Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. NP and M Proteins Prediction Structure and Amino Acid Alignment Comparison
2.2. PRV NP and M Genes Amplification
2.3. Bacterial Transformation with the Vector pJET 1.2/Blunt
2.4. Cloning and Construction of the pET SUMO-NP and pET SUMO-M Plasmids
2.5. Expression and Purification of the Recombinant NP and M Proteins
2.6. Antigenicity of NP and M Recombinant Proteins by Mice Immunization
2.7. Immunoreactivity of the NP and M Recombinant Proteins with Pig Serum Samples
3. Results
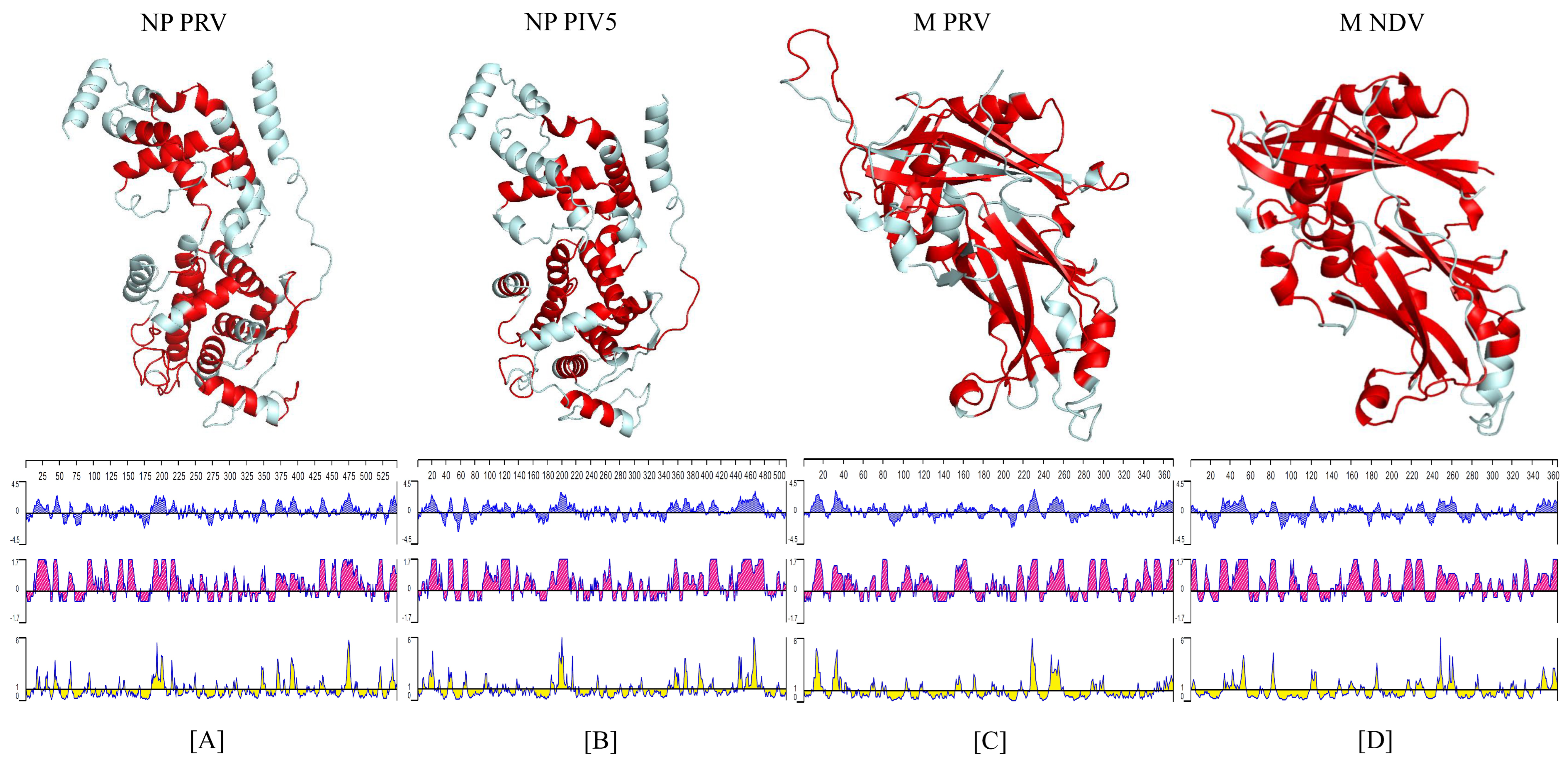
3.1. Tertiary Structure of the NP and M proteins and Amino Acid Alignment Comparison
3.2. Construction of an Expression System for NP and M Proteins
3.3. Expression of the PRV-NP and M Proteins in E. coli
3.4. Purification of the NP and M Recombinant Proteins
3.5. Antigenicity Assays
3.6. Immunoreactivity Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Buchholz, U.J. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef] [PubMed]
- Santos-López, G.; Hernández, J.; Borraz-Argüello, M.T.; Ramírez-Mendoza, H.; Vallejo, V.; Reyes-Leyva, J. Estructura, función e implicaciones patológicas de las proteínas del Rubulavirus porcino. Arch. Med. Vet. 2004, 36, 119–136. [Google Scholar] [CrossRef]
- Sundqvist, A.; Berg, M.; Hernandez-Jauregui, P.; Linn, T.; Moreno-Lopez, J. The structural proteins of a porcine paramyxovirus (LPMV). J. Gen. Virol. 1990, 71, 609–613. [Google Scholar] [CrossRef]
- Sundqvist, A.; Berg, M.; Moreno-Lopez, J.; Linne, T. The hemagglutinin-neuraminidase glycoprotein of the porcine paramyxovirus LPMV: Comparison with other paramyxoviruses revealed the closest relationship to simian virus 5 and mumps virus. Arch. Virol. 1992, 122, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Sundqvist, A.; Moreno-López, J.; Linné, T. Identification of the porcine paramyxovirus LPMV matrix protein gene: Comparative sequence analysis with other paramyxoviruses. J. Gen. Virol. 1991, 72, 1045–1050. [Google Scholar] [CrossRef]
- Berg, M.; Hjertner, B.; Moreno-López, J.; Linné, T. The P gene of the porcine paramyxovirus LPMV encodes three possible polypeptides P, V and C: The P protein mRNA is edited. J. Gen. Virol. 1992, 73, 1195–1200. [Google Scholar] [CrossRef]
- Berg, M.; Bergvall, A.C.; Svenda, M.; Sundqvist, A.; Moreno-López, J.; Linné, T. Analysis of the fusion protein gene of the porcine rubulavirus LPMV: Comparative analysis of paramyxovirus F proteins. Virus Genes 1997, 14, 55–61. [Google Scholar] [CrossRef]
- Moreno-López, J.; Correa-Girón, P.; Martinéz, A.; Ericsson, A. Characterization of a paramyxovirus isolated from the brain of a piglet in Mexico. Arch. Virol. 1986, 91, 221–231. [Google Scholar] [CrossRef]
- Stephano, H.A.; Gay, G.M.; Ramirez, T.Z. Encephalomyelitis, reproductive failure and corneal opacity (blue eyes) in pigs, associated with a paramyxovirus infection. Vet. Rec. 1988, 122, 6–10. [Google Scholar] [CrossRef]
