Undiagnosed HIV Infections May Drive HIV Transmission in the Era of “Treat All”: A Deep-Sampling Molecular Network Study in Northeast China during 2016 to 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data and Sample Collection
2.3. Definition of ART Status
2.4. HIV-1 Limiting Antigen (LAg) Avidity Enzyme Immunoassay
2.5. Sequence Analysis
2.6. Estimating Effective Reproductive Number (Re) for Dominant Subtypes
2.7. Identification and Analysis of the Molecular Networks
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics and ART Status of the Cases
3.2. Re of Dominant HIV Strains
3.3. Molecular Networks of Dominant HIV Strains
3.4. Drivers of HIV Transmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Fact Sheet: Treat All Policy and Implementation Status in Countries; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/treat-all-policy-adoption-and-implementation-status-in-countries (accessed on 1 June 2021).
- UNAIDS. Global HIV & AIDS Statistics—2020 Fact Sheet; UNAIDS: Geneva, Switzerland, 2020; Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 1 June 2021).
- UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic; The Joint United Nations Programme on HIV/AIDS. Available online: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (accessed on 1 October 2014).
- Commission, G.; Oonha, F.P. Notice of the General Office of the National Health and Family Planning Commission on Adjusting the Standard of Free Antiretroviral Treatment for AIDS. Available online: http://www.nhc.gov.cn/yzygj/s3593/201606/0b0fa78e10dc41328e842b1bf9cd433e.shtml (accessed on 15 July 2016).
- China DCBoNHCo. New Progress in AIDS Prevention and Control in China in 2019. Available online: http://www.nhc.gov.cn/jkj/s3586/201911/c2388ce70bdd404ea6dfcd886591784d.shtml (accessed on 5 January 2020).
- Chuai, Z.; Zhang, Y.; Zhao, Y.; Yan, J.; Sun, Z.; Wang, Y.; Jiao, Y.; Wang, F. Latest AIDS epidemic in global and China. Infect. Dis. Info. 2020, 33, 501–503. [Google Scholar]
- China DCBoNHCo. Regular Press Conference of the National Health Commission on 23 November 2018. Available online: https://ncaids.chinacdc.cn/zxzx/mtsd3/201811/t20181123_197487.htm (accessed on 25 November 2018).
