Heart Disease and Arboviruses: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Collection and Extraction
2.4. Statistical Analysis
3. Results
3.1. Study Quality
3.2. Data Synthesis
3.3. Studies on Zika
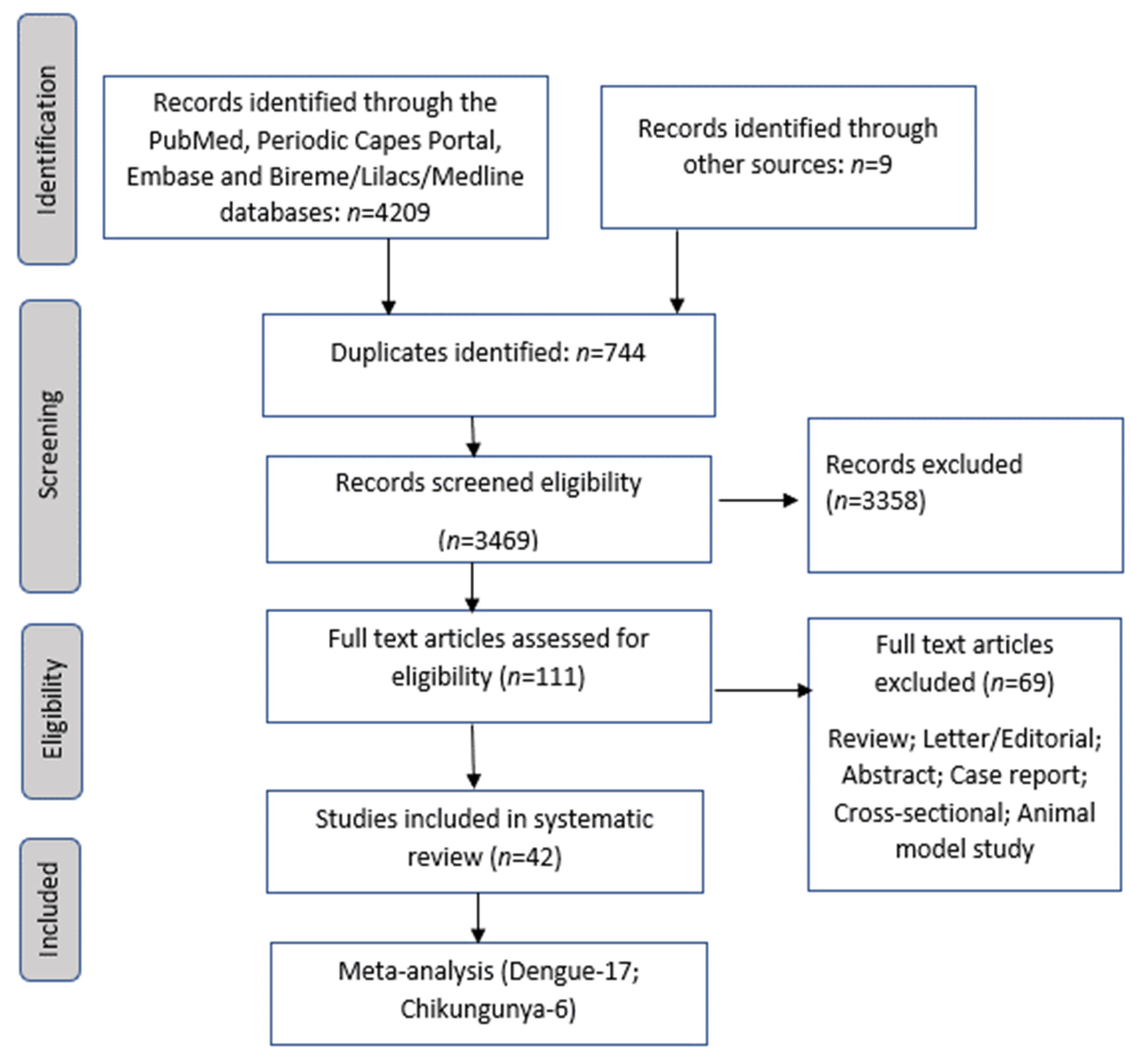
3.4. Meta-Analyses of Studies on Chikungunya
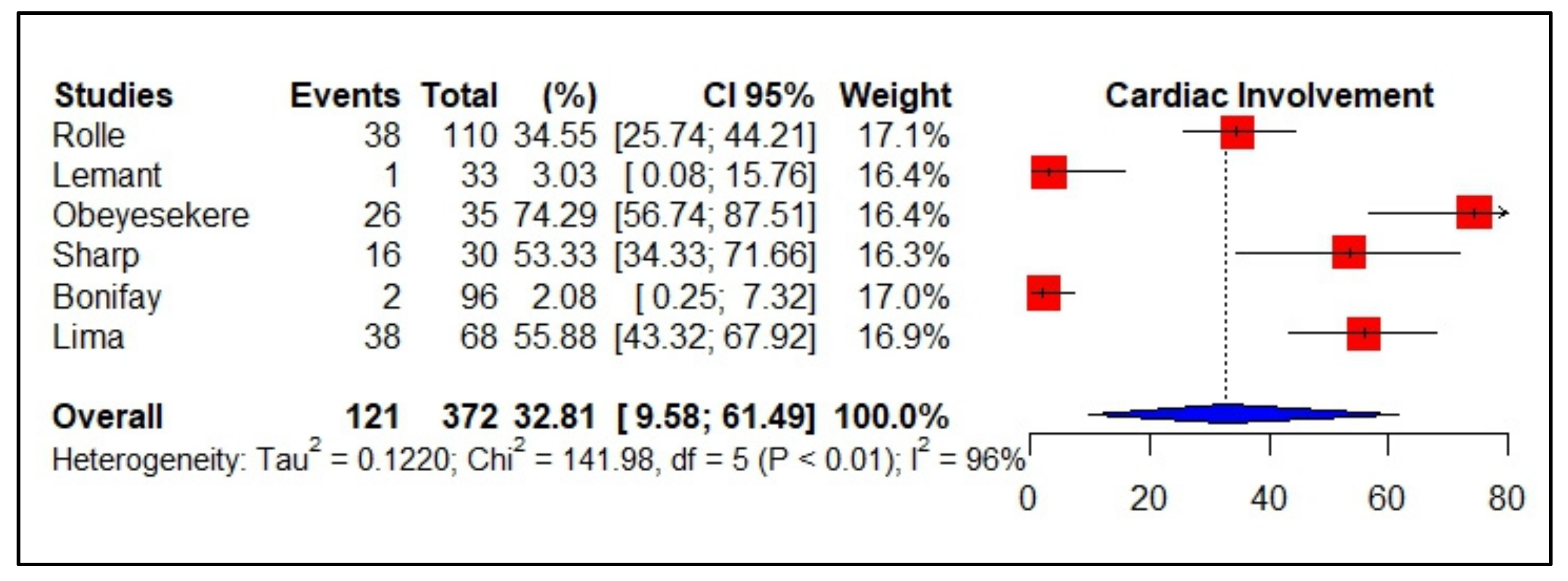
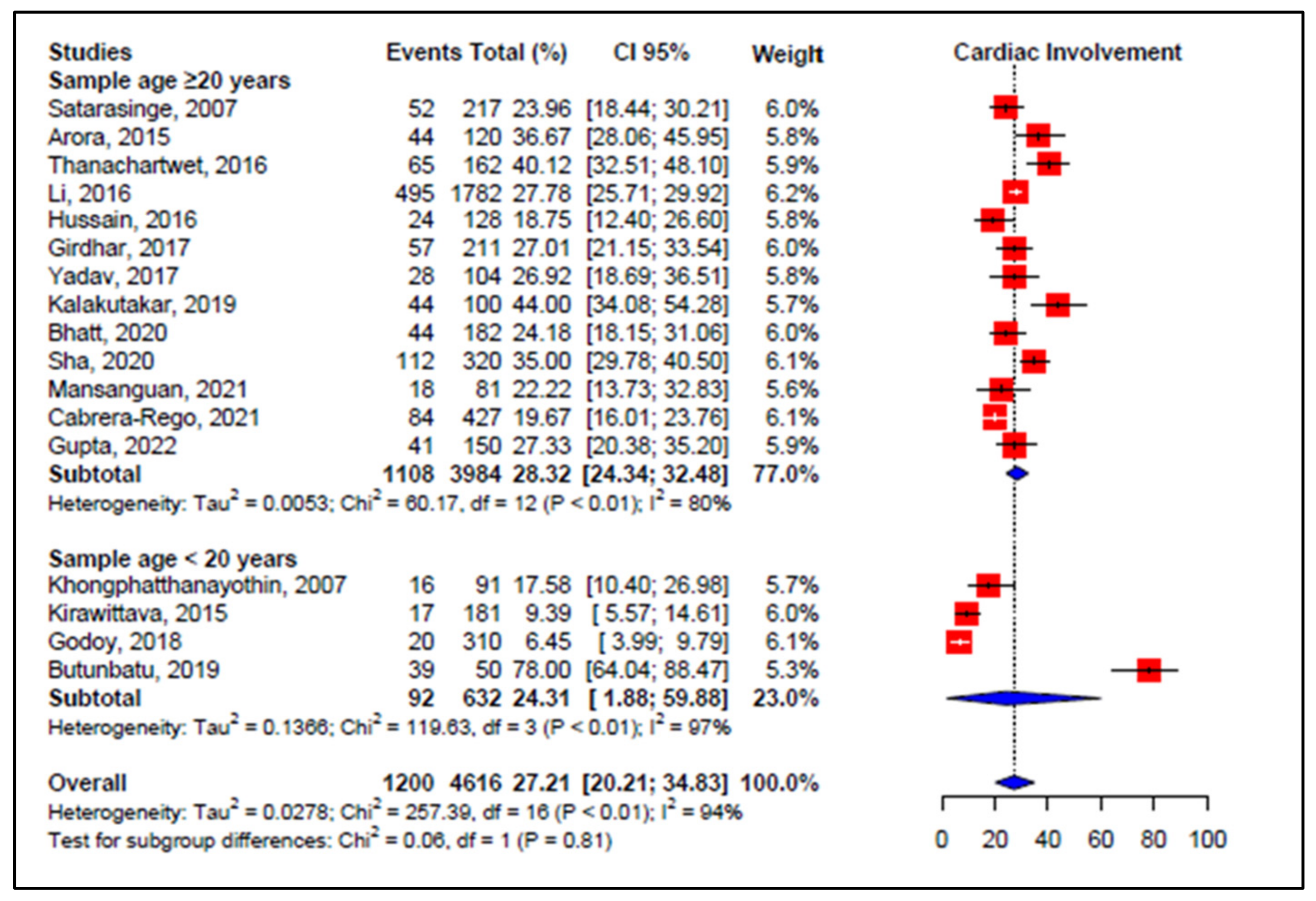
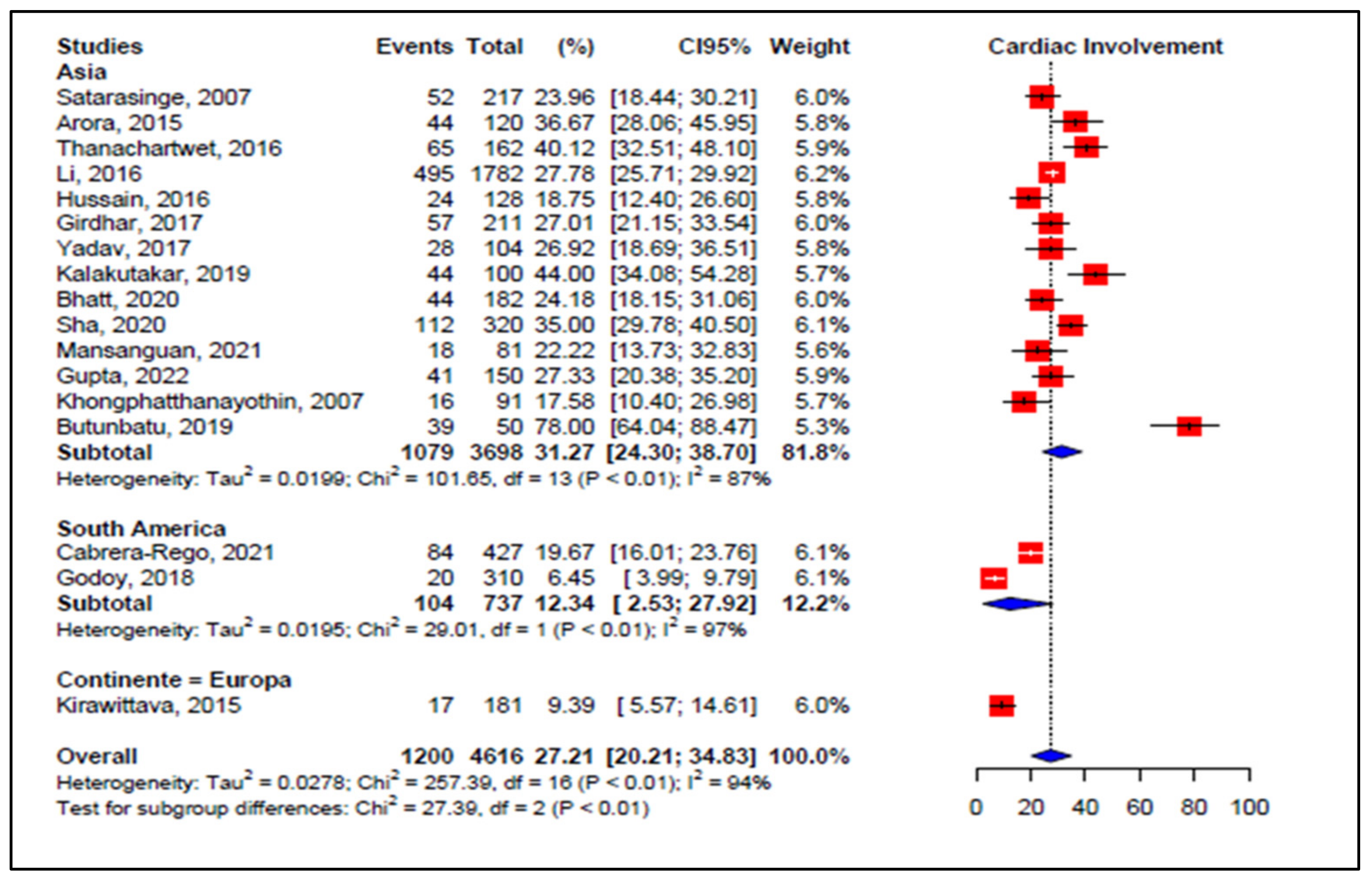
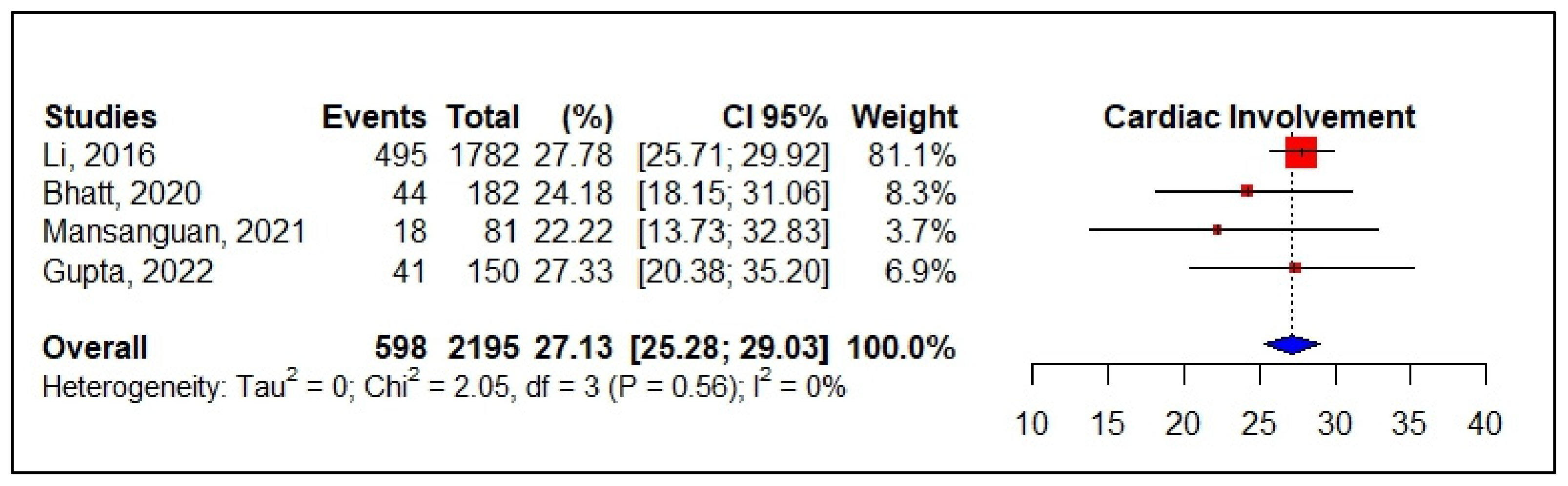
3.5. Meta-Analyses of Studies on Dengue
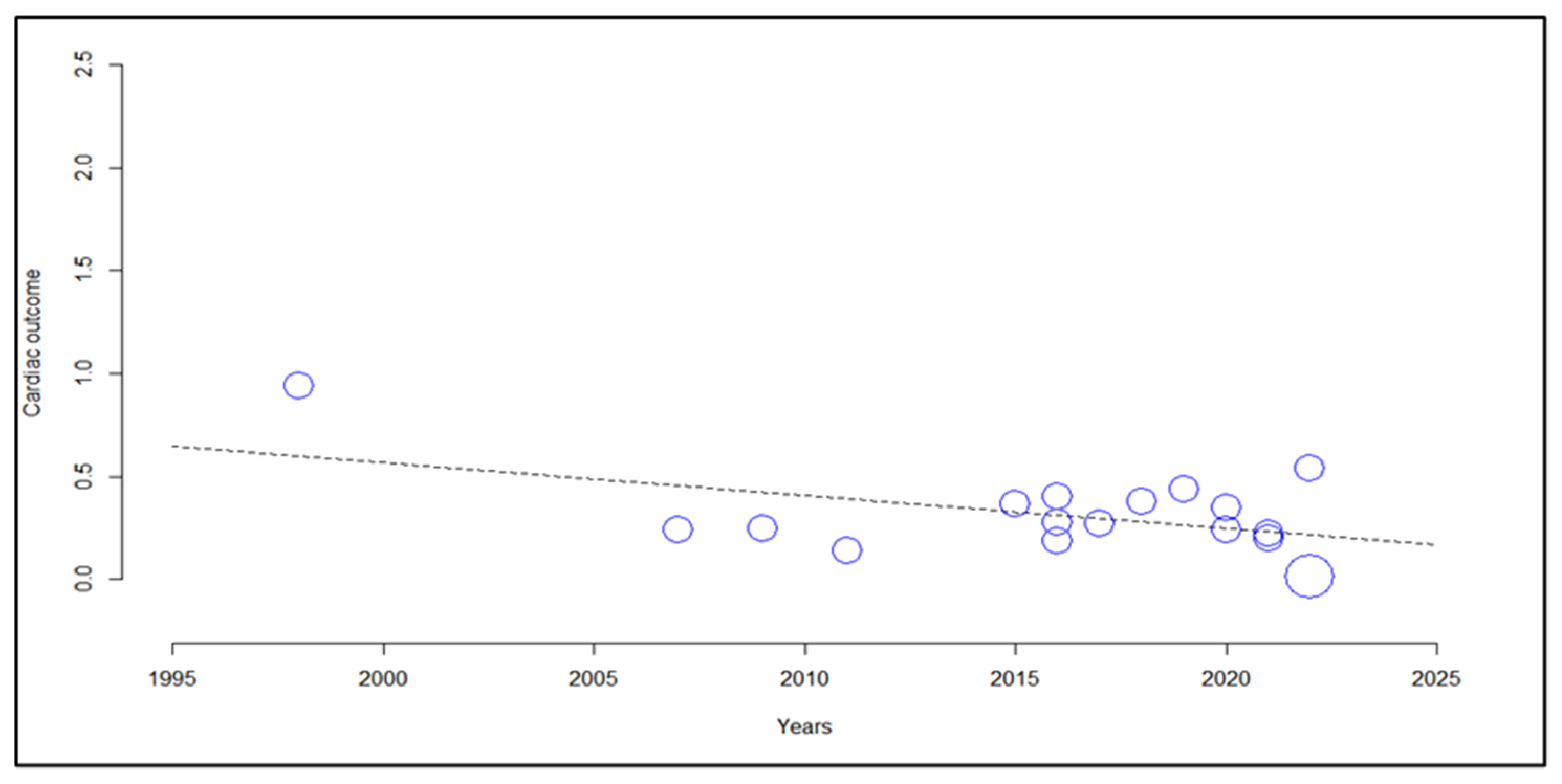
3.6. Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A global public health threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Ajide-Bamigboye, N.T. Tackling the global health threat of arboviruses: An appraisal of the three holistic approaches to health. Health Promot. Perspect. 