Evaluation of the Hepatocellular Carcinoma Predictive Scores PAGE-B and mPAGE-B among Brazilian Patients with Chronic Hepatitis B Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Design and Patients’ Selection
2.2. Variables
- •
- Type 2 diabetes mellitus (T2D): fasting blood glucose and/or glycated hemoglobin above 126 mg/dL and 6.5%, respectively, confirmed in a second measurement or use of medications for glucose control;
- •
- Dyslipidemia: high-density lipoprotein (HDL) < 40 mg/dL in men, HDL < 50 mg/dL in women, low-density lipoprotein (LDL) > 160 mg/dL, triglycerides > 150 mg/dL or use of statin/fibrate;
- •
- Systemic arterial hypertension (SAH): systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg in at least two appointments or use of medication to treat SAH;
- •
- Chronic kidney disease (CKD): estimated creatinine clearance ≤ 60 mL/min for at least 3 months or presence of kidney transplantation;
- •
- Significant alcohol use: >20 g/day for women or >30 g/day for men [22];
- •
- •
2.3. Assessment of Risk Scores for HCC
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. HCC Occurrence and Associated Factors
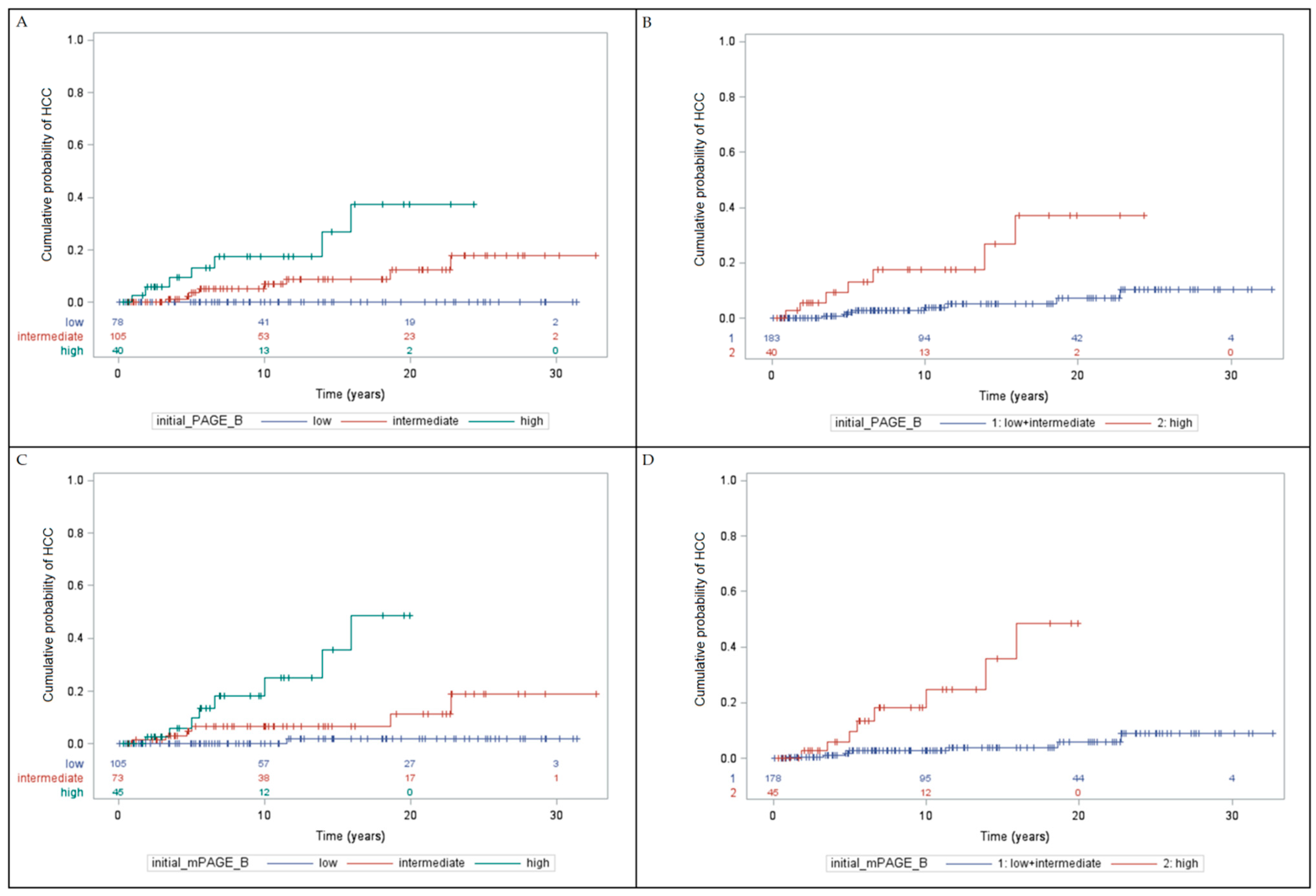
3.3. Cumulative Incidence of HCC
3.4. Performance of HCC Risk Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 1 July 2022).
- Alvarado-Mora, M.V.; Pinho, J.R. Epidemiological update of hepatitis B, C and delta in Latin America. Antivir. Ther. 2013, 18, 429–433. [Google Scholar] [CrossRef]
- Ministério da Saúde. Boletim Epidemiológico Hepatites Virais 2021. Available online: http://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2021/boletim-epidemiologico-de-hepatite-2021.pdf (accessed on 27 August 2022).
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64, S84–S101. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Prim. 2018, 4, 18035. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Pol, S. Review article: Immunisation against hepatitis B virus infection and the prevention of hepatocellular carcinoma. Aliment. Pharm. Ther. 2021, 53, 1166–1182. [Google Scholar] [CrossRef]
- Beasley, R.P.; Lin, C.C.; Chien, C.S.; Chen, C.J.; Hwang, L.Y. Geographic distribution of HBsAg carriers in China. Hepatology 1982, 2, 553–556. [Google Scholar] [CrossRef]
- Liang, T.J. Hepatitis B: The virus and disease. Hepatology 2009, 49, S13–S21. [Google Scholar] [CrossRef]
- Raffetti, E.; Fattovich, G.; Donato, F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis. Liver Int. 2016, 36, 1239–1251. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Chan, H.L.; Hansen, B.E.; Janssen, H.L.; Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J. Hepatol. 2015, 62, 956–967. [Google Scholar] [CrossRef]
