Changes in Body Mass Index after Initiation of Antiretroviral Treatment: Differences by Class of Core Drug
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Statistical Analysis
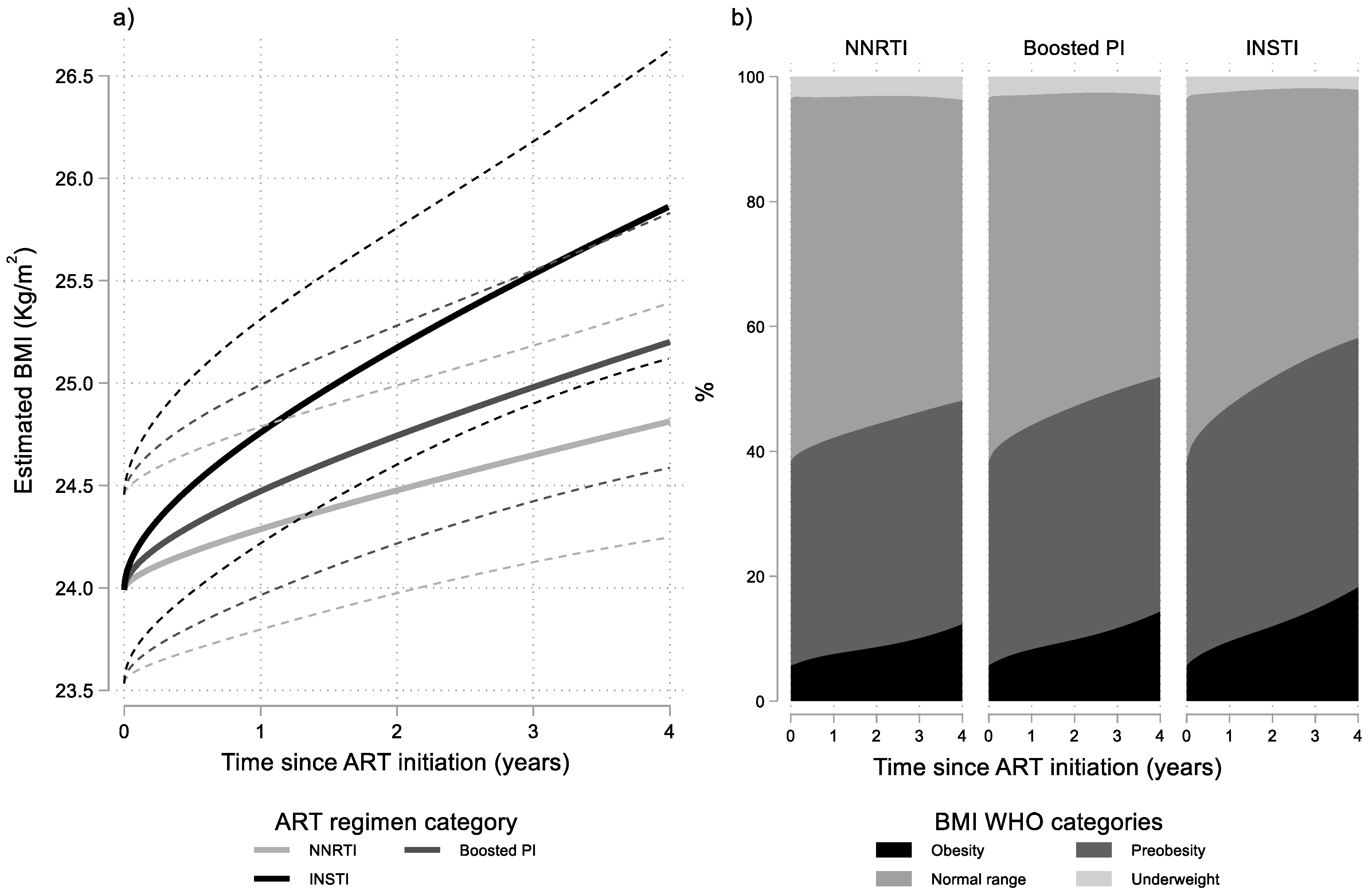
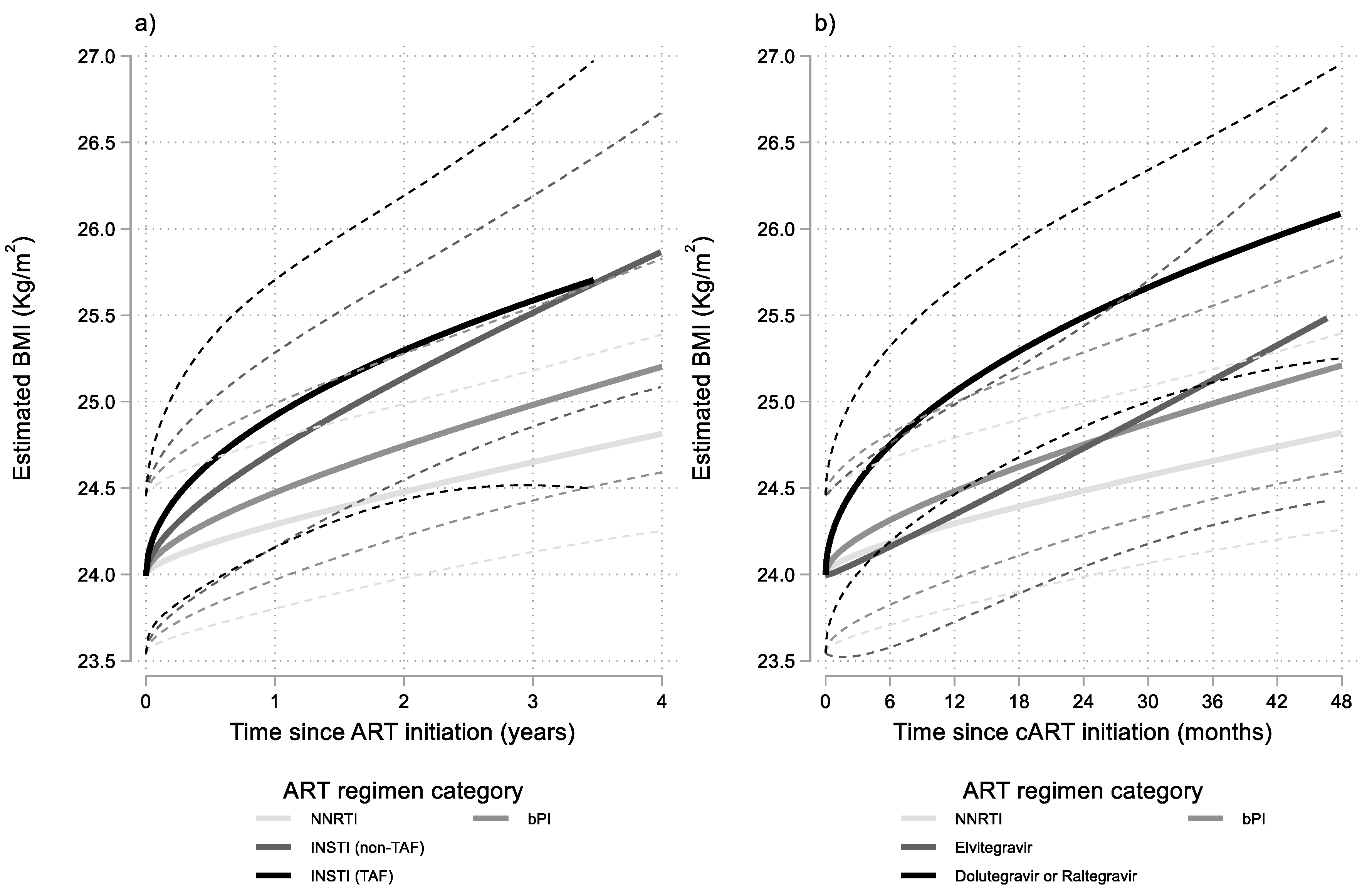
3. Results
Sensitivity Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Coodley, G.O.; Loveless, M.O.; Merrill, T.M. The HIV wasting syndrome: A review. J. Acquir. Immune. Defic. Syndr. 1994, 7, 681–694. [Google Scholar] [PubMed]
- Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef] [Green Version]
- Smit, E.; Skolasky, R.L.; Dobs, A.S.; Calhoun, B.C.; Visscher, B.R.; Palella, F.J.; Jacobson, L.P. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am. J. Epidemiol. 2002, 156, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schouten, J.; Wit, F.W.; Stolte, I.G.; Kootstra, N.A.; van der Valk, M.; Geerlings, S.E.; Prins, M.; Reiss, P.; AGEhIV Cohort Study Group. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clin. Infect. Dis. 2014, 59, 1787–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Samaras, K. The Impact of Weight Gain During HIV Treatment on Risk of Pre-diabetes, Diabetes Mellitus, Cardiovascular Disease, and Mortality. Front. Endocrinol. 2018, 9, 705. [Google Scholar] [CrossRef] [Green Version]
- Morse, C.G.; Kovacs, J.A. Metabolic and skeletal complications of HIV infection: The price of success. JAMA 2006, 296, 844–854. [Google Scholar] [CrossRef]
- Barnighausen, T.; Welz, T.; Hosegood, V.; Batzing-Feigenbaum, J.; Tanser, F.; Herbst, K.; Hill, C.; Newell, M.L. Hiding in the shadows of the HIV epidemic: Obesity and hypertension in a rural population with very high HIV prevalence in South Africa. J. Hum. Hypertens. 2008, 22, 236–239. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.; Tejidor, R.; Medina, S.; Barahona, I.; Ganesan, A. Obesity among patients with HIV: The latest epidemic. AIDS Patient Care STDS 2008, 22, 925–930. [Google Scholar] [CrossRef]