- Duong, Y.T.; Qiu, M.; De, A.K.; Jackson, K.; Dobbs, T.; Kim, A.A.; Nkengasong, J.N.; Parekh, B.S. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: Potential for HIV-1 incidence estimates and avidity maturation studies. PLoS ONE 2012, 7, e33328. [Google Scholar] [CrossRef] [PubMed]
- Poon, A.F.Y.; Gustafson, R.; Daly, P.; Zerr, L.; Demlow, S.E.; Wong, J.; Woods, C.K.; Hogg, R.; Krajden, M.; Moore, D.; et al. Near real-time monitoring of HIV transmission hotspots from routine HIV genotyping: An implementation case study. Lancet HIV 2016, 3, e231–e238. [Google Scholar] [CrossRef]
- Oster, A.M.; Wertheim, J.O.; Hernandez, A.L.; Ocfemia, M.C.; Saduvala, N.; Hall, H.I. Using Molecular HIV Surveillance Data to Understand Transmission Between Subpopulations in the United States. J. Acquir. Immune Defic. Syndr. 2015, 70, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Leigh Brown, A.J.; Hepler, N.L.; Mehta, S.R.; Richman, D.D.; Smith, D.M.; Pond, S.L.K. The global transmission network of HIV-1. J. Infect. Dis. 2014, 209, 304–313. [Google Scholar] [CrossRef]
- Kang, R.; Li, J.; Chen, H.; Tang, Z.; Pan, S.W.; Luo, L.; Zhu, Q.; Lan, G.; Liang, S.; Lu, H.; et al. Using longitudinal genetic-network study to understand HIV treatment-as-prevention. AIDS 2021, 35, 947–955. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Scott, S.R.; McGoogan, J.M. History of the HIV Epidemic in China. Curr. HIV/AIDS Rep. 2019, 16, 458–466. [Google Scholar] [CrossRef]
- Zhao, B.; Song, W.; Kang, M.; Dong, X.; Li, X.; Wang, L.; Liu, J.; Ding, H.; Chu, Z.; Wang, L.; et al. Molecular Network Analysis Reveals Transmission of HIV-1 Drug-Resistant Strains Among Newly Diagnosed HIV-1 Infections in a Moderately HIV Endemic City in China. Front. Microbiol. 2021, 12, 797771. [Google Scholar] [CrossRef]
- Wang, L.; Qin, Q.Q.; Ding, Z. Current Situation of AIDS epidemic in China. Chin. J. AIDS STD 2017, 23, 330–333. [Google Scholar]
- AIDS and Hepatitis C Professional Group SoID; Chinese Medical Association Chinese Center for Disease Control and Prevention. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2018). Zhonghua Nei Ke Za Zhi 2018, 57, 867–884. [Google Scholar]
- Biomedical Maxim. HIV-1 Limiting Antigen Avidity EIA: Single Well Avidity Enzyme Immunoassay for Detection of Recent HIV-1 Infection, Cat. No.92001; Biomedical Maxim: Rockville, MD, USA, 2013. [Google Scholar]