2021, 11, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parida, M.; Dash, P.K. Impact of transmission cycles and vector competence on global expansion and emergence of arboviruses. Rev. Med. Virol. 2017, 27, e1941. [Google Scholar] [CrossRef]
- Mohan, A.; Kiran, D.H.N.; Manohar, I.C.; Kumar, D.P. Epidemiology, clinical manifestations, and diagnosis of Chikungunya fever: Lessons learned from the re-emerging epidemic. Indian J. Dermatol. 2010, 55, 54–63. [Google Scholar] [CrossRef]
- An, W.; Ge, N.; Cao, Y.; Sun, J.; Jin, X. Recent progress on chikungunya virus research. Virol. Sin. 2017, 32, 441–453. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Ruckert, C.; Cavany, S.M.; Perkins, T.A.; Ebel, G.D.; Grubaugh, N.D. Arbovirus coinfection and co-transmission: A neglected public health concern? PLoS Biol. 2019, 17, e3000130. [Google Scholar] [CrossRef]
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.V.; RADAM-LAC Research Team; Talbot, B. Arbovirus vectors of epidemiological concern in the Americas: A scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef]
- Tandale, B.V.; Sathe, P.S.; Arankalle, V.A.; Wadia, R.S.; Kulkarni, R.; Shah, S.V.; Shah, S.K.; Sheth, J.K.; Sudeep, A.B.; Tripathy, A.S.; et al. Systemic involvements and fatalities during Chikungunya epidemic in India, 2006. J. Clin. Virol. 2009, 46, 145–149. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Pan American Health Organization. Guidelines for the Clinical Diagnosis and Treatment of Dengue, Chikungunya, and Zika; Pan American Health Organization: Washington, DC, USA, 2022; Available online: https://iris.paho.org/handle/10665.2/55867 (accessed on 10 July 2022).