- Dorey-Stein, Z.; Amorosa, V.K.; Kostman, J.R.; Lo Re, V., 3rd; Shannon, R.P. Severe weight gain, lipodystrophy, dyslipidemia, and obstructive sleep apnea in a human immunodeficiency virus-infected patient following highly active antiretroviral therapy. J. Cardiometab. Syndr. 2008, 3, 111–114. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.; Roediger, M.P.; Eberly, L.; Headd, M.; Marconi, V.; Ganesan, A.; Weintrob, A.; Barthel, R.V.; Fraser, S.; Agan, B.K.; et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS ONE 2010, 5, e10106. [Google Scholar] [CrossRef]
- Achhra, A.C.; Mocroft, A.; Reiss, P.; Sabin, C.; Ryom, L.; de Wit, S.; Smith, C.J.; d’Arminio Monforte, A.; Phillips, A.; Weber, R.; et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: The D:A:D study. HIV Med. 2016, 17, 255–268. [Google Scholar] [CrossRef]
- Koethe, J.R.; Jenkins, C.A.; Lau, B.; Shepherd, B.E.; Justice, A.C.; Tate, J.P.; Buchacz, K.; Napravnik, S.; Mayor, A.M.; Horberg, M.A.; et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res. Hum. Retrovir. 2016, 32, 50–58. [Google Scholar] [CrossRef]
- McComsey, G.A.; Moser, C.; Currier, J.; Ribaudo, H.J.; Paczuski, P.; Dube, M.P.; Kelesidis, T.; Rothenberg, J.; Stein, J.H.; Brown, T.T. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin. Infect. Dis. 2016, 62, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Menard, A.; Meddeb, L.; Tissot-Dupont, H.; Ravaux, I.; Dhiver, C.; Mokhtari, S.; Tomei, C.; Brouqui, P.; Colson, P.; Stein, A. Dolutegravir and weight gain: An unexpected bothering side effect? AIDS 2017, 31, 1499–1500. [Google Scholar] [CrossRef]
- Norwood, J.; Turner, M.; Bofill, C.; Rebeiro, P.; Shepherd, B.; Bebawy, S.; Hulgan, T.; Raffanti, S.; Haas, D.W.; Sterling, T.R.; et al. Brief Report: Weight Gain in Persons With HIV Switched From Efavirenz-Based to Integrase Strand Transfer Inhibitor-Based Regimens. J. Acquir. Immune Defic. Syndr. 2017, 76, 527–531. [Google Scholar] [CrossRef]
- Bakal, D.R.; Coelho, L.E.; Luz, P.M.; Clark, J.L.; De Boni, R.B.; Cardoso, S.W.; Veloso, V.G.; Lake, J.E.; Grinsztejn, B. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J. Antimicrob. Chemother. 2018, 73, 2177–2185. [Google Scholar] [CrossRef]
- Bourgi, K.; Rebeiro, P.F.; Turner, M.; Castilho, J.L.; Hulgan, T.; Raffanti, S.P.; Koethe, J.R.; Sterling, T.R. Greater Weight Gain in Treatment-naive Persons Starting Dolutegravir-based Antiretroviral Therapy. Clin. Infect. Dis. 2020, 70, 1267–1274. [Google Scholar] [CrossRef]