- Huang, Z.H.; Lu, G.Y.; Qiu, L.X.; Zhong, G.H.; Huang, Y.; Yao, X.M.; Liu, X.H.; Huang, S.J.; Wu, T.; Yuan, Q.; et al. Risk of hepatocellular carcinoma in antiviral treatment-naïve chronic hepatitis B patients treated with entecavir or tenofovir disoproxil fumarate: A network meta-analysis. BMC Cancer 2022, 22, 287. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tseng, C.M.; Wu, J.L.; Hsu, Y.C.; El-Serag, H.B. Magnitude of and prediction for risk of hepatocellular carcinoma in patients with chronic hepatitis B taking entecavir or tenofovir therapy: A systematic review. J. Gastroenterol. Hepatol. 2020, 35, 1684–1693. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Chagas, A.L.; Mattos, A.A.; Carrilho, F.J.; Bittencourt, P.L.; Members of the Panel of the 2nd Consensus of the Brazilian Society of Hepatology on the Diagnosis and Management of Hepatocellular Carcinoma. Brazilian society of hepatology updated recommendations for diagnosis and treatment of hepatocellular carcinoma. Arq. Gastroenterol. 2020, 57, 1–20. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Taddei, T.H.; Serper, M.; Mehta, R.; Dieperink, E.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Pedrosa, M.; et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017, 65, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Geh, D.; Watson, R.; Sen, G.; French, J.J.; Hammond, J.; Turner, P.; Hoare, T.; Anderson, K.; McNeil, M.; McPherson, S.; et al. COVID-19 and liver cancer: Lost patients and larger tumours. BMJ Open Gastroenterol. 2022, 9, e000794. [Google Scholar] [CrossRef]
- Akbulut, S.; Garzali, I.U.; Hargura, A.S.; Aloun, A.; Yilmaz, S. Screening, Surveillance, and Management of Hepatocellular Carcinoma During the COVID-19 Pandemic: A Narrative Review. J. Gastrointest. Cancer 2022, 1–12. [Google Scholar] [CrossRef]
- Papatheodoridis, G.; Dalekos, G.; Sypsa, V.; Yurdaydin, C.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; Mangia, G.; et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 2016, 64, 800–806. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.D.; Lee, M.; Jun, B.G.; Kim, T.S.; Suk, K.T.; Kang, S.H.; Kim, M.Y.; Cheon, G.J.; Kim, D.J.; et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 2018, 69, 1066–1073. [Google Scholar] [CrossRef]
- Costa, A.P.M.; da Silva, M.A.C.N.; Castro, R.S.; Sampaio, A.L.O.; Alencar Júnior, A.M.; da Silva, M.C.; Ferreira, A.S.P. PAGE-B and REACH-B Predicts the Risk of Developing Hepatocellular Carcinoma in Chronic Hepatitis B Patients from Northeast, Brazil. Viruses 2022, 14, 732. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef] [Green Version]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Simonovský, V. The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. Br. J. Radiol. 1999, 72, 29–34. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Silva, M.; Marinho, F.R.T.; Oliveira, P.F.; Lopes, T.M.; Sevá-Pereira, T.; Lorena, S.L.S.; Almeida, J.R.S. Retrospective analysis of hepatitis B virus chronic infection in 247 patients: Clinical stages, response to treatment and poor prognostic factors. Braz. J. Infect. Dis. 2017, 21, 441–447. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef]