- Sangkaew, S.; Ming, D.; Boonyasiri, A.; Honeyford, K.; Kalayanarooj, S.; Yacoub, S.; Dorigatti, I.; Holmes, A. Risk predictors of progression to severe disease during the febrile phase of dengue: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1014–1026. [Google Scholar] [CrossRef]
- Chastel, C. Human Infections in Cambodia by the Chikungunya Virus or an Apparently Closely Related Agent. I. Clinical Aspects. Isolations and Identification of the Viruses. Serology. Bull. Soc. Pathol. Exot. Filiales. 1963, 56, 892–915. [Google Scholar]
- ECDC European Centre for Disease Prevention and Control. Chikungunya Worldwide Overview. 2022. Available online: https://www.ecdc.europa.eu/en/chikungunya-monthly (accessed on 20 August 2022).
- De Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; de Oliveira Franca, R.F. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Fernanda Urbano-Garzon, S.; Sebastian Hurtado-Zapata, J. Prevalence of Post-Chikungunya Infection Chronic Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2016, 68, 1849–1858. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, J.A.; Ramirez-Arroyo, G.F. Chikungunya Virus: History, Geographic Distribution, Clinical Picture, and Treatment. P. R. Health Sci. J. 2018, 37, 187–194. [Google Scholar]
- Badawi, A.; Ryoo, S.G.; Vasileva, D.; Yaghoubi, S. Prevalence of chronic comorbidities in chikungunya: A systematic review and meta-analysis. Int J. Infect. Dis. 2018, 67, 107–113. [Google Scholar] [CrossRef]
- Ozden, S.; Huerre, M.; Riviere, J.-P.; Coffey, L.L.; Afonso, P.V.; Mouly, V.; de Monredon, J.; Roger, J.-C.; El Amrani, M.; Yvin, J.-L.; et al. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS ONE 2007, 2, e527. [Google Scholar] [CrossRef]
- Brito, C.A.A.; Azevedo, F.; Cordeiro, M.T.; Marques, E.T.A., Jr.; Franca, R.F.O. Central and peripheral nervous system involvement caused by Zika and chikungunya coinfection. PLoS Negl. Trop. Dis. 2017, 11, e0005583. [Google Scholar] [CrossRef]
- Brito Ferreira, M.L.; Militao de Albuquerque, M.F.P.; de Brito, C.A.A.; de Oliveira Franca, R.F.; Porto Moreira, A.J.; de Morais Machado, M.I.; da Paz Melo, R.; Medialdea-Carrera, R.; Dornelas Mesquita, S.; Lopes Santos, M.; et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: A prospective observational study. Lancet Neurol. 2020, 19, 826–839. [Google Scholar] [CrossRef]
- Hills, S.L.; Fischer, M.; Petersen, L.R. Epidemiology of Zika Virus Infection. J. Infect. Dis. 2017, 216 (Suppl. 10), S868–S874. [Google Scholar] [CrossRef]
- World Health Organization. Zika—OPAS/OMS Organização Pan-Americana da Saúde Washington, DC: PAHO/WHO. 2022. Available online: https://www.paho.org/pt/topicos/zika (accessed on 14 July 2022).
- Mlakar, J.; Korva, M.; Tul, N.; Popovic, M.; Poljsak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodusek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Plourde, A.R.; Bloch, E.M. A Literature Review of Zika Virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scatularo, C.E.; Ballesteros, O.A.; Saldarriaga, C.; Mendoza, I.; Wyss, F.; Liprandi, A.S.; Munera, A.; Liendro, M.C.; Baranchuk, A. Neglected Tropical Diseases and other Infectious Diseases affecting the Heart (NET-Heart project). Zika & heart: A systematic review. Trends Cardiovasc. Med. 2020, 32, 52–58. [Google Scholar] [PubMed]
- WHO/World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Available online: https://www.who.int/publications/i/item/9789240010352 (accessed on 14 July 2022).
- Boletim Epidemiológico Vol.53 N°18—Português (Brasil). GOV.BR—Português (Brasil). Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/boletins-epidemiologicos/edicoes/2022/boletim-epidemiologico-vol-53-no18/view (accessed on 19 July 2022).
- Burgos, L.M.; Farina, J.; Liendro, M.C.; Saldarriaga, C.; Liprandi, A.S.; Wyss, F.; Mendoza, I.; Baranchuk, A. On behalf of the Neglected Tropical Diseases and other Infectious Diseases affecting the Heart (NET-Heart project). Neglected Tropical Diseases and Other Infectious Diseases Affecting the Heart. The NET-Heart Project: Rationale and Design. Glob. Heart 2020, 15, 60. [Google Scholar] [CrossRef]
- Araiza-Garaygordobil, D.; Garcia-Martinez, C.E.; Burgos, L.M.; Saldarriaga, C.; Liblik, K.; Mendoza, I.; Martinez-Selles, M.; Scatularo, C.E.; Farina, J.M.; Baranchuk, A.; et al. Dengue and the heart. Cardiovasc. J. Afr. 2021, 32, 276–283. [Google Scholar] [CrossRef]
- Pothapregada, S.; Kamalakannan, B.; Thulasingam, M. Clinical Profile of Atypical Manifestations of Dengue Fever. Indian J. Pediatr. 2016, 83, 493–499. [Google Scholar] [CrossRef]
- Obeyesekere, I.; Hermon, Y. Arbovirus heart disease: Myocarditis and cardiomyopathy following dengue and chikungunya fever—A follow-up study. Am. Heart J. 1973, 85, 186–194. [Google Scholar] [CrossRef]
- Lo, C.K.-L.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Cohen, A. Comparison of correlated correlations. Stat. Med. 1989, 8, 1485–1495. [Google Scholar] [CrossRef]
- CEBM. Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford [Section]. 2020. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence (accessed on 19 July 2022).