- Bourgi, K.; Jenkins, C.A.; Rebeiro, P.F.; Palella, F.; Moore, R.D.; Altoff, K.N.; Gill, J.; Rabkin, C.S.; Gange, S.J.; Horberg, M.A.; et al. Weight gain among treatment-naive persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J. Int. AIDS Soc. 2020, 23, e25484. [Google Scholar] [CrossRef] [Green Version]
- Sax, P.E.; Erlandson, K.M.; Lake, J.E.; McComsey, G.A.; Orkin, C.; Esser, S.; Brown, T.T.; Rockstroh, J.K.; Wei, X.; Carter, C.C.; et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin. Infect. Dis. 2020, 71, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Venter, W.D.F.; Sokhela, S.; Simmons, B.; Moorhouse, M.; Fairlie, L.; Mashabane, N.; Serenata, C.; Akpomiemie, G.; Masenya, M.; Qavi, A.; et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): Week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 2020, 7, e666–e676. [Google Scholar] [CrossRef]
- Ruderman, S.A.; Crane, H.M.; Nance, R.M.; Whitney, B.M.; Harding, B.N.; Mayer, K.H.; Moore, R.D.; Eron, J.J.; Geng, E.; Mathews, W.C.; et al. Brief Report: Weight Gain Following ART Initiation in ART-Naive People Living With HIV in the Current Treatment Era. J. Acquir. Immune Defic. Syndr. 2021, 86, 339–343. [Google Scholar] [CrossRef]
- Venter, W.D.F.; Moorhouse, M.; Sokhela, S.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef]
- Mallon, P.W.; Brunet, L.; Hsu, R.K.; Fusco, J.S.; Mounzer, K.C.; Prajapati, G.; Beyer, A.P.; Wohlfeiler, M.B.; Fusco, G.P. Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J. Int. AIDS Soc. 2021, 24, e25702. [Google Scholar] [CrossRef]
- Gomez, M.; Seybold, U.; Roider, J.; Harter, G.; Bogner, J.R. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015–2017. Infection 2019, 47, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Plum, P.E.; Maes, N.; Sauvage, A.S.; Frippiat, F.; Meuris, C.; Uurlings, F.; Lecomte, M.; Leonard, P.; Paquot, N.; Fombellida, K.; et al. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect. Dis. 2021, 21, 910. [Google Scholar] [CrossRef]
