Action Plan Summary; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 1993.
- Horak, I.G.; Biggs, H.C.; Hanssen, T.S.; Hanssen, R.E. The prevalence of helminth and arthropod parasites of warthog, Phacochoerus aethiopicus, in South West Africa/Namibia. Onderstepoort J. Vet. Res. 1983, 50, 145–148. [Google Scholar]
- Horak, I.G.; Boomker, J.; De Vos, V.; Potgieter, F.T. Parasites of domestic and wild animals in South Africa. XXIII. Helminth and arthropod parasites of warthogs, Phacochoerus aethiopicus, in the eastern Transvaal lowveld. Onderstepoort J. Vet. Res. 1988, 55, 145–152. [Google Scholar]
- Boomker, J.; Horak, I.G.; Booyse, D.G.; Meyer, S. Parasites of South African wildlife. VIII. Helminth and arthropod parasites ofwarthogs, Phacochoerus aethiopicus, in the eastern Transvaal. Onderstepoort J. Vet. Res. 1991, 58, 195–202. [Google Scholar]
- Cumming, D.H.M. Order: Suiformes. In The Mammals of the Southern African Sub-Region, 3rd ed.; Skinner, J., Chimimba, C., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 547–555. [Google Scholar] [CrossRef]
- Whittington-Jones, G.M.; Bernard, R.T.F.; Parker, D.M. Aardvark burrows: A potential resource for animals in arid and semi-arid environments. Afr. Zool. 2011, 46, 362–370. [Google Scholar] [CrossRef]
- Kada, S.; McCoy, K.D.; Boulinier, T. Impact of life stage-dependent dispersal on the colonization dynamics of host patches by ticks and tick-borne infectious agents. Parasites Vectors 2017, 4, 375. [Google Scholar] [CrossRef] [Green Version]
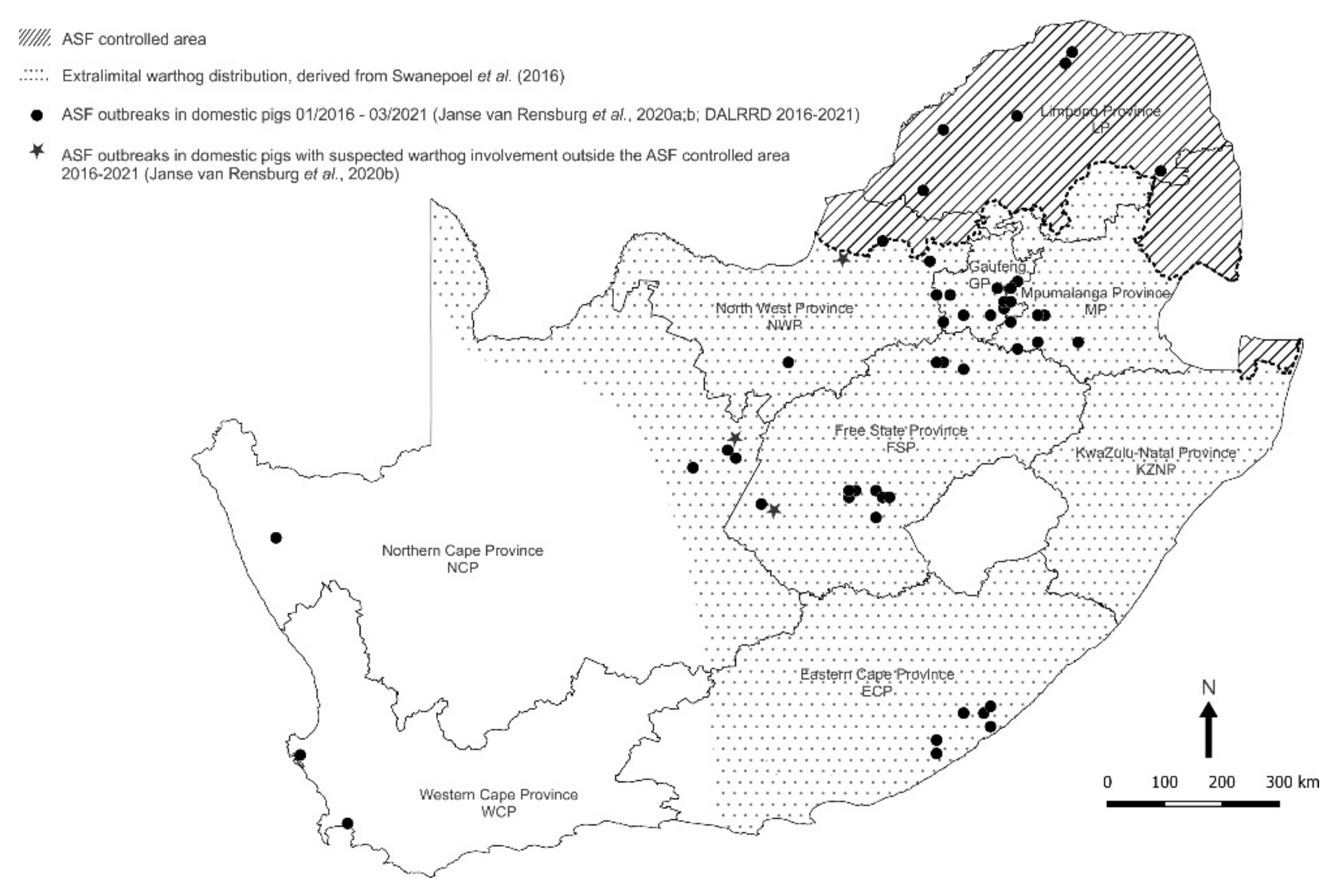





| Location | Collection | Ticks | Pools | ASFV qPCR | ASFV p72 | 16S rRNA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Location | Province 1 | Type | QDGC 2 | Sites | Collected | Tested | Positive | Genotype | Sequences | Tick Species |
| Locations inside ASF controlled area | ||||||||||
| LP01 | LP | Farm | E029S022CB | 1 | 218 | 7 | 6 | O. (O.) waterbergensis | ||
| LP02 | LP | Farm | E027S024CA | 1 | 2 | 1 | O. (O.) waterbergensis | |||
| LP03 | LP | Farm | E026S024DB | 3 | 160 | 27 | 1 | XXII | 2 | O. (O.) waterbergensis |
| GKNP01 | LP | Nature Reserve | E031S024AC | 1 | 82 | 12 | 1 | XX | 12 | O. (O.) phacochoerus |