- Zhao, B.; Han, X.; Dai, D.; Liu, J.; Ding, H.; Xu, J.; Chu, Z.; Bice, T.; Diao, Y.; Shang, H. New trends of primary drug resistance among HIV type 1-infected men who have sex with men in Liaoning Province, China. AIDS Res. Hum. Retrovir. 2011, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.K.; Brumme, C.J.; Liu, T.F.; Chui, C.K.; Chu, A.L.; Wynhoven, B.; Hall, T.A.; Trevino, C.; Shafer, R.W.; Harrigan, P.R. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J. Clin. Microbiol. 2012, 50, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Stadler, T.; Kuhnert, D.; Bonhoeffer, S.; Drummond, A.J. Birth-death skyline plot reveals temporal changes of epidemic spread in HIV and hepatitis C virus (HCV). Proc. Natl. Acad. Sci. USA 2013, 110, 228–233. [Google Scholar] [CrossRef]
- Vasylyeva, T.I.; du Plessis, L.; Pineda-Pena, A.C.; Kuhnert, D.; Lemey, P.; Vandamme, A.M.; Gomes, P.; Camacho, R.J.; Pybus, O.; Abecasis, A.; et al. Tracing the Impact of Public Health Interventions on HIV-1 Transmission in Portugal Using Molecular Epidemiology. J. Infect. Dis. 2019, 220, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.M.; Hue, S.; Billock, R.; Levintow, S.; Sebastian, J.; Miller, W.C.; Eron, J.J. Human Immunodeficiency Virus Type 1 Phylodynamics to Detect and Characterize Active Transmission Clusters in North Carolina. J. Infect. Dis. 2020, 221, 1321–1330. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Weaver, S.; Leigh Brown, A.J.; Wertheim, J.O. HIV-TRACE (TRAnsmission Cluster Engine): A Tool for Large Scale Molecular Epidemiology of HIV-1 and Other Rapidly Evolving Pathogens. Mol. Biol. Evol. 2018, 35, 1812–1819. [Google Scholar] [CrossRef]
- Zhao, B.; Song, W.; An, M.; Dong, X.; Li, X.; Wang, L.; Liu, J.; Tian, W.; Wang, Z.; Ding, H.; et al. Priority Intervention Targets Identified Using an In-Depth Sampling HIV Molecular Network in a Non-Subtype B Epidemics Area. Front. Cell. Infect. Microbiol. 2021, 11, 642903. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar]
- Ratmann, O.; van Sighem, A.; Bezemer, D.; Gavryushkina, A.; Jurriaans, S.; Wensing, A.; de Wolf, F.; Reiss, P.; Fraser, C.; Cohort, A.O. Sources of HIV infection among men having sex with men and implications for prevention. Sci. Transl. Med. 2016, 8, 320ra322. [Google Scholar] [CrossRef]