- Lomiak, M.; Stepnicki, J.; Mikula, T.; Wiercinska-Drapalo, A. Weight and body mass index increase after switch from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate-containing treatment in an antiretroviral therapy-experienced group. Int. J. STD AIDS 2021, 32, 570–577. [Google Scholar] [CrossRef]
- Pantazis, N.; Papastamopoulos, V.; Paparizos, V.; Metallidis, S.; Adamis, G.; Antoniadou, A.; Psichogiou, M.; Chini, M.; Sambatakou, H.; Sipsas, N.V.; et al. Long-term evolution of CD4+ cell count in patients under combined antiretroviral therapy. AIDS 2019, 33, 1645–1655. [Google Scholar] [CrossRef]
- Harrison, L.; Dunn, D.T.; Green, H.; Copas, A.J. Modelling the association between patient characteristics and the change over time in a disease measure using observational cohort data. Stat. Med. 2009, 28, 3260–3275. [Google Scholar] [CrossRef]
- Bernardino, J.I.; Mocroft, A.; Wallet, C.; de Wit, S.; Katlama, C.; Reiss, P.; Mallon, P.W.; Richert, L.; Molina, J.M.; Knobel, H.; et al. Body composition and adipokines changes after initial treatment with darunavir-ritonavir plus either raltegravir or tenofovir disoproxil fumarate-emtricitabine: A substudy of the NEAT001/ANRS143 randomised trial. PLoS ONE 2019, 14, e0209911. [Google Scholar] [CrossRef] [PubMed]
- Calmy, A.; Tovar Sanchez, T.; Kouanfack, C.; Mpoudi-Etame, M.; Leroy, S.; Perrineau, S.; Lantche Wandji, M.; Tetsa Tata, D.; Omgba Bassega, P.; Abong Bwenda, T.; et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): Week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020, 7, e677–e687. [Google Scholar] [CrossRef]
- Martinez-Sanz, J.; Blanco, J.R.; Muriel, A.; Perez-Elias, M.J.; Rubio-Martin, R.; Berenguer, J.; Peraire, J.; Bernal, E.; Martinez, O.J.; Serrano-Villar, S.; et al. Weight changes after antiretroviral therapy initiation in CoRIS (Spain): A prospective multicentre cohort study. J. Int. AIDS Soc. 2021, 24, e25732. [Google Scholar] [CrossRef] [PubMed]
- Bansi-Matharu, L.; Phillips, A.; Oprea, C.; Grabmeier-Pfistershammer, K.; Gunthard, H.F.; De Wit, S.; Guaraldi, G.; Vehreschild, J.J.; Wit, F.; Law, M.; et al. Contemporary antiretrovirals and body-mass index: A prospective study of the RESPOND cohort consortium. Lancet HIV 2021, 8, e711–e722. [Google Scholar] [CrossRef]