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Moher, D.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Enst, W.A.; Ochodo, E.; Scholten, R.J.; Hooft, L.; Leeflang, M.M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: A meta-epidemiological study. BMC Med. Res. Methodol. 2014, 14, 70. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rucker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P.T.; Hedges, L.V.; Rothstein, H.R. Basics of meta-analysis: I2is not an absolute measure of heterogeneity. Res. Synth. Methods 2017, 8, 5–18. [Google Scholar] [CrossRef]
- Lakshman, A.; Balasubramanian, P.; Nampoothiri, R.V.; Vijayvergiya, R.; Bhalla, A.; Varma, S.C. Elevated cardiac biomarkers and echocardiographic left ventricular dysfunction at admission in patients with dengue fever: Report from a tertiary care center in Northwest India. Trop. Dr. 2018, 48, 261–265. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, M.; Kashyap, J.R.; Arora, S.K. Early cardiovascular involvement in dengue fever: A prospective study with two-dimensional speckle tracking echocardiography. Trop. Dr. 2022, 52, 285–292. [Google Scholar] [CrossRef]
- Wei, K.-C.; Sy, C.-L.; Wang, W.-H.; Wu, C.-L.; Chang, S.-H.; Huang, Y.-T. Major acute cardiovascular events after dengue infection–A population-based observational study. PLoS Negl. Trop. Dis. 2022, 16, e0010134. [Google Scholar] [CrossRef]
- Mansanguan, C.; Hanboonkunupakarn, B.; Muangnoicharoen, S.; Huntrup, A.; Poolcharoen, A.; Mansanguan, S.; Piyaphanee, W.; Phumratanaprapin, W. Cardiac evaluation in adults with dengue virus infection by serial echocardiography. BMC Infect. Dis. 2021, 21, 940. [Google Scholar] [CrossRef]
- Lee, I.-K.; Chen, Y.-H.; Huang, C.-H.; Hsu, J.-C.; Chang, Y.-C.; Kuo, H.-J.; Tai, C.-H.; Lee, N.-Y. A multicenter cohort study of severe dengue and critically ill influenza patients with elevated cardiac troponin-I: Difference clinical features and high mortality. Travel Med. Infect. Dis. 2022, 47, 102281. [Google Scholar] [CrossRef]
- Kabra, S.K.; Junela, R.; Madhulyka Jain, Y.; Singhal, T.; Dar, L.; Khotari, S.S.; Broor, S. Myocardial dysfunction in children with dengue haemorrhagic fever. Natl. Med. J. India 1988, 11, 59–61. [Google Scholar]
- Wichmann, D.; Kularatne, S.; Ehrhardt, S.; Wijesinghe, S.; Brattig, N.W.; Abel, W.; Burchard, G.D. Cardiac involvement in dengue virus infections during the 2004/2005 dengue fever season in Sri Lanka. Southeast Asian J. Trop. Med. Public Health 2009, 40, 727–730. [Google Scholar]
- Arora, M.; Patil, R.S. Cardiac Manifestation in Dengue Fever. J. Assoc. Physicians India 2016, 64, 40–44. [Google Scholar]
- Thanachartwet, V.; Wattanathum, A.; Sahassananda, D.; Wacharasint, P.; Chamnanchanunt, S.; Khine Kyaw, E.; Jittmittraphap, A.; Naksomphun, M.; Surabotsophon, M.; Desakorn, V. Dynamic Measurement of Hemodynamic Parameters and Cardiac Preload in Adults with Dengue: A Prospective Observational Study. PLoS ONE 2016, 11, e0156135. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Z.; Huang, Y.; Li, J.; Hong, W.; Qin, Z.; Tong, Y.; Li, J.; Lv, M.; Li, M.; et al. Characterization of the Myocarditis during the worst outbreak of dengue infection in China. Medicine 2016, 95, e4051. [Google Scholar] [CrossRef]
- Hussain, R.; Jamil, S.A.; Javed, S. Dengue Deaths: A clinical experience in critical unit. Pak. J. Med. Health Sci. 2016, 10, 77–79. [Google Scholar]
- Lal Yadav, B.; Harshvardhan, L.; Singh Yadav, K. Magnitude of Cardiac Involvement in Dengue Fever. J. Evol. Med. Dent. Sci. 2017, 6, 570–574. [Google Scholar] [CrossRef]
- Bhatt, M.; Soneja, M.; Farooqui, F.A.; Singla, P.; Vikram, N.K.; Biswas, A.; Roy, A.; Wig, N. Myocarditis in admitted patients with dengue fever. Infection 2020, 48, 899–903. [Google Scholar] [CrossRef]
- Shah, C.; Vijayaraghavan, G.; Kartha, C.C. Spectrum of cardiac involvement in patients with dengue fever. Int. J. Cardiol. 2020, 324, 180–185. [Google Scholar] [CrossRef]
- Girdhar, R.; Kothari, Y.; Raj, R.A.; Rahul, P.; Kenchappa, K.; Raju, N.; Rai, P.; Kodmad, C. Cardiac Manifestation involvement in Dengue Infection. J. Med. Sci. Clin. Res. 2017, 5, 30413–30420. [Google Scholar] [CrossRef]
- Cabrera-Rego, J.O.; Rojas-Quiroz, A.F.; Vidal-Turruelles, Y.; Yanes-Quintana, A.A. Manifestaciones cardiovasculares en pacientes hospitalizados con dengue. Enferm. Infecc. Microbiol. Clínica 2020, 39, 115–118. [Google Scholar] [CrossRef]
- Khongphatthanayothin, A.; Lertsapcharoen, P.; Supachokchaiwattana, P.; La-orkhun, V.; Khumtonvong, A.; Boonlarptaveechoke, C.; Pancharoen, C. Myocardial depression in dengue hemorrhagic fever: Prevalence and clinical description. Pediatric Crit. Care Med. 2007, 8, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Satarasinghe, R.L.; Kanagasinham, A.; Amerasena, N.L.; Bulugahapitiya, U.S.; Sahayam, D.V. Asymptomatic myocardial involvement in acute dengue virus infection in a cohort of adult Sri Lankans admitted to a tertiary referral centre. Br. J. Cardiol. 2007, 14, 171–173. [Google Scholar]
- Kirawittaya, T.; Yoon, I.-K.; Wichit, S.; Green, S.; Ennis, F.A.; Gibbons, R.V.; Thomas, S.J.; Rothman, A.L.; Kalayanarooj, S.; Srikiatkhachorn, A. Evaluation of Cardiac Involvement in Children with Dengue by Serial Echocardiographic Studies. PLoS Negl. Trop. Dis. 2015, 9, e0003943. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.; Arce, M.; Pavlicich, V.; Mesquita, M. Dengue y Bradicardia: Caracterización clínica y evolución. Pediatría 2018, 45, 115–118. [Google Scholar] [CrossRef]