- Dennis, A.M.; Frost, S.D.W.; Enders, K.; Cressman, A.E.; Volz, E.; Adams, N.; Miller, W.C.; Cohen, M.S.; Mobley, V.; Samoff, E.; et al. HIV-1 Transmission linkages among persons with incident infection to inform public health surveillance. EClinicalMedicine 2021, 37, 100968. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M.; Detels, R. The Enigma of the Human Immunodeficiency Virus (HIV) Epidemic in China. Clin. Infect. Dis. 2021, 72, 876–881. [Google Scholar] [CrossRef]
- Pybus, O.G.; Rambaut, A. Evolutionary analysis of the dynamics of viral infectious disease. Nat. Rev. Genet. 2009, 10, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Vinken, L.; Fransen, K.; Cuypers, L.; Alexiev, I.; Balotta, C.; Debaisieux, L.; Seguin-Devaux, C.; Ribas, S.G.; Gomes, P.; Incardona, F.; et al. Earlier Initiation of Antiretroviral Treatment Coincides with an Initial Control of the HIV-1 Sub-Subtype F1 Outbreak Among Men-Having-Sex-With-Men in Flanders, Belgium. Front. Microbiol. 2019, 10, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodger, A.J.; Cambiano, V.; Bruun, T.; Vernazza, P.; Collins, S.; Degen, O.; Corbelli, G.M.; Estrada, V.; Geretti, A.M.; Beloukas, A.; et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet 2019, 393, 2428–2438. [Google Scholar] [CrossRef]
- World Health Organization. HIV Data and Statistics; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 14 July 2022).
- Skarbinski, J.; Rosenberg, E.; Paz-Bailey, G.; Hall, H.I.; Rose, C.E.; Viall, A.H.; Fagan, J.L.; Lansky, A.; Mermin, J.H. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern. Med. 2015, 175, 588–596. [Google Scholar] [CrossRef]
- Bachmann, N.; Kusejko, K.; Nguyen, H.; Chaudron, S.E.; Kadelka, C.; Turk, T.; Böni, J.; Perreau, M.; Klimkait, T.; Yerly, S.; et al. Phylogenetic Cluster Analysis Identifies Virological and Behavioral Drivers of Human Immunodeficiency Virus Transmission in Men Who Have Sex with Men. Clin. Infect. Dis. 2021, 72, 2175–2183. [Google Scholar] [CrossRef]
- National Center for HIV/AIDS VH, STD, and TB Prevention; Division of HIV/AIDS. Prevention Detecting and Responding to HIV Transmission Clusters: A Guide for Health Department; 2018. Available online: https://www.cdc.gov/hiv/pdf/funding/announcements/ps18-1802/cdc-hiv-ps18-1802-attachmente-detecting-investigating-and-responding-to-hiv-transmission-clusters.pdf (accessed on 14 July 2022).