- Allan, G.M.; McNeilly, F.; Walker, I.; Linne, T.; Moreno-Lopez, J.; Hernandez, P.; Kennedy, S.; Carroll, B.P.; Herron, B.; Foster, J.C.; et al. A sequential study of experimental porcine paramyxovirus (LPMV) infection of pigs: Immunostaining of cryostat sections and virus isolation. J. Vet. Diagn. Investig. 1996, 8, 405–413. [Google Scholar] [CrossRef]
- Escobar-López, A.C.; Rivera-Benítez, J.F.; Castillo-Juárez, H.; Ramírez-Mendoza, H.; Trujillo-Ortega, M.E.; Sánchez-Betancourt, J.I. Identification of antigenic variants of the porcine rubulavirus in sera of field swine and their seroprevalence. Transbound. Emerg. Dis. 2012, 59, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Benitez, J.F.; Luz-Armendáriz, J.D.; Saavedra-Montañez, M.; Jasso-Escutia, M.A.; Sánchez-Betancourt, I.; Pérez-Torres, A.; Reyes-Leyva, J.; Hernández, J.; Martínez-Lara, A.; Ramírez-Mendoza, H. Co-infection of classic swine H1N1 influenza virus in pigs persistently infected with porcine rubulavirus. Vet. Microbiol. 2016, 184, 31–39. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, F.; McNeilly, F.; Walker, I.; Allan, G.M.; Foster, J.C.; Linne, T.; Merza, M.; Hernandez, P.; Kennedy, S.; Adair, B. A comparative study on the use of virus and antibody detection techniques for the diagnosis of La Piedad Michoacan paramyxovirus (LPMV) infection in pigs. J. Vet. Diagn. Investig. 1997, 9, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Nordengrahn, A.; Svenda, M.; Moreno-López, J.; Bergvall, A.; Hernández, P.; McNeilly, F.; Allan, G.; Merza, M. Development of a blocking ELISA for screening anti-bodies to porcine rubulavirus, La Piedad Michoacán Virus. J. Vet. Diagn. Investig. 1999, 11, 319–323. [Google Scholar] [CrossRef]
- Rivera-Benitez, J.F.; Martínez-Bautista, R.; Pérez-Torres, A.; García-Contreras, A.D.C.; Reyes-Leyva, J.; Hernández, J.; Ramírez-Mendoza, H. Persistence of porcine rubulavirus in experimentally infected boars. Vet. Microbiol. 2013, 162, 491–498. [Google Scholar] [CrossRef]
- Wiman, A.C.; Hjertner, B.; Linné, T.; Herron, B.; Allan, G.; McNeilly, F.; Berg, M. Porcine rubulavirus LPMV RNA persists in the central nervous system of pigs after recovery from acute infection. J. Neurovirol. 1998, 4, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Romero, S.; Blomström, A.-L.; Alvarado, I.A.; Hernández-Jauregui, P.; Rivera-Benitez, F.; Ramírez-Mendoza, H.; Berg, M. Development of a real-time RT-PCR method for detection of porcine rubulavirus (PoRV-LPMV). J. Virol. Methods 2013, 189, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Benitez, J.F.; García-Contreras, A.C.; Reyes-Leyva, J.; Hernández, J.; Sánchez-Betancourt, J.I.; Ramírez-Mendoza, H. Efficacy of quantitative RT-PCR for detection of the nucleoprotein gene from different porcine rubulavirus strains. Arch. Virol. 2013, 158, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Stephano, H.A. Blue eye disease: Clinical signs and lesions. In Trends in Emerging Viral Infections of Swine; Morilla, A., Yoon, K., Zimmerman, J., Eds.; University Press: Ames, IA, USA, 2002; pp. 47–50. [Google Scholar]