- Bai, R.; Lv, S.; Wu, H.; Dai, L. Effects of different integrase strand transfer inhibitors on body weight in patients with HIV/AIDS: A network meta-analysis. BMC Infect. Dis. 2022, 22, 118. [Google Scholar] [CrossRef]
- Kerchberger, A.M.; Sheth, A.N.; Angert, C.D.; Mehta, C.C.; Summers, N.A.; Ofotokun, I.; Gustafson, D.; Weiser, S.D.; Sharma, A.; Adimora, A.A.; et al. Weight Gain Associated With Integrase Stand Transfer Inhibitor Use in Women. Clin. Infect. Dis. 2020, 71, 593–600. [Google Scholar] [CrossRef]
| ART Regimen Category | Overall 982 (100.0%) | p-Value | |||
|---|---|---|---|---|---|
| Boosted PI 1 352 (35.85%) | NNRTI 2 364 (37.07%) | INSTI 3 266 (27.09%) | |||
| Age at ART initiation (years) | 35.6 (29.9, 43.6) | 34.2 (29.8, 41.3) | 35.4 (29.3, 44.1) | 35.2 (29.7, 42.9) | 0.194 |
| Female sex | 38 (10.80%) | 25 (6.87%) | 17 (6.39%) | 80 (8.15%) | 0.075 |
| Transmission mode | <0.001 | ||||
| MSM 4 | 192 (54.55%) | 259 (71.15%) | 214 (80.45%) | 665 (67.72%) | |
| PWID 5 | 70 (19.89%) | 37 (10.16%) | 22 (8.27%) | 129 (13.14%) | |
| Heterosexual | 71 (20.17%) | 48 (13.19%) | 25 (9.40%) | 144 (14.66%) | |
| NA 6 | 19 (5.40%) | 20 (5.49%) | 5 (1.88%) | 44 (4.48%) | |
| Ethnic/racial group | 0.015 | ||||
| White | 320 (90.91%) | 339 (93.13%) | 259 (97.37%) | 918 (93.48%) | |
| Black | 3 (0.85%) | 4 (1.10%) | 2 (0.75%) | 9 (0.92%) | |
| Asian | 0 (0.00%) | 2 (0.55%) | 1 (0.38%) | 3 (0.31%) | |
| NA 6 | 29 (8.24%) | 19 (5.22%) | 4 (1.50%) | 52 (5.30%) | |
| Baseline 7 CD4 (cells/μL) | 263 (128, 376) | 342 (269, 434) | 358 (249, 499) | 325 (212, 428) | <0.001 |
| Baseline 7 HIV-RNA (log10 copies/mL) | 4.59 (4.00, 5.09) | 4.45 (3.95, 4.82) | 4.46 (3.71, 5.03) | 4.50 (3.94, 4.99) | 0.022 |
| Year of ART initiation | 2012 (2011, 2013) | 2012 (2011, 2013) | 2016 (2014, 2018) | 2013 (2011, 2015) | <0.001 |
| Months from diagnosis to ART | 2.71 (0.82, 7.82) | 9.36 (2.68, 28.45) | 1.58 (0.62, 4.47) | 3.45 (1.12, 13.11) | <0.001 |
| ART NRTI 2 backbone | <0.001 | ||||
| Tenofovir disoproxil fumarate & Emtricitabine | 282 (80.11%) | 355 (97.53%) | 144 (54.14%) | 781 (79.53%) | |