| GKNP02 | LP | Nature Reserve | E031S022CA | 1 | 316 | 46 | 2 | O. (O.) phacochoerus | ||
| GKNP03 | LP | Nature Reserve | E031S022CA | 1 | 26 | 4 | 2 | O. (O.) phacochoerus | ||
| GKNP04 | LP | Nature Reserve | E031S023CC | 1 | 192 | 64 | 2 | O. (O.) phacochoerus | ||
| GKNP05 | LP | Nature Reserve | E031S023DC | 1 | 115 | 15 | 2 | O. (O.) phacochoerus | ||
| GKNP06 | LP | Nature Reserve | E031S023CD | 1 | 60 | 11 | 1 | O. (O.) phacochoerus | ||
| GKNP07 | MP | Nature Reserve | E031S024BA | 1 | 104 | 31 | 2 | XXI | 31 | O. (O.) phacochoerus |
| GKNP08 | MP | Nature Reserve | E031S024BA | 1 | 150 | 26 | 1 | O. (O.) phacochoerus | ||
| GKNP09 | MP | Nature Reserve | E031S024BC | 1 | 58 | 10 | 1 | O. (O.) phacochoerus | ||
| GKNP10 | MP | Nature Reserve | E031S024BC | 1 | 23 | 8 | 1 | O. (O.) phacochoerus | ||
| GKNP11 | MP | Nature Reserve | E031S024DB | 1 | 129 | 36 | 1 | O. (O.) phacochoerus | ||
| GKNP12 | MP | Nature Reserve | E031S024DD | 1 | 84 | 8 | 1 | O. (O.) phacochoerus | ||
| GKNP13 | MP | Nature Reserve | E031S024DB | 1 | 157 | 15 | 1 | O. (O.) phacochoerus | ||
| GKNP14 | MP | Nature Reserve | E031S025BA | 4 | 558 | 25 | 2 | O. (O.) phacochoerus | ||
| GKNP15 | MP | Nature Reserve | E031S025BA | 1 | 56 | 10 | 2 | O. (O.) phacochoerus | ||
| GKNP16 | MP | Nature Reserve | E031S025BC | 1 | 219 | 57 | 1 | O. (O.) phacochoerus | ||
| GKNP17 | MP | Nature Reserve | E031S025BD | 1 | 120 | 14 | 1 | O. (O.) phacochoerus | ||
| GKNP18 | MP | Nature Reserve | E031S025BD | 4 | 254 | 14 | 4 | XX, XXI | 4 | O. (O.) phacochoerus |
| NWP01 | NWP | Farm | E026S024CD | 5 | 148 | 25 | 3 | Ia | 3 | O. (O.) moubata |
| NWP02 | NWP | Farm | E026S025AA | 3 | 12 | 1 | 1 | O. (O.) moubata | ||
| NWP03 | NWP | Farm | E026S025AA | 1 | 136 | 10 | 2 | Ib | 3 | O. (O.) moubata |
| Locations outside ASF controlled area | ||||||||||
| NWP04 | NWP | Nature Reserve | E026S025CA | 1 | 7 | 2 | 1 | Ib | 1 | O. (O.) moubata |
| NWP05 | NWP | Farm | E026S025CA | 2 | 227 | 37 | 6 | Ib | 5 | O. (O.) moubata |
| MP01 | MP | Nature Reserve | E029S025CA | 10 | 0 | |||||
| GP01 | GP | Nature Reserve | E028S025BC | 1 | 8 | 5 | 4 | O. (O.) moubata | ||
| GP02 | GP | Nature Reserve | E028S025AD | 3 | 92 | 6 | 2 | O. (O.) moubata | ||