- Ghany, M.G.; Feld, J.J.; Chang, K.M.; Chan, H.L.Y.; Lok, A.S.F.; Visvanathan, K.; Janssen, H.L.A. Serum alanine aminotransferase flares in chronic hepatitis B infection: The good and the bad. Lancet. Gastroenterol. Hepatol. 2020, 5, 406–417. [Google Scholar] [CrossRef]
- Lee, Y.B.; Moon, H.; Lee, J.H.; Cho, E.J.; Yu, S.J.; Kim, Y.J.; Zoulim, F.; Lee, J.; Yoon, J.H. Association of Metabolic Risk Factors With Risks of Cancer and All-Cause Mortality in Patients With Chronic Hepatitis B. Hepatology 2021, 73, 2266–2277. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Lam, L.; Fontaine, H.; Bourliere, M.; Lusivika-Nzinga, C.; Dorival, C.; Thabut, D.; Zoulim, F.; Habersetzer, F.; Asselah, T.; Duclos-Vallee, J.C.; et al. Predictive factors for hepatocellular carcinoma in chronic hepatitis B using structural equation modeling: A prospective cohort study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101713. [Google Scholar] [CrossRef]
- Yuen, M.F.; Tanaka, Y.; Fong, D.Y.; Fung, J.; Wong, D.K.; Yuen, J.C.; But, D.Y.; Chan, A.O.; Wong, B.C.; Mizokami, M.; et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 2009, 50, 80–88. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, N.; Sun, F.; Zhou, J.; Wu, X.; Sun, Y.; Wang, B.; Zhan, S.; Kong, Y.; Jia, J.; et al. Hepatocellular Carcinoma Prediction Models in Chronic Hepatitis B: A Systematic Review of 14 Models and External Validation. Clin. Gastroenterol. Hepatol. 2021, 19, 2499–2513. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Yoon, J.S.; Park, S.Y.; Lee, H.A.; Jang, M.J.; Kim, S.U.; Sinn, D.H.; Seo, Y.S.; Kim, H.Y.; Kim, S.E.; et al. Impact of HBeAg on Hepatocellular Carcinoma Risk During Oral Antiviral Treatment in Patients with Chronic Hepatitis B. Clin. Gastroenterol. Hepatol. 2022, 20, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Wu, S.; Ye, W.; Lou, L. Metabolic syndrome and the incidence of hepatocellular carcinoma: A meta-analysis of cohort studies. OncoTargets Ther. 2018, 11, 6277–6285. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Zhang, W.; Tang, L.; Yang, H.; Yan, K.; Jiang, L.; Yang, J.; Li, C.; Yang, J.; et al. The Influence of Metabolic Syndrome on the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B Infection in Mainland China. Cancer Epidemiol. Biomark. Prev. 2019, 28, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.W.; Hyun, J.J.; Kim, B.; Han, K.D. Influence of Metabolic Syndrome on Cancer Risk in HBV Carriers: A Nationwide Population Based Study Using the National Health Insurance Service Database. J. Clin. Med. 2021, 10, 2401. [Google Scholar] [CrossRef]