- Sánchez-Betancourt, J.I.; Santos-López, G.; Alonso, R.; Doporto, J.M.; Ramírez-Mendoza, H.; Mendoza, S.; Hernández, J.; Reyes-Leyva, J.; Trujillo, M.E. Molecular characterization of the hemagglutinin-neuraminidase gene of porcine rubulavirus isolates associated with neurological disorders in fattening and adult pigs. Res. Vet. Sci. 2008, 85, 359–367. [Google Scholar] [CrossRef]
- Sánchez-Betancourt, J.I.; Trujillo, M.E.; Mendoza, S.E.; Reyes-Leyva, J.; Alonso, R.A. Genetic and antigenic changes in porcine rubulavirus. Can. J. Vet. Res. 2012, 76, 33–37. [Google Scholar]
- Hernández, J.J.; Reyes-Leyva, J.; Zenteno, R.; Ramírez, H.; Hernández-Jauregui, P.; Zenteno, E. Immunity to porcine rubulavirus infection in adult swine. Vet. Immunol. Immunopathol. 1998, 64, 367–381. [Google Scholar] [CrossRef]
- Svenda, M.; Hjertner, B.; Linné, T.; Berg, M. Both the P and V proteins of the porcine rubulavirus LPMV interact with the NP protein via their respective C-terminal unique parts. Virus Res. 2002, 83, 31–41. [Google Scholar] [CrossRef]
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The Viruses and Their Replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott-Raven Publishers: Philadelphia, NY, USA, 2007; pp. 1449–1496. [Google Scholar]
- Pentecost, M.; Vashisht, A.A.; Lester, T.; Voros, T.M.; Beaty, S.M.; Park, A.; Wang, Y.E.; Yun, T.E.; Freiberg, A.N.; Wohlschlegel, J.A.; et al. Evidence for ubiquitin-regulated nuclear and subnuclear trafficking among Paramyxovirinae matrix proteins. PLoS Pathog. 2015, 11, e1004739. [Google Scholar] [CrossRef]
- Cuevas-Romero, S.; Rivera-Benítez, J.F.; Blomström, A.-L.; Ramliden, M.; Hernández-Baumgarten, E.; Hernández-Jauregui, P.; Ramírez-Mendoza, H.; Berg, M. Molecular characterisation of Porcine rubulavirus (PorPV) isolates from different outbreaks in Mexico. Virus Genes 2016, 52, 81–90. [Google Scholar] [CrossRef]
- Sui, Z.; Chen, Q.; Fang, F.; Zheng, M.; Chen, Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine 2010, 28, 7690–7698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Grund, C.; Beer, M.; Harder, T.C. Engineered recombinant protein products of the avian paramyxovirus type-1 nucleocapsid and phosphoprotein genes for serological diagnosis. Virol. J. 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Jameson, B.A.; Wolf, H. The antigenic index: A novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 1988, 4, 181–186. [Google Scholar] [CrossRef]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Emini, E.A.; Hughes, J.V.; Perlow, D.S.; Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985, 55, 836–839. [Google Scholar] [CrossRef]