- Buntubatu, S.; Prawirohartono, E.P.; Arguni, E. Myocarditis Prevalence in Paediatric Dengue Infection: A Prospective Study in Tertiary Hospital in Yogyakarta, Indonesia. J. Trop. Pediatrics 2019, 65, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Khongphatthanayothin, A.; Suesaowalak, M.; Muangmingsook, S.; Bhattarakosol, P.; Pancharoen, C. Hemodynamic profiles of patients with dengue hemorrhagic fever during toxic stage: An echocardiographic study. Intensive Care Med. 2003, 29, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Choudhary, S.; Gupta, P.K.; Beniwal, M.K.; Agarwal, S.; Shukla, U.; Dubey, N.K.; Sankar, J.; Kumar, P. The Tei Index and Asymptomatic Myocarditis in Children with Severe Dengue. Pediatric Cardiol. 2013, 34, 1307–1313. [Google Scholar] [CrossRef]
- Kalakutakar, A.; Suresh, H.; Ashok, G. Study of Cardiac Manifestations in Dengue Fever. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 636–644. [Google Scholar] [CrossRef]
- Saldarriaga, C.G.; Roncancio, G.; González, N.; Fortich, F. Manifestaciones cardiacas del dengue. Reporte de una serie de casos durante la epidemia colombiana de 2010. Rev. Colomb. de Cardiol. 2013, 20, 366–369. [Google Scholar]
- Lee, J.-C.; Cia, C.-T.; Lee, N.-Y.; Ko, N.-Y.; Chen, P.-L.; Ko, W.-C. Causes of death among dengue patients causes of death among hospitalized adults with dengue fever in Tainan, 2015: Emphasis on cardiac events and bacterial infections. J. Microbiol. Immunol. Infect. 2021, 55, 207–214. [Google Scholar] [CrossRef]
- Weerakoon, K.G.; Kularatne, S.A.; Edussuriya, D.H.; Kodikara, S.K.; Gunatilake, L.P.; Pinto, V.G.; Seneviratne, A.B.; Gunasena, S. Histopathological diagnosis of myocarditis in a dengue outbreak in Sri Lanka, 2009. BMC Res. Notes 2011, 4, 268. [Google Scholar] [CrossRef]
- Salgado, D.M.; Eltit, J.M.; Mansfield, K.; Panqueba, C.; Castro, D.; Vega, M.R.; Xhaja, K.; Schmidt, D.; Martin, K.J.; Allen, P.D.; et al. Heart and Skeletal Muscle Are Targets of Dengue Virus Infection. Pediatric Infect. Dis. J. 2010, 29, 238–242. [Google Scholar] [CrossRef]
- Yacoub, S.; Trung, T.H.; Lam, P.K.; Thien, V.H.N.; Hai, D.H.T.; Phan, T.Q.; Nguyet, O.P.K.; Quyen, N.T.H.; Simmons, C.P.; Broyd, C.; et al. Cardio-haemodynamic assessment and venous lactate in severe dengue: Relationship with recurrent shock and respiratory distress. PLoS Negl. Trop. Dis. 2017, 11, e0005740. [Google Scholar] [CrossRef]
- Wali, J.P.; Biswas, A.; Chandra, S.; Malhotra, A.; Aggarwal, P.; Handa, R.; Wig, N.; Bahl, V.K. Cardiac involvement in Dengue Haemorrhagic Fever. Int. J. Cardiol. 1998, 64, 31–36. [Google Scholar] [CrossRef]
- Khositseth, A.; Tangnararatchakit, K.; Chuansumrit, A.; Wanitkun, S.; Kuptanon, T.; Chaiyaratana, W.; Yoksan, S. Cardiovascular change in children with dengue shock syndrome. J. Pediatr. Intensive Care 2012, 1, 153–160. [Google Scholar]
- La-Orkhun, V.; Supachokchaiwattana, P.; Lertsapcharoen, P.; Khongphatthanayothin, A. Spectrum of cardiac rhythm abnormalities and heart rate variability during the convalescent stage of dengue virus infection: A Holter study. Ann. Trop. Paediatr. 2011, 31, 123–128. [Google Scholar] [CrossRef]
- Miranda, C.H.; Borges, M.d.C.; Matsuno, A.K.; Vilar, F.C.; Gali, L.G.; Volpe, G.J.; Schmidt, A.; Pazin-Filho, A.; da Silva, F.M.F.; de Castro-Jorge, L.A.; et al. Evaluation of Cardiac Involvement During Dengue Viral Infection. Clin. Infect. Dis. 2013, 57, 812–819. [Google Scholar] [CrossRef]
- Rollé, A.; Schepers, K.; Cassadou, S.; Curlier, E.; Madeux, B.; Hermann-Storck, C.; Fabre, I.; Lamaury, I.; Tressières, B.; Thiery, G.; et al. Severe Sepsis and Septic Shock Associated with Chikungunya Virus Infection, Guadeloupe, 2014. Emerg. Infect. Dis. 2016, 22, 891–894. [Google Scholar] [CrossRef]
- Sharp, T.M.; Keating, M.K.; Shieh, W.J.; Bhatnagar, J.; Bollweg, B.C.; Levine, R.; Blau, D.M.; Torres, J.V.; Rivera, A.; Perez-Padilla, J.; et al. Clinical Characteristics, Histopathology, and Tissue Immunolocalization of Chikungunya Virus Antigen in Fatal Cases. Clin. Infect. Dis. 2021, 73, e345–e354. [Google Scholar] [CrossRef]
- De Lima, S.T.S.; de Souza, W.M.; Cavalcante, J.W.; Cândido, D.S.; Fumagalli, M.J.; Carrera, J.P.; Mello, L.M.S.; Araújo, F.M.C.; Ramalho, I.L.C.; Barreto, F.K.A.; et al. Fatal Outcome of Chikungunya Virus Infection in Brazil. Clin. Infect. Dis. 2021, 73, e2436–e2443. [Google Scholar] [CrossRef]
- Lemant, J.; Boisson, V.; Winer, A.; Thibault, L.; André, H.; Tixier, F.; Lemercier, M.; Antok, E.; Cresta, M.P.; Grivard, P.; et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit. Care Med. 2008, 36, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Bonifay, T.; Prince, C.; Neyra, C.; Demar, M.; Rousset, D.; Kallel, H.; Nacher, M.; Djossou, F.; Epelboin, L.; The Char Chik Working group. Atypical and severe manifestations of chikungunya virus infection in French Guiana: A hospital-based study. PLoS ONE 2018, 13, e0207406. [Google Scholar] [CrossRef] [PubMed]
- Orofino, D.H.G.; Passos, S.R.L.; Pone, S.M.; da Silva Pone, M.V.; de Aguiar, E.B.; de Araújo, I.O.; Ramos, T.M.; e Silva, L.M.L.; de Oliveira, B.M.; da Silva, L.N.; et al. 24-hour Holter findings in infants with in-utero exposure to the Zika virus: A series of cases. Rev. Inst. Med. Trop. S. Paulo 2020, 62, e50. [Google Scholar] [CrossRef] [PubMed]
- Di Cavalcanti, D.; Alves, L.V.; Furtado, G.J.; Santos, C.C.; Feitosa, F.G.; Ribeiro, M.C.; Menge, P.; Lira, I.M.; Alves, J.G. Echocardiographic findings in infants with presumed congenital Zika syndrome: Retrospective case series study. PLoS ONE 2017, 12, e0175065. [Google Scholar]
- McHug, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Alvarez, M.F.; Bolívar-Mejía, A.; Rodriguez-Morales, A.J.; Ramirez-Valejjo, E. Cardiovascular involvement and manifestations of systemic Chikungunya virus infection: A systematic review. F1000Research 2017, 6, 390. [Google Scholar] [CrossRef]