- Kirino, S.; Tamaki, N.; Kaneko, S.; Kurosaki, M.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Watakabe, K.; et al. Validation of hepatocellular carcinoma risk scores in Japanese chronic hepatitis B cohort receiving nucleot(s)ide analog. J. Gastroenterol. Hepatol. 2020, 35, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.A.; Kowgier, M.; Hansen, B.E.; Brouwer, W.P.; Maan, R.; Wong, D.; Shah, H.; Khalili, K.; Yim, C.; Heathcote, E.J.; et al. Toronto HCC risk index: A validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J. Hepatol. 2018, 68, 92–99. [Google Scholar] [CrossRef]
- Wong, V.W.; Chan, S.L.; Mo, F.; Chan, T.C.; Loong, H.H.; Wong, G.L.; Lui, Y.Y.; Chan, A.T.; Sung, J.J.; Yeo, W.; et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J. Clin. Oncol. 2010, 28, 1660–1665. [Google Scholar] [CrossRef]
- Yang, H.I.; Yuen, M.F.; Chan, H.L.; Han, K.H.; Chen, P.J.; Kim, D.Y.; Ahn, S.H.; Chen, C.J.; Wong, V.W.; Seto, W.K.; et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): Development and validation of a predictive score. Lancet Oncol. 2011, 12, 568–574. [Google Scholar] [CrossRef]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef]
- Yip, T.C.; Wong, G.L.; Wong, V.W.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.; Lee, H.W.; Lui, G.C.; Chan, H.L. Reassessing the accuracy of PAGE-B-related scores to predict hepatocellular carcinoma development in patients with chronic hepatitis B. J. Hepatol. 2020, 72, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Q.; Cui, T.; Huang, J.; Jin, H. Reporting and Performance of Hepatocellular Carcinoma Risk Prediction Models: Based on TRIPOD Statement and Meta-Analysis. Can. J. Gastroenterol. Hepatol. 2021, 2021, 9996358. [Google Scholar] [CrossRef] [PubMed]
- Voulgaris, T.; Papatheodoridi, M.; Lampertico, P.; Papatheodoridis, G.V. Clinical utility of hepatocellular carcinoma risk scores in chronic hepatitis B. Liver Int. 2020, 40, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, M.F.; Feist, C.; Koch, S.; Kremer, W.M.; Lackner, K.J.; Weinmann, A.; Galle, P.R. Cost evaluation of PAGE-B risk score guided HCC surveillance in patients with treated chronic hepatitis B. BMC Health Serv. Res. 2021, 21, 846. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | n (%) or Mean ± SD |
|---|---|
| Men/women | 148 (66.1%)/76 (33.9%) |
| Age at diagnosis (years) | 38.71 ± 14.19 |
| Systemic arterial hypertension | 91 (40.6%) |
| Dyslipidemia | 65 (29.0%) |
| Chronic kidney disease | 55 (24.6%) |
| Type 2 diabetes mellitus | 39 (17.4%) |
| Alcohol use (n = 221) | 30 (13.6%) |
| HBeAg positive at diagnosis | 72 (32.1%) |
| Cirrhosis at diagnosis (n = 205) | 45 (21.9%) |
| Outpatient follow-up period (months) | 133.59 ± 102.26 |
| Hepatitis B treatment during follow-up | 123/224 (54.9%) |
| Interferon | 6/123 (4.9%) |
| IFN followed by nucleos(t)ide analogs | 18/123 (14.6%) |
| Nucleos(t)ide analogs | 99/123 (80.5%) |
| HBeAg seroclearance (n = 72) | 40 (55.6%) |
| HBsAg seroclearance | 33 (14.7%) |
| Cirrhosis at end of follow-up | 86 (38.4%) |
| HCC development | 15 (6.7%) |