- Battisti, A.J.; Meng, G.; Winkler, D.C.; McGinnes, L.W.; Plevka, P.; Steven, A.C.; Rossmann, M.G. Structure and assembly of a paramyxovirus matrix protein. Proc. Natl. Acad. Sci. USA 2012, 109, 13996–14000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alayyoubi, M.; Leser, G.P.; Kors, C.A.; Lamb, R.A. Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein–RNA complex. Proc. Natl. Acad. Sci. USA 2015, 112, E1792–E1799. [Google Scholar] [CrossRef] [PubMed]
- Cerriteño-Sánchez, J.L.; Santos-López, G.; Rosas-Murrieta, N.H.; Reyes-Leyva, J.; Cuevas-Romero, S.; Herrera-Camacho, I. Production of an enzymatically active and immunogenic form of ectodomain of Porcine rubulavirus hemagglutinin-neuraminidase in the yeast Pichia pastoris. J. Biotechnol. 2016, 223, 52–61. [Google Scholar] [CrossRef]
- Cuevas-Romero, J.S.; Rivera-Benítez, J.F.; Hernández-Baumgarten, E.; Hernández-Jaúregui, P.; Blomström, A.-L.; Berg, M.; Baule, C. Cloning, expression and characterization of potential immunogenic recombinant hemagglutinin-neuraminidase protein of Porcine rubulavirus. Protein Expr. Purif. 2016, 128, 1–7. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Kevin, W.E. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Barrera, A.A.; Del Valle, A.; Montaño-Hirose, J.A.; Barrón, B.L.; Salinas-Trujano, J.; Torres-Flores, J. Full-genome sequencing and phylogenetic analysis of four neurovirulent Mexican isolates of porcine rubulavirus. Arch. Virol. 2017, 162, 1765–1768. [Google Scholar] [CrossRef]
- Hidalgo-Lara, D.R.; Luz-Armendáriz, J.D.; Rivera-Benítez, J.F.; Gomez-Nuñez, L.; Salazar-Jiménez, E.N.; Madrigal-Valencia, T.L.; Ramírez-Mendoza, H. Comparison of hemagglutination inhibition tests, immunoperoxidase monolayer assays, and serum neutralizing tests in detecting antibodies against blue eye disease in pigs. J. Immunol. Methods 2021, 496, 113088. [Google Scholar] [CrossRef]
- Siañez-Estrada, L.; Rivera-Benítez, J.F.; Rosas-Murrieta, H.R.; Reyes-Leyva, J.; Santos-López, G.; Herrera-Camacho, I. Immunoinformatics approach for predicting epitopes in HN and F proteins of Porcine rubulavirus. PLoS ONE 2020, 15, e0239785. [Google Scholar] [CrossRef]
- Iram, N.; Shah, M.S.; Ismat, F.; Habib, M.; Iqbal, M.; Hasnain, S.S.; Rahman, M. Heterologous expression, characterization and evaluation of the matrix protein from Newcastle disease virus as a target for antiviral therapies. Appl. Microbiol. Biotechnol. 2014, 98, 1691–1701. [Google Scholar] [CrossRef]
- Bugli, F.; Caprettini, V.; Cacaci, M.; Martini, C.; Sterbini, F.P.; Torelli, R.; Longa, S.D.; Papi, M.; Palmieri, V.; Giardina, B.; et al. Synthesis and characterization of different immunogenic viral nanoconstructs from rotavirus VP6 inner capsid protein. Int. J. Nanomed. 2014, 9, 2727–2739. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Yan, H.; Geng, X.; Wang, Y.; Xu, X.; Wang, M.; Zhang, H.; Huang, B.; Pang, W.; et al. The High Immunity Induced by the Virus-Like Particles of Foot-and-Mouth Disease Virus Serotype O. Front. Vet. Sci. 2021, 8, 633–706. [Google Scholar] [CrossRef] [PubMed]