| Tenofovir alafenamide & Emtricitabine | 0 (0.00%) | 3 (0.82%) | 69 (25.94%) | 72 (7.33%) | |
| Lamivudine & Abacavir | 64 (18.18%) | 6 (1.65%) | 43 (16.17%) | 113 (11.51%) | |
| Other | 6 (1.70%) | 0 (0.00%) | 10 (3.76%) | 16 (1.63%) | |
| AIDS before baseline | 22 (6.25%) | 12 (3.30%) | 8 (3.01%) | 42 (4.28%) | 0.072 |
| Baseline 7 Weight (kg) | 72.0 (65.0, 80.0) | 74.5 (68.0, 83.0) | 75.0 (67.0, 82.0) | 74.0 (66.0, 82.0) | 0.005 |
| Baseline 7 Height (cm) | 176 (171, 180) | 178 (173, 183) | 178 (172, 182) | 1.78 (1.72, 1.82) | 0.002 |
| Baseline 7 BMI 8 (kg/m 2) | 23.36 (21.47, 25.13) | 23.66 (21.69, 25.85) | 23.67 (21.71, 25.93) | 23.53 (21.67, 25.66) | 0.182 |
| Baseline 7 BMI 8 classification | 0.293 | ||||
| Underweight | 19 (5.40%) | 10 (2.75%) | 11 (4.14%) | 40 (4.07%) | |
| Normal range | 237 (67.33%) | 238 (65.38%) | 167 (62.78%) | 642 (65.38%) | |
| Preobesity | 83 (23.58%) | 93 (25.55%) | 75 (28.20%) | 251 (25.56%) | |
| Obesity | 13 (3.69%) | 23 (6.32%) | 13 (4.89%) | 49 (4.99%) | |
| Follow-up time (years) | 1.73 (0.09, 3.44) | 2.91 (0.54, 3.68) | 0.78 (0.00, 2.43) | 1.80 (0.02, 3.47) | <0.001 |
| Number of BMI measurements | 5.0 (2.0, 9.0) | 6.0 (2.0, 9.0) | 3.0 (1.0, 5.0) | 4.0 (2.0, 8.0) | <0.001 |
| Covariate | Coefficient | 95% CI | p-Value (df 1) |
|---|---|---|---|
| Intercept (reference category 2) | 3.178 | (3.158, 3.197) | <0.001 (1) |
| Time (in years) trend (reference category 2) | <0.001 (1) | ||
| Time-1: Square root of time | 0.008 | (−0.008, 0.023) | |
| Time-2: Time | 0.005 | (−0.004, 0.013) | |
| Interaction: ART regimen category on time trend | 0.014 (4) | ||
| ART regimen category X Time-1 | |||
| Boosted PI 3 /NNRTI 4 | 0.007 | (−0.015, 0.030) | |
| INSTI 5/NNRTI 4 | 0.018 | (−0.009, 0.045) | |
| ART regimen category X Time-2 | |||
| Boosted PI 3 /NNRTI 4 | 0.000 | (−0.012, 0.013) | |
| INSTI 5/NNRTI 4 | 0.002 | (−0.014, 0.017) | |
| Main effect: Age (years) | <0.001 (3) | ||
| 20–29/30–39 | −0.059 | (−0.081, −0.037) | |
| 40–49/30–39 | 0.035 | (0.012, 0.058) | |
| 50+/30–39 | 0.010 | (−0.023, 0.043) | |
| Main effect: HIV-RNA at ART initiation (copies/mL) | 0.010 (4) | ||
| <500/10,000–49,999 | 0.040 | (−0.001, 0.080) | |