| Follow-up period to HCC diagnosis (months) | 103.13 ± 78.99 |
| Initial PAGE-B (value) | 11.58 ± 6.05 |
| Initial mPAGE-B (value) | 8.90 ± 4.16 |
| Initial PAGE-B (stratified) | |
| 1 | 78 (34.8%) |
| 2 | 106 (47.3%) |
| 3 | 40 (17.9%) |
| Initial mPAGE-B (stratified) | |
| 1 | 105 (46.9%) |
| 2 | 74 (33.0%) |
| 3 | 45 (20.1%) |
| Characteristics | With HCC (n = 15) n (%) or Mean ± SD | Without HCC (n = 209) n (%) or Mean ± SD | p Value |
|---|---|---|---|
| Men/women | 14 (93.3%)/1 (6.7%) | 134 (64.1%)/75 (35.9%) | 0.0210 1 |
| Age at HBV diagnosis (years) | 50.00 ± 12.58 | 37.89 ± 13.98 | <0.0021 1 |
| Systemic arterial hypertension | 8 (53.3%) | 83 (39.7%) | 0.2995 |
| Dyslipidemia | 4 (26.7%) | 61 (29.2%) | 1.0000 |
| Chronic kidney disease | 2 (13.3%) | 53 (25.4%) | 0.3701 |
| Type 2 diabetes mellitus | 4 (26.7%) | 35 (16.7%) | 0.3037 |
| Alcohol use (n = 221) | 0 (0.0%) | 30 (14.6%) | 0.2322 |
| HBeAg positive at diagnosis | 3 (20.0%) | 69 (33.0%) | 0.3971 |
| Cirrhosis at HBV diagnosis (n = 205) | 11 (73.3%) | 34 (17.9%) | <0.0001 1 |
| Follow-up period (months) | 105.79 ± 79.10 | 135.46 ± 103.52 | 0.3923 |
| HBeAg seroclearance | 1 (33.3%) | 39 (56.5%) | 0.5815 |
| HBsAg seroclearance | 2 (13.3%) | 31 (14.8%) | 1.0000 |
| Cirrhosis at end of follow-up | 15 (100.0%) | 71 (34.0%) | <0.0001 1 |
| Initial PAGE-B (value) | 17.07 ± 3.03 | 11.18 ± 6.02 | 0.0002 1 |
| Initial mPAGE-B (value) | 12.67 ± 2.55 | 8.63 ± 4.12 | 0.0002 1 |
| Initial PAGE-B (stratification) | 0.0014 1 | ||
| 1 | 0 (0.0%) | 78 (37.3%) | |
| 2 | 8 (53.3%) | 98 (46.9%) | |
| 3 | 7 (46.7%) | 33 (15.8%) | |
| Initial mPAGE-B (stratification) | 0.0003 1 | ||
| 1 | 1 (6.7%) | 104 (49.8%) | |
| 2 | 6 (40.0%) | 68 (32.5%) | |
| 3 | 8 (53.3%) | 37 (17.7%) |
| Characteristics | Hazard Ratio | CI 95% | p Value |
|---|---|---|---|
| Men | 7.879 | 1.036–59.933 | 0.0461 1 |
| Age at HBV diagnosis | 1.076 | 1.037–1.117 | 0.0001 1 |
| Systemic arterial hypertension | 1.346 | 0.487–3.722 | 0.5669 |
| Dyslipidemia | 0.677 | 0.215–2.130 | 0.5049 |
| Chronic kidney disease | 0.359 | 0.081–1.596 | 0.1783 |
| Type 2 diabetes mellitus | 1.484 | 0.472–4.666 | 0.4994 |
| HBeAg negative at diagnosis | 2.516 | 0.848–7.463 | 0.0962 |
| Cirrhosis at HBV diagnosis | 21.531 | 6.585–70.401 | <0.0001 1 |
| Lack of HBsAg seroclearance | 2.419 | 0.680–8.608 | 0.1727 |
| Initial PAGE-B (value) | 1.321 | 1.143–1.528 | 0.0002 1 |
| Initial mPAGE-B (value) | 1.408 | 1.201–1.652 | <0.0001 1 |
| Initial PAGE-B (stratification) (3 vs. 1 + 2) | 5.870 | 2.107–16.352 | 0.0007 1 |
| Initial mPAGE-B (stratification) (3 vs. 1 + 2) | 9.125 | 3.084–27.000 | <0.0001 1 |
| % (Standard Error) | ||||
|---|---|---|---|---|
| Time | 3 Years | 5 Years | 7 Years | p Value |
| PAGE-B 1 1 | 0.0% (0.0%) | 0.0% (0.0%) | 0.0% (0.0%) | |
| PAGE-B 2 | 0.0% (0.0%) | 3.67% (2.08%) | 5.01% (2.45%) | |