- Cotella, J.I.; Sauce, A.L.; Saldarriaga, C.I.; Perez, G.E.; Farina, J.M.; Wyss, F.; Sosa Liprandi, A.; Mendoza, I.; Múnera, A.G.; Alexander, B.; et al. Chikungunya and the Heart. Cardiology 2021, 146, 324–334. [Google Scholar] [CrossRef]
- Pomiato, E.; Perrone, M.A.; Palmieri, R.; Gagliardi, M.G. Pediatric Myocarditis: What Have We Learnt So Far? J. Cardiovasc. Dev. Dis. 2022, 9, 143. [Google Scholar] [CrossRef]
- Akgül, F.; Er, A.; Ulusoy, E.; Çağlar, A.; Vuran, G.; Seven, P.; Yılmazer, M.M.; Ağın, H.; Apa, H. Are clinical features and cardiac biomarkers at admission related to severity in pediatric acute myocarditis?: Clinical features and cardiac biomarkers in pediatric acute myocarditis. Arch. Pediatrie 2022, 29, 376–380. [Google Scholar] [CrossRef]
- Canter, C.E.; Simpson, K.E. Diagnosis and Treatment of Myocarditis in Children in the Current Era. Circulation 2014, 129, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef]
- Da Silveira, L.T.C.; Tura, B.; Santos, M. Systematic review of dengue vaccine efficacy. BMC Infect. Dis. 2019, 19, 8. [Google Scholar] [CrossRef]
| First Author Year Journal | Country /Region | Type of Study Follow Up | Study Group(n) Age/Sex(M/F) | Cardiovascular Findings | Severity Arboviruses (WHO) | Cardiac Involvement (n) Comorbidities |
|---|---|---|---|---|---|---|
| DENGUE | ||||||
| Wali et al., 1998 [70] International Journal of Cardiology | India | Observational study, prospective Follow-up: 3 weeks | n: 17 M: 12/F: 5 Mean age: 29.7 yrs | EF 33–44%: 7 EF 41–50%: 9 Sinus bradycardia: 3 | DSS-8 DHF-9 WHO, 1997 | n: 16 Related to gravity Comorbidities: not reported |
| Kabra et al., 1998 [46] The National Medical Journal Of India | India | Prospective observational study Follow-up: 2 months | n: 54 Mean age: 6.3 yrs M: 22/F: 32 | EF # < 50%: 9 (EF 35–50%: 7 EF < 35%: 2) First degree atrioventricular block: 1 | Dengue fever, dengue, DHF *, and DSS ** WHO, 1986 | n: 9 Not related severity Comorbidities: not reported |
| Khongphatthanaythin et al., 2003 [62] Intensive Care Med | Thailand | Prospective observational study Follow-up 2 weeks | n: 24 Mean age: 11 yrs M: 11/F: 13 | EF lower during toxic stage | DHF: 24 Comorbidities: not reported WHO, 1997 | n: 24 Amount of cardiac involvement not reported |
| Khongphatthanayothin et al., 2007 [57] Pediatr Crit Care Med | Thailand | Prospective observational study Follow up: 17.5 days | n: 91 Age: 5–15 years Male: 52/F: 39 | EF < 50%: 16 | Dengue fever-30 DHF-36 DSS-25 WHO, 1997 | n: 16 Related to gravity Comorbidities: not |
| Satarasinghe et al., 2007 [58] British Journal of Cardiology | Sri Lanka | Observational, prospective study Follow-up: 3 months | n: 217 Age distribution: 12–65 yrs M: 144/F: 73 | Myocarditis: 52 Sinus bradycardia: 52 | Dengue classification not reported | n: 52 Not correlation severity Comorbidities: not reported |
| Wichmann et al., 2009 [47] South Asian J Trop Med Public Health | Sri Lanka | Hospital based observational descriptive study | n: 133 Age: 18–76 years M 69/F: 64 | Myoglobin: 60 CK-MB: 17 Troponin-1 NT-Pro BNP: 25 | Dengue fever Secondary dengue: 66 WHO, 1997 | n: 33 Comordities: not reported |
| Salgado et al., 2010 [68] Pediatr Infect Dis | Colombia | Observational, prospective Follow-up: 1 year | n: 102 Mean age: 6 yrs M/F: not reported | Myocarditis: 11 Pleural effusion: 5 Sinus bradycardia: 9 Sinus tachycardia: 2 CK-MB: 6 | DHF-79 Dengue fever: 23 WHO, 2009 | n: 11 Related to gravity Comorbidities: not reported |
| La-Orkhun et al., 2011 [72] Annals of Tropical Paediatrics | Thailand | Prospective Follow up: by 14 days | n: 35 Mean age: 11.7 yrs M: 20/F: 15 | First degree atrioventricular block: 2 First degree atrioventricular block: 3 Other abnormalities: 10 | Dengue fever-12 DHF-18 DSS-5 WHO,1997 | n: 15 Not related to gravity Comorbidities: not |
| Weerakon et al., 2011 [67] BMC Research | Sri Lanka | Observational prospective Follow up: not reported | n: 319 Age: 13–31 yrs | Death: 11 Myocarditis: 21 Pleural effusion: 41 Abnormal ECG: 5 Troponin T: 5 | Dengue fever: 153 Severe dengue: 166 WHO, 1997 Secondary infection | n: 45 Related to gravity Comorbidities: not reported |
| Khositseth et al., 2012 [71] Journal of Pediatric Intensive Care | Thailand | Prospective observational study Follow up: 3–44 days | n: 8 M: 5/F: 3 Mean age: 6.5 yrs | Impaired systolic function: 1 | Death: 06 DSS WHO, 1997 | n: 1 (impaired systolic function) Comorbidities: not reported |
| Kumar Yadav et al., 2013 [63] Pediatr Cardiol | India | Prospective observational study | n: 67 Mean age: 10.4 yrs Male: 65% | Myocarditis-32 Pericardial effusion: 1 EF< 35%: 3 Tei index & abnormal: 48 | Death: 01 Dengue severe Secondary WHO, 2009 | n: 48 Related to gravity Comorbidities: not |
| Saldarriaga et al., 2013 [65] RevistaColombian de Cardiologí | Colombia | Observational descriptive prospective study | n: 7 M: 4/F: 3 Mean age: 55.7 yrs | Systolic dysfunction: 2 Pericardial effusion: 1 | Not clinical classification. WHO, 2011 | n: 3 Comorbidities: coronary heart disease and heart failure |
| Miranda et al., 2013 [73] Clinical Infectious Diseases | Brazil | Observational prospective descriptive study Follow up: 3–6 days | n: 81 M: 39/F: 42 Mean age: 32 yrs | Myocarditis: 3 Impaired systolic function: 4 Troponin: 6 NT Pro BNP: 10 | Dengue fever-54 Dengue hemorragic fever-27 WHO, 1997 | n: 12 Comorbidities: not reported |
| Arora et al., 2015 [48] JAPI | India | Prospective observational Follow up not reported | n: 120 Mean age-32 yrs M: 85/F: 35 | Myocarditis: 45 Sinus bradycardia: 10 Sinus tachycardia: 4 | Dengue fever-20 DHF-85 * DSS-15 ** WHO, 2009 | n: 45 Related to gravity Comorbidities: not reported |