| 500–9999/10,000–49,999 | 0.010 | (−0.014, 0.035) | |
| 50,000–99,999/10,000–49,999 | −0.008 | (−0.034, 0.019) | |
| 100,000+/10,000–49,999 | −0.025 | (−0.049, −0.002) | |
| Main effect: AIDS before ART initiation | 0.047 (1) | ||
| Yes/No | −0.045 | (−0.090, −0.001) | |
| Interaction: AIDS before ART initiation on time trend | <0.001 (2) | ||
| AIDS before ART X Time-1: Yes/No | 0.178 | (0.123, 0.233) | |
| AIDS before ART X Time-2: Yes/No | −0.057 | (−0.088, −0.025) | |
| Main effect: Sex &Transmission category | <0.001 (5) | ||
| Male PWID 6 /MSM 7 | −0.066 | (−0.094, −0.038) | |
| Female PWID 6/MSM 1 | −0.100 | (−0.171, −0.028) | |
| Male Heterosexual/MSM 7 | 0.027 | (−0.005,−0.060) | |
| Female Heterosexual/MSM 7 | 0.004 | (−0.033, 0.042) | |
| NA 8/MSM 7 | 0.057 | (0.013, 0.100) |
| Time since ART Initiation (Years) | ART Regimen Category | Estimated Probabilities (%) | |||
|---|---|---|---|---|---|
| Underweight | Normal Range | Preobesity | Obesity | ||
| NNRTI 1 | 3.3 | 58.1 | 32.8 | 5.7 | |
| 0 (Baseline) | Boosted PI 2 | 3.3 | 58.1 | 32.8 | 5.7 |
| INSTI 3 | 3.3 | 58.1 | 32.8 | 5.7 | |
| NNRTI 1 | 3.2 | 54.7 | 34.7 | 7.5 | |
| 1 | Boosted PI | 2.8 | 53.0 | 36.0 | 8.2 |
| INSTI 3 | 2.3 | 50.3 | 37.9 | 9.5 | |
| NNRTI 1 | 3.0 | 52.7 | 35.8 | 8.5 | |
| 2 | Boosted PI 2 | 2.5 | 50.3 | 37.5 | 9.7 |
| INSTI 3 | 1.9 | 46.2 | 40.0 | 11.9 | |
| NNRTI 1 | 3.0 | 50.7 | 36.4 | 9.9 | |
| 3 | Boosted PI 2 | 2.5 | 47.7 | 38.2 | 11.6 |
| INSTI 3 | 1.8 | 42.8 | 40.9 | 14.6 | |
| NNRTI 1 | 3.6 | 48.3 | 36.0 | 12.2 | |
| 4 | Boosted PI 2 | 2.9 | 45.2 | 37.7 | 14.2 |
| INSTI 3 | 2.0 | 39.8 | 40.2 | 18.1 | |
| Baseline BMI (kg/m2) | ART Regimen Category | Time since ART Initiation | |||
|---|---|---|---|---|---|
| 1 Year | 2 Years | 3 Years | 4 Years | ||
| NNRTI 1 | 1.79 (0.95, 2.63) | 2.48 (1.52, 3.43) | 2.99 (1.85, 4.13) | 3.41 (1.95, 4.86) | |
| 18 | Boosted PI 2 | 2.23 (1.36, 3.10) | 3.12 (2.12, 4.12) | 3.79 (2.58, 4.99) | 4.34 (2.79, 5.89) |
| INSTI 3 | 2.92 (1.94, 3.89) | 4.15 (3.02, 5.28) | 5.12 (3.71, 6.52) | 5.94 (4.07, 7.80) | |
| NNRTI 1 | 1.09 (0.46, 1.73) | 1.71 (0.99, 2.42) | 2.25 (1.41, 3.08) | 2.74 (1.69, 3.80) | |