- The State Council of the People’s Republic of China. Available online: http://www.gov.cn/guoqing/2015-07/23/content_2901627.htm (accessed on 10 May 2020).
- Mao, X.; Wang, Z.; Hu, Q.; Huang, C.; Yan, H.; Wang, Z.; Lu, L.; Zhuang, M.; Chen, X.; Fu, J.; et al. HIV incidence is rapidly increasing with age among young men who have sex with men in China: A multicentre cross-sectional survey. HIV Med. 2018, 19, 513–522. [Google Scholar] [CrossRef]
- Dovel, K.; Shaba, F.; Offorjebe, O.A.; Balakasi, K.; Nyirenda, M.; Phiri, K.; Gupta, S.K.; Wong, V.; Tseng, C.-H.; E Nichols, B.; et al. Effect of facility-based HIV self-testing on uptake of testing among outpatients in Malawi: A cluster-randomised trial. Lancet Glob. Health 2020, 8, e276–e287. [Google Scholar] [CrossRef]
- Hu, Q.H.; Meyers, K.; Xu, J.J.; Chu, Z.X.; Zhang, J.; Ding, H.B.; Han, X.X.; Jiang, Y.J.; Geng, W.Q.; Shang, H. Efficacy and cost-effectiveness of early antiretroviral therapy and partners’ pre-exposure prophylaxis among men who have sex with men in Shenyang, China: A prospective cohort and costing study. BMC Infect. Dis. 2019, 19, 663. [Google Scholar] [CrossRef]
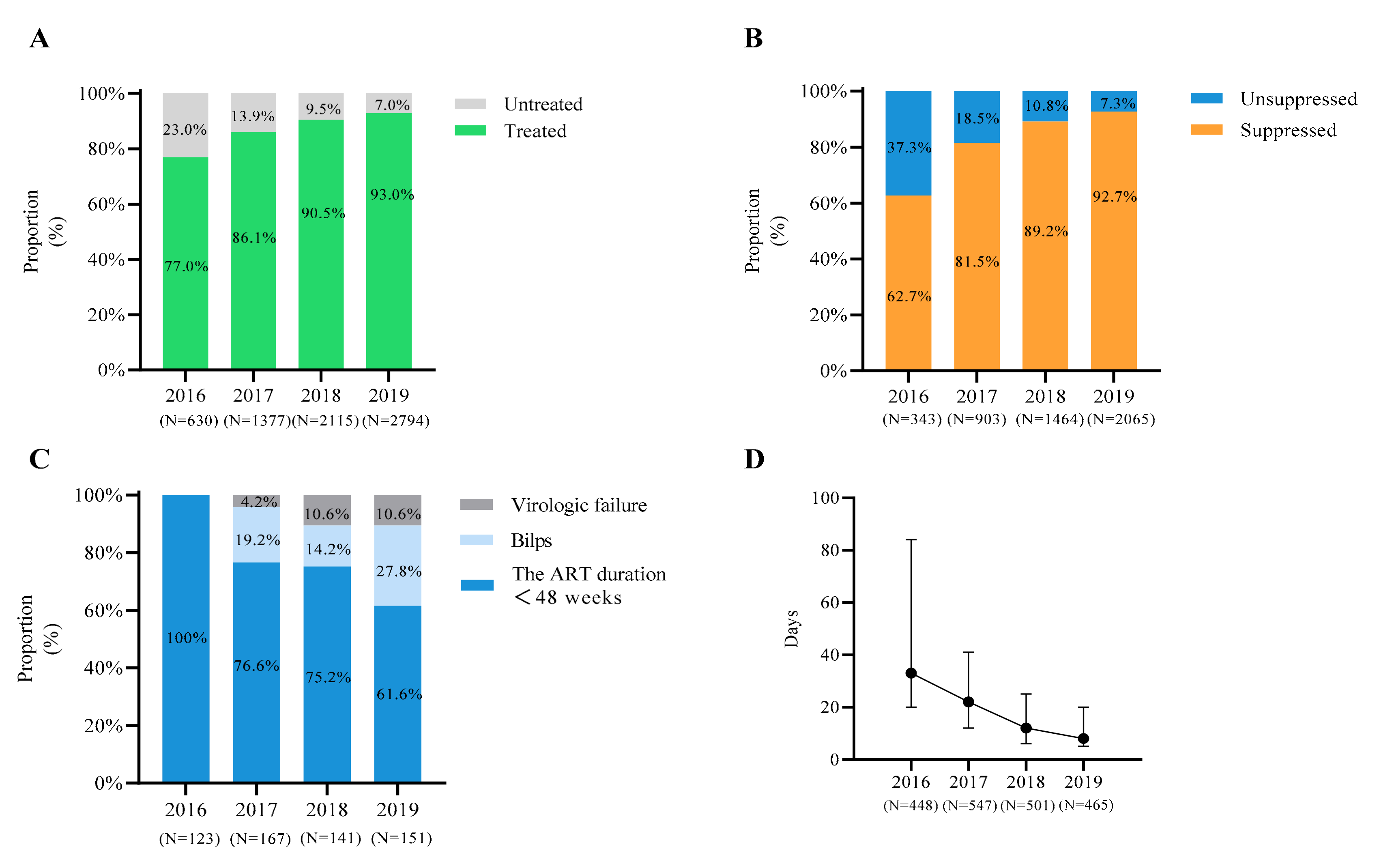
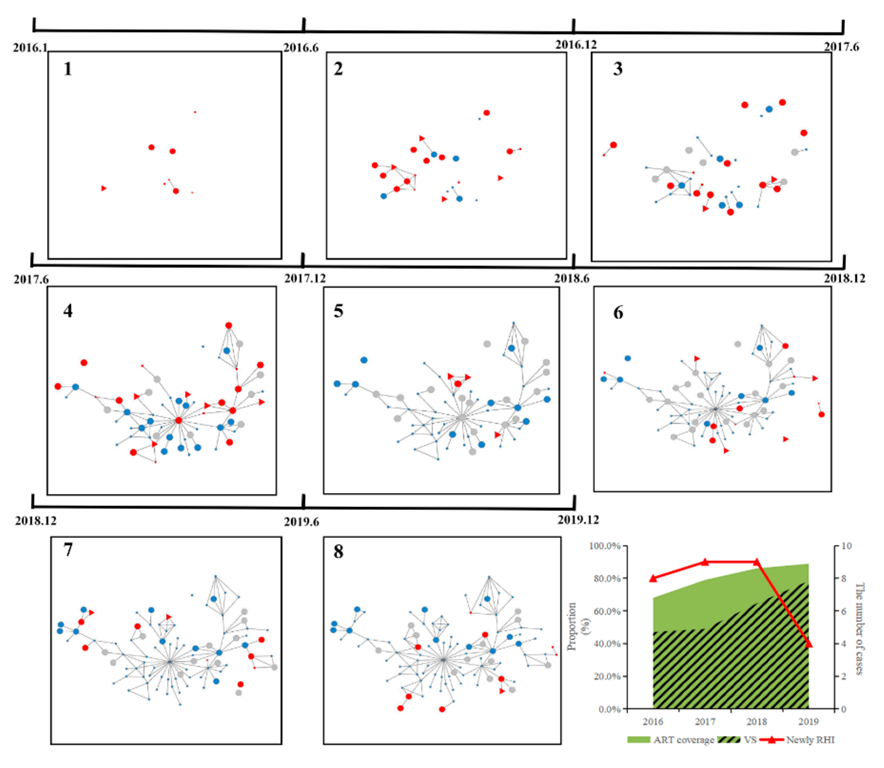
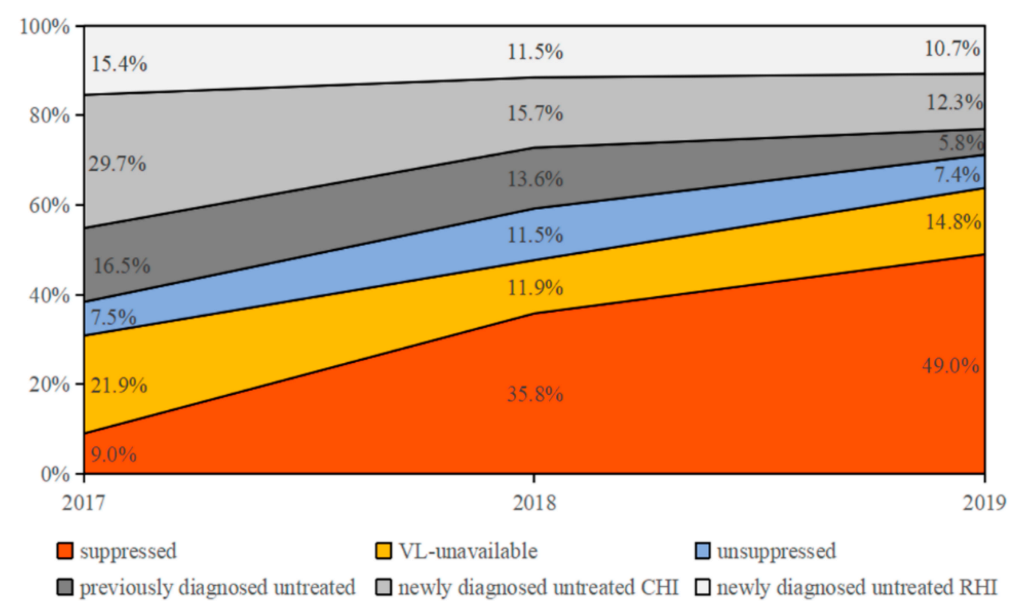
| Characteristics | Total (N, %) | 2016 (N, %) | 2017 (N, %) | 2018 (N, %) | 2019 N (%) |
|---|---|---|---|---|---|
| Total | 2882 | 666(23.1%) | 755(26.2%) | 750(26.0%) | 711(25.7%) |
| Age | |||||
| Median (IQR) | 32(26–44) | 30(25–44) | 33(26–46) | 31(26–43) | 32(26–45) |
| ≤25 | 710(24.6) | 183(27.5) | 178(23.6) | 184(24.5) | 165(23.2) |
| >25 | 2172(75.4) | 483(72.5) | 577(76.4) | 566(75.5) | 546(76.8) |
| Gender | |||||
| Male | 2686(93.2) | 633(95.0) | 700(92.7) | 706(94.1) | 647(91.0) |
| Female | 196(6.8) | 33(5.0) | 55(7.3) | 44(5.9) | 64(9.0) |
| Ethnicity | |||||
| Han | 2482(86.1) | 579(86.9) | 660(87.4) | 642(85.6) | 601(84.5) |
| Others | 400(13.9) | 87(13.1) | 95(12.6) | 108(14.4) | 110(15.5) |
| Marital status | |||||
| Unmarried | 1818(62.7) | 428(64.3) | 459(60.8) | 479(63.9) | 442(62.2) |