| PAGE-B 3 | 5.72% (3.93%) | 13.29% (6.29%) | 17.63% (7.32%) | |
| mPAGE-B 1 | 0.0% (0.0%) | 0.0% (0.0%) | 0.0% (0.0%) | <0.0001 |
| mPAGE-B 2 | 1.45% (1.44%) | 6.57% (3.19%) | 6.57% (3.19%) | |
| mPAGE-B 3 | 2.70% (2.67%) | 9.82% (5.45%) | 18.12% (7.50%) | |
| Risk 1 + 2 vs. 3 | ||||
| PAGE-B 1 + 2 | 0.0% (0.0%) | 2.08% (1.19%) | 2.83% (1.39%) | 0.0001 |
| PAGE-B 3 | 5.72% (3.93%) | 13.29% (6.29%) | 17.63% (7.32%) | |
| mPAGE-B 1 + 2 | 0.6% (0.6%) | 2.70% (1.33%) | 2.70% (1.33%) | <0.0001 |
| mPAGE-B 3 | 2.70% (2.67%) | 9.82% (5.45%) | 18.12% (7.50%) | |
| Score | AUROC (95%CI) | Cutoff | Accuracy |
|---|---|---|---|
| PAGE-B | 0.7906 (0.7007–0.8805) | >14 | Sensitivity: 93.33% |
| Specificity: 55.50% | |||
| NPV: 99.14% PPV: 13.08% | |||
| PLR: 2.10% NLR: 0.12% | |||
| mPAGE-B | 0.7904 (0.7026–0.8783) | >10.5 | Sensitivity: 86.67% |
| Specificity: 66.51% | |||
| NPV: 98.58% PPV: 15.66% | |||
| PLR: 2.59% NLR: 0.20% |
| Score | AUROC (95%CI) | Cutoff | Accuracy | |
|---|---|---|---|---|
| HBV therapy during follow-up | PAGE-B | 0.7634 (0.6395–0.8873) | >14 | Sensitivity: 100% |
| Specificity: 47.32% | ||||
| NPV: 100% PPV: 15.71% | ||||
| PLR: 1.90% NLR: 0.00% | ||||
| mPAGE-B | 0.7597 (0.6276–0.8919) | >11 | Sensitivity: 81.82% | |
| Specificity: 62.50% | ||||
| NPV: 97.22% PPV: 17.65% | ||||
| PLR: 2.18% NLR: 0.29% | ||||
| Absence of HBV therapy during follow-up | PAGE-B | 0.8119 (0.6558–0.9679) | >17 | Sensitivity: 75% |
| Specificity: 83.51% | ||||
| NPV: 98.78% PPV: 15.79% | ||||
| PLR: 4.55% NLR: 0.30% | ||||
| mPAGE-B | 0.8273 (0.7306–0.9240) | >11 | Sensitivity: 100% | |
| Specificity: 71.13% | ||||
| NPV: 100% PPV: 12.50% | ||||
| PLR: 3.46% NLR: 0.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira da Silva, A.C.; Cunha-Silva, M.; Sevá-Pereira, T.; Mazo, D.F. Evaluation of the Hepatocellular Carcinoma Predictive Scores PAGE-B and mPAGE-B among Brazilian Patients with Chronic Hepatitis B Virus Infection. Viruses 2022, 14, 1968. https://doi.org/10.3390/v14091968
Ferreira da Silva AC, Cunha-Silva M, Sevá-Pereira T, Mazo DF. Evaluation of the Hepatocellular Carcinoma Predictive Scores PAGE-B and mPAGE-B among Brazilian Patients with Chronic Hepatitis B Virus Infection. Viruses. 2022; 14(9):1968. https://doi.org/10.3390/v14091968
Chicago/Turabian StyleFerreira da Silva, Ana Caroline, Marlone Cunha-Silva, Tiago Sevá-Pereira, and Daniel F. Mazo. 2022. "Evaluation of the Hepatocellular Carcinoma Predictive Scores PAGE-B and mPAGE-B among Brazilian Patients with Chronic Hepatitis B Virus Infection" Viruses 14, no. 9: 1968. https://doi.org/10.3390/v14091968
APA StyleFerreira da Silva, A. C., Cunha-Silva, M., Sevá-Pereira, T., & Mazo, D. F. (2022). Evaluation of the Hepatocellular Carcinoma Predictive Scores PAGE-B and mPAGE-B among Brazilian Patients with Chronic Hepatitis B Virus Infection. Viruses, 14(9), 1968. https://doi.org/10.3390/v14091968