| 23 | Boosted PI 2 | 1.65 (0.96, 2.34) | 2.51 (1.72, 3.30) | 3.24 (2.29, 4.19) | 3.91 (2.69, 5.14) |
| INSTI 3 | 2.51 (1.63, 3.39) | 3.81 (2.79, 4.83) | 4.91 (3.62, 6.20) | 5.91 (4.17, 7.65) | |
| NNRTI 1 | NS 4 | NS 4 | 1.45 (0.35, 2.55) | 2.01 (0.62, 3.40) | |
| 27 | Boosted PI 2 | 1.03 (0.15, 1.92) | 1.83 (0.82, 2.85) | 2.61 (1.39, 3.82) | 3.36 (1.79, 4.94) |
| INSTI 3 | 2.03 (0.94, 3.12) | 3.34 (2.08, 4.60) | 4.53 (2.94, 6.12) | 5.67 (3.54, 7.81) | |
| NNRTI 1 | NS 4 | NS 4 | NS 4 | NS 4 | |
| 33 | Boosted PI 2 | NS 4 | NS 4 | NS 4 | NS 4 |
| INSTI 3 | NS 4 | 2.37 (0.28, 4.46) | 3.68 (1.11, 6.26) | 5.03 (1.63, 8.44) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantazis, N.; Papastamopoulos, V.; Antoniadou, A.; Adamis, G.; Paparizos, V.; Metallidis, S.; Sambatakou, H.; Psichogiou, M.; Chini, M.; Chrysos, G.; et al. Changes in Body Mass Index after Initiation of Antiretroviral Treatment: Differences by Class of Core Drug. Viruses 2022, 14, 1677. https://doi.org/10.3390/v14081677
Pantazis N, Papastamopoulos V, Antoniadou A, Adamis G, Paparizos V, Metallidis S, Sambatakou H, Psichogiou M, Chini M, Chrysos G, et al. Changes in Body Mass Index after Initiation of Antiretroviral Treatment: Differences by Class of Core Drug. Viruses. 2022; 14(8):1677. https://doi.org/10.3390/v14081677
Chicago/Turabian StylePantazis, Nikos, Vasilios Papastamopoulos, Anastasia Antoniadou, Georgios Adamis, Vasilios Paparizos, Simeon Metallidis, Helen Sambatakou, Mina Psichogiou, Maria Chini, Georgios Chrysos, and et al. 2022. "Changes in Body Mass Index after Initiation of Antiretroviral Treatment: Differences by Class of Core Drug" Viruses 14, no. 8: 1677. https://doi.org/10.3390/v14081677
APA StylePantazis, N., Papastamopoulos, V., Antoniadou, A., Adamis, G., Paparizos, V., Metallidis, S., Sambatakou, H., Psichogiou, M., Chini, M., Chrysos, G., Panagopoulos, P., Sipsas, N. V., Barbunakis, E., Gogos, C., & Touloumi, G., on behalf of the Athens Multicenter AIDS Cohort Study (AMACS). (2022). Changes in Body Mass Index after Initiation of Antiretroviral Treatment: Differences by Class of Core Drug. Viruses, 14(8), 1677. https://doi.org/10.3390/v14081677