| Married | 548(19.0) | 102(15.3) | 161(21.3) | 132(17.6) | 153(21.5) |
| Divorced | 519(18.0) | 136(20.4) | 134(17.8) | 133(17.7) | 116(16.3) |
| Not available | 7(0.3) | 0(0.0) | 1(0.1) | 6(0.8) | 0(0.0) |
| Education | |||||
| Senior high school and above | 2019(70.0) | 483(72.5) | 532(70.5) | 522(69.6) | 482(67.8) |
| Junior high school and below | 853(29.6) | 181(27.2) | 221(29.2) | 222(29.6) | 229(32.2) |
| Not available | 10(0.4) | 2(0.3) | 2(0.3) | 6(0.8) | 0(0.0) |
| Infection Route | |||||
| Men who have sex with men | 2367(82.1) | 592(88.9) | 616(81.6) | 598(79.8) | 561(78.9) |
| Heterosexual transmission | 434(15.1) | 63(9.5) | 115(15.2) | 118(15.7) | 138(19.4) |
| Injection drug users | 44(1.5) | 9(1.3) | 13(1.7) | 13(1.7) | 9(1.3) |
| Other/Not available | 37(1.3) | 2(0.3) | 11(1.5) | 21(2.8) | 3(0.4) |
| Subtype | |||||
| CRF01_AE | 2019(70.0) | 471(70.7) | 554(73.4) | 517(68.9) | 477(67.1) |
| CRF07_BC | 526(18.3) | 120(18.0) | 125(16.5) | 148(19.8) | 133(18.7) |
| B | 132(4.6) | 33(5.0) | 31(4.1) | 31(4.1) | 37(5.2) |
| Others | 205(7.1) | 42(6.3) | 45(6.0) | 54(7.2) | 64(9.0) |
| Infection status | |||||
| Recent HIV infection | 953(33.1) | 205(30.8) | 252(33.4) | 295(39.3) | 201(28.3) |
| Chronic HIV infection | 1868(64.8) | 435(65.3) | 494(65.4) | 450(60.0) | 489(68.7) |
| Not available | 61(2.1) | 26(3.9) | 9(1.2) | 5(0.7) | 21(3.0) |
| CD4+T cell (cells/μL) | |||||
| <350 | 1244(43.2) | 288(43.2) | 340(45.0) | 410(54.6) | 206(29.0) |
| 350–500 | 419(14.5) | 90(13.5) | 122(16.2) | 131(17.5) | 76(10.7) |
| >500 | 323(11.2) | 81(12.3) | 73(9.7) | 108(14.4) | 61(8.6) |
| Not available | 896(31.1) | 207(31.0) | 220(29.1) | 101(13.5) | 368(51.7) |
| Viral Load (copies/mL) | |||||
| <10,000 | 194(6.7) | 51(7.7) | 60(7.9) | 48(6.4) | 35(4.9) |
| ≥10,000 | 1383(48.0) | 357(53.6) | 339(44.9) | 391(52.1) | 296(41.7) |
| Not available | 1305(45.3) | 258(38.7) | 356(47.2) | 311(41.5) | 380(53.4) |
| Characteristics | N | (%) | Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-Value | Adjusted Odds Ratio | 95% Confidence Interval | p-Value | |||
| Gender | ||||||||
| Female | 86 | 6.6 | Ref | Ref | ||||
| Male | 1223 | 93.4 | 3.148 | 1.26–7.867 | 0.014 | 3.332 | 1.205–9.211 | 0.020 |
| Age at diagnosis | ||||||||