- Makadiya, N.; Brownlie, R.; Van den Hurk, J.; Berube, N.; Allan, B.; Gerdts, V.; Zakhartchouk, A. S1 domain of the porcine epidemic diarrhea virus spike protein as a vaccine antigen. Virol. J. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, C.; Rodriguez, F.; Oviedo, J.M.; Eiras, A.; De Diego, M.; Alonso, C.; Escribano, J.M. Highly specific confirmatory western blot test for African swine fever virus antibody detection using the recombinant virus protein p54. J. Virol. Methods 1995, 52, 111–119. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, L.; Peng, B.; Wang, Y.; Yang, Z.; Yao, X.; Hu, L.; Lin, X. Prokaryotic expression, purification and antigenicity analysis of African swine fever virus pK205R protein. Pol. J. Vet. Sci. 2016, 19, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yabukarski, F.; Lawrence, P.; Tarbouriech, N.; Bourhis, J.M.; Delaforge, E.; Jensen, M.R.; Ruigrok, R.W.; Blackledge, M.; Volchkov, V.; Jamin, M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat. Struct. Mol. Biol. 2014, 21, 754–759. [Google Scholar] [CrossRef]
- Varsanyi, M.; Jörnvall, H.; Norrby, E. Isolation and characterization of the measles virus F1 polypeptide: Comparison with other paramyxovirus fusion proteins. Virology 1985, 147, 110–117. [Google Scholar] [CrossRef]






| Gene | Forward Primer | Reverse Primer | Amplicon |
|---|---|---|---|
| M | ACTGCACAACTTCAACCATTTCC | GCTACTACTTACGGAAAGGATTCCAG | 1066 |
| NP | CTCCAGGATAGAGGAGCCGA | CTACTAAAGCTGCTGAAACATGGC | 1563 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Romero, R.; Cerriteño-Sánchez, J.L.; Mendoza-Elvira, S.; García-Cambrón, J.B.; Castañeda-Montes, M.A.; Pérez-Aguilar, J.M.; Cuevas-Romero, J.S. Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction. Viruses 2022, 14, 1946. https://doi.org/10.3390/v14091946
Lara-Romero R, Cerriteño-Sánchez JL, Mendoza-Elvira S, García-Cambrón JB, Castañeda-Montes MA, Pérez-Aguilar JM, Cuevas-Romero JS. Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction. Viruses. 2022; 14(9):1946. https://doi.org/10.3390/v14091946
Chicago/Turabian StyleLara-Romero, Rocío, José Luis Cerriteño-Sánchez, Susana Mendoza-Elvira, José Bryan García-Cambrón, María Azucena Castañeda-Montes, José Manuel Pérez-Aguilar, and Julieta Sandra Cuevas-Romero. 2022. "Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction" Viruses 14, no. 9: 1946. https://doi.org/10.3390/v14091946
APA StyleLara-Romero, R., Cerriteño-Sánchez, J. L., Mendoza-Elvira, S., García-Cambrón, J. B., Castañeda-Montes, M. A., Pérez-Aguilar, J. M., & Cuevas-Romero, J. S. (2022). Development of Novel Recombinant Antigens of Nucleoprotein and Matrix Proteins of Porcine orthorubulavirus: Antigenicity and Structural Prediction. Viruses, 14(9), 1946. https://doi.org/10.3390/v14091946