| GP03 | GP | Farm | E028S025CB | 1 | 1 | 1 | 1 | O. (O.) moubata | ||
| GP04 | GP | Nature Reserve | E028S025BA | 1 | 10 | 5 | 5 | O. (O.) moubata | ||
| GP05 | GP | Nature Reserve | E028S025BC | 1 | 10 | 4 | 2 | O. (O.) moubata | ||
| GP06-GP07 | GP | Farms | E027S026CB | 2 | 0 | |||||
| GP08-GP11 | GP | Farms | E027S026CA | 4 | 0 | |||||
| GP12-GP13 | GP | Farms | E028S025DA | 2 | 0 | |||||
| GP14-GP18 | GP | Farms | E028S025CB | 5 | 0 | |||||
| MNP01 | NCP | Nature Reserve | E024S029AB | 6 | 287 | 54 | 4 | Ia, Ic | 53 | O. (O.) compactus |
| NCP01 | NCP | Farm | E024S028BC | 3 | 316 | 51 | 19 | Ia, Ib, Ic 3 | 19 | O. (O.) compactus |
| NCP02 | NCP | Farm | E024S028AD | 2 | 86 | 11 | 3 | Ia,b | 4 | O. (O.) compactus |
| NCP03 | NCP | Nature Reserve | E024S028BC | 6 | 65 | 9 | 1 | O. (O.) compactus | ||
| NCP04 4 | NCP | Nature Reserve | E024S028CB | 1 | 32 | 3 | 1 | Ia | 3 | O. (O.) compactus |
| NCP04 4 | NCP | Nature Reserve | E024S028CB | 1 | 27 | 1 | 1 | O. (O.) kalahariensis | ||
| NCP05 | NCP | Farm | E023S028DD | 1 | 53 | 2 | 1 | O. (O.) compactus | ||
| NCP06 | NCP | Farm | E022S028CC | 3 | 31 | 3 | 1 | I | 1 | O. (O.) compactus |
| FSP01 | FSP | Nature Reserve | E024S028DD | 1 | 2 | 1 | 1 | O. (O.) compactus | ||
| FSP02 | FSP | Farm | E025S029AC | 3 | 97 | 7 | 1 | Ic | 3 | O. (O.) compactus |
| FSP03 | FSP | Farm | E024S029BD | 1 | 8 | 1 | 1 | III | 1 | O. (O.) compactus |
| AENP01 | ECP | Nature Reserve | E025S033BD | 1 | 7 | 1 | 1 | O. (P.) zumpti | ||
| AENP02 | ECP | Nature Reserve | E025S033BD | 1 | 234 | 23 | 23 | O. (P.) zumpti | ||
| AENP03 | ECP | Nature Reserve | E025S033DB | 2 | 91 | 5 | 5 | O. (P.) zumpti | ||
| ECP01 | ECP | Farm | E024S033AB | 2 | 8 | 2 | 1 | O. (O.) noorsveldensis | ||
| 105 | 5078 | 711 | 50 | 221 | ||||||
| Original Description | Walton, 1962 | Van der Merwe, 1968 | Walton, 1979 | Bakkes et al., 2018 (Type locality) |
|---|---|---|---|---|
| Eyes absent: Ornithodoros moubata group/complex | ||||
| Argas moubata Murray, 1877 | O. moubata | O. moubata moubata | O. moubata | O. moubata (South Africa) |
| O. porcinus porcinus | O. moubata porcinus | O. porcinus porcinus | O. porcinus (Tanzania) | |
| O. phacochoerus (South Africa) | ||||