| ≥25 | 1052 | 80.4 | Ref | Ref | ||||
| <25 | 255 | 19.5 | 1.667 | 1.18–2.353 | 0.004 | 1.596 | 1.090–2.336 | 0.016 |
| Marriage | ||||||||
| Unmarried | 806 | 61.6 | Ref | Ref | ||||
| Married | 268 | 20.5 | 0.668 | 0.442–1.009 | 0.055 | 0.867 | 0.552–1.362 | 0.537 |
| Divorced/Widowed | 231 | 17.7 | 0.91 | 0.609–1.357 | 0.642 | 1.115 | 0.720–1.728 | 0.625 |
| Nationality | ||||||||
| Han | 1134 | 86.6 | Ref | Ref | ||||
| Manchu | 128 | 9.8 | 1.851 | 1.196–2.863 | 0.006 | 1.746 | 1.121–2.719 | 0.014 |
| Others | 46 | 3.5 | 0.869 | 0.363–2.081 | 0.752 | 0.837 | 0.348–2.018 | 0.692 |
| Infection Route | ||||||||
| Men who have sex with men | 1084 | 82.8 | Ref | Ref | ||||
| Heterosexual transmission | 210 | 16.1 | 0.707 | 0.452–1.106 | 0.129 | 1.064 | 0.642–1.764 | 0.808 |
| Injection drug users | 15 | 1.1 | 2.615 | 0.883–7.744 | 0.083 | 3.765 | 1.201–11.801 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Qiu, Y.; Song, W.; Kang, M.; Dong, X.; Li, X.; Wang, L.; Liu, J.; Ding, H.; Chu, Z.; et al. Undiagnosed HIV Infections May Drive HIV Transmission in the Era of “Treat All”: A Deep-Sampling Molecular Network Study in Northeast China during 2016 to 2019. Viruses 2022, 14, 1895. https://doi.org/10.3390/v14091895
Zhao B, Qiu Y, Song W, Kang M, Dong X, Li X, Wang L, Liu J, Ding H, Chu Z, et al. Undiagnosed HIV Infections May Drive HIV Transmission in the Era of “Treat All”: A Deep-Sampling Molecular Network Study in Northeast China during 2016 to 2019. Viruses. 2022; 14(9):1895. https://doi.org/10.3390/v14091895
Chicago/Turabian StyleZhao, Bin, Yu Qiu, Wei Song, Mingming Kang, Xue Dong, Xin Li, Lu Wang, Jianmin Liu, Haibo Ding, Zhenxing Chu, and et al. 2022. "Undiagnosed HIV Infections May Drive HIV Transmission in the Era of “Treat All”: A Deep-Sampling Molecular Network Study in Northeast China during 2016 to 2019" Viruses 14, no. 9: 1895. https://doi.org/10.3390/v14091895
APA StyleZhao, B., Qiu, Y., Song, W., Kang, M., Dong, X., Li, X., Wang, L., Liu, J., Ding, H., Chu, Z., Wang, L., Tian, W., Shang, H., & Han, X. (2022). Undiagnosed HIV Infections May Drive HIV Transmission in the Era of “Treat All”: A Deep-Sampling Molecular Network Study in Northeast China during 2016 to 2019. Viruses, 14(9), 1895. https://doi.org/10.3390/v14091895