| O. waterbergensis (South Africa) | ||||
| O. porcinus. domesticus | O. porcinus domesticus | |||
| O. porcinus avivora | ||||
| O. apertus | O. moubata apertus | O. apertus | O. apertus (Kenya) | |
| O. compactus | O. compactus | O. compactus | O. compactus (South Africa) | |
| Eyes present: Ornithodoros savignyi group/complex | ||||
| Argas savignyi Audouin, 1827 * | O. savignyi | O. savignyi | O. savignyi | O. savignyi (Egypt) |
| O. pavimentosus, Neumann, 1901 † | O. pavimentosus (South Africa) | |||
| O. kalahariensis (South Africa) | ||||
| O. noorsveldensis (South Africa) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craig, A.F.; Schade-Weskott, M.L.; Rametse, T.; Heath, L.; Kriel, G.J.P.; de Klerk-Lorist, L.-M.; van Schalkwyk, L.; Trujillo, J.D.; Crafford, J.E.; Richt, J.A.; et al. Detection of African Swine Fever Virus in Ornithodoros Tick Species Associated with Indigenous and Extralimital Warthog Populations in South Africa. Viruses 2022, 14, 1617. https://doi.org/10.3390/v14081617
Craig AF, Schade-Weskott ML, Rametse T, Heath L, Kriel GJP, de Klerk-Lorist L-M, van Schalkwyk L, Trujillo JD, Crafford JE, Richt JA, et al. Detection of African Swine Fever Virus in Ornithodoros Tick Species Associated with Indigenous and Extralimital Warthog Populations in South Africa. Viruses. 2022; 14(8):1617. https://doi.org/10.3390/v14081617
Chicago/Turabian StyleCraig, Anthony F., Mathilde L. Schade-Weskott, Thapelo Rametse, Livio Heath, Gideon J. P. Kriel, Lin-Mari de Klerk-Lorist, Louis van Schalkwyk, Jessie D. Trujillo, Jan E. Crafford, Juergen A. Richt, and et al. 2022. "Detection of African Swine Fever Virus in Ornithodoros Tick Species Associated with Indigenous and Extralimital Warthog Populations in South Africa" Viruses 14, no. 8: 1617. https://doi.org/10.3390/v14081617
APA StyleCraig, A. F., Schade-Weskott, M. L., Rametse, T., Heath, L., Kriel, G. J. P., de Klerk-Lorist, L. -M., van Schalkwyk, L., Trujillo, J. D., Crafford, J. E., Richt, J. A., & Swanepoel, R. (2022). Detection of African Swine Fever Virus in Ornithodoros Tick Species Associated with Indigenous and Extralimital Warthog Populations in South Africa. Viruses, 14(8), 1617. https://doi.org/10.3390/v14081617