| Kirawittaya et al., 2015 [59] PloS NT | France | Observational analytical longitudinal prospective | n: 181 M/F: not reported Follow up: 1 week | EF < 56%: 17 Pericardial effusions: 7 Without myocarditis | Dengue fever DHF-23 WHO, 1997; 2009 | n: 17 Related to gravity Comorbidities: not reported |
| Li et al., 2016 [50] Medicin | China | Observational analytical longitudinal prospective | n: 1782 M: 203/: 224 Mean age: 60.7 yrs | Myocarditis: 201 ST-T abnormality: 59 Atrial fibrillation: 26 Troponin I: 13 CK-MB: 54 NT Pro BNP: 81 | DF *** without warning signs 1707 DF with warning signs +severe cases-75 WHO, 2009 | n: 495 Related to gravity Comorbidities: not reported |
| Pothapregada et al., 2016 [31] Indian J Pediat | India | Observational retrospective study Follow up: 8.1 days | n: 254 Mean age: 6.9 yrs M: 132/F: 122 | Myocarditis: 5 Diastolic dysfunction: 2 Pericardial effusion: 3 Paroxysmal supraventricular tachycardia: 3 Sinus bradycardia: 2 | Death-6 Dengue fever-159 Severe dengue-95 WHO, 2011 | n: 5 Related to gravity Comorbidities: not reported |
| Thanachartwet et al., 2016 [49] PloS One | Thailand | Prospective observational study Follow-up total: 15 days | n: 162 Median age: 24.5 yrs M: 87/F: 75 | Abnormal ECG ##: 65 Troponin T: 2 NT Pro BNP: 23 | Death: 02 DSS-17 Not DSS-145 WHO, 2009 | n: 65 Related to gravity Comorbidities: not reported |
| Hussain et al., 2016 [51] P J M H S | Pakistan | Observational, prospective study. Follow up: two weeks | n: 128 Above 12 yrs | Myocarditis: 24 | Death: 20 Dengue fever DHF and DSS-122 WHO, 2011 | n: 24 Related to gravity Comorbidities: not |
| Girdhar et al., 2017 [55] Jour of Med Science and Clinical Search | India | Hospital based observational descriptive study | n: 211 M: 110/F: 101 Mean age: 30.4 yrs | Pericardial effusion: 10 EF # < 50%: 10 Sinus bradycardia: 18 Sinus tachycardia: 14 Troponin T: 12 CK-MB: 79 | Dengue fever: (no clinical severity classification) WHO, 2009 | n: 57 Related to gravity Comorbidities: not reported |
| Yacoub et al., 2017 [69] PLoS NTD English | Vietnam | Prospective observational study Follow up: 14 days | n: 102 Median age: 11 yrs F: 102 | Left ventricular dysfunction: 24 Right ventricular dysfunction: 6 | DSS-80 WHO, 2009 | n: 30 Related to gravity Comorbidities: not reported |
| Yadav et al., 2017 [52] J. Evolution Med. Dent. Sci | India | Observational prospective descriptive study | n: 104 Age: 21–30 yrs | Left ventricular dysfunction: 4 Right ventricle dilation: 2 Sinus bradycardia: 9 Sinus tachycardia: 7 Troponin T: 9 | Not clinical Dengue classification WHO, 2012 | n: 28 Related to gravity Comorbidities: not |
| Lakshman et al., 2018 [41] Tropical Doctor | India | Prospective observational Follow up: not reported | n: 50 Median age: 38 yrs M: 35/F: 15 | Left ventricular dysfunction: 8 Sinus tachycardia: 10 CK-MB: 10 Troponin I: 3 | DF *** not warning-10 DF with warning-33 Severe DF-7 WHO, 2014 | n: 16 Not related to gravity Comorbidities: not reported |
| Godoy et al., 2018 [60] Pediatr. (Asunción) | Paraguay | Prospective observational study Follow up: 2 weeks | n: 310 Mean age: 13 yrs M: 16/F: 29 | Pericardial effusion: 2 Sinus bradycardia: 19 first degree atrioventricular block: 1 | Dengue fever-12 DF with warning signs-8 WHO, 1997 | n: 20 Related to gravity Comorbidities: not reported |
| Buntubatu et al., 2019 [61] Jour of Tropical Pediatrics | Indonesia | Prospective observational Follow-up: 2 to 7 days (median 3 days) | n: 50 Median age: 8 yrs M: 15/F: 35 | Myocarditis: 39 Sinus tachycardia: 11 Sinus tachycardia: 4 CK-MB: 35 Troponin I: 12 | Death: 0 Dengue fever-15 DHF-12 DSS-23 WHO, 2011 | n: 39 Related to gravity Comorbidities: not reported |
| Kalakutakar et al., 2019 [64] Inter Journal of Current Microb | India | Observational prospective descriptive study | n: 100 M: 59/F: 41 Mean age: 30 yrs | Myocarditis: 2 Sinus bradycardia: 15 Sinus tachycardia: 9 | Dengue fever: (no clinical severity) WHO, 2009 | n: 44 Not studied relationship with clinical severity. |
| Bhatt et al., 2020 [53] Infection | India | Prospective observational Follow up: 7 days | n: 182 Mean age: 30 yrs M: 126/F: 56 | Myocarditis: 13 EF < 50%: 11 Sinus bradycardia: 10 Sinus tachycardia: 30 Troponini I: 25 NT Pro BNP: 22 | Death: 5 Dengue fever: 37 DF warning signs: 85 -DF severe: 60 WHO, 2014 | n: 44 Related to gravity Comorbidities: not reported |
| Sha et al., 2020 [54] International Journal of Cardiology | India | Observational analytical longitudinal prospective | n: 320 M: 198/F: 122 Age: 18–80 yrs | Myocarditis: 56 Pleural effusion: 3 | Death-14 Dengue classification not reported WHO, 2009 | n: 112 Not related to gravity Comorbidities: not reported |
| Cabrera-Rego et al., 2021 [56] Enf Infec.Microb Clin | Cuba | Observational longitudinal prospective | n: 427 Age: <25–>65 yrs M: 237/F: 190 | Myocarditis: 1 Pericarditis: 7 Sinus bradycardia: 59 Atrial fibrillation: 2 | Dengue classification not reported Comorbidities: not WHO, 2009 | n: 84 Related to gravity Comorbidities: not |
| Mansanguan et al., 2021 [44] BMC Infect Dis | Thailand | Observational prospective descriptive study Follow up: 2 weeks | n: 81 Mean age: 33 yrs | Myocarditis-2 Left ventricular systolic dysfunction: 3 Troponin: 2 | Dengue fever: 39 Dengue hemorragic fever: 42 WHO, 1997 | n: 18 Comorbidities: diabetes mellitus, hypertension Related to gravity |
| Lee et al., 2021 [71] Jour of Microb Immunology and Infection | Taiwan | Observational retrospective study | n: 4488 Mean age of those who died: 73 yrs | Tachycardia and ventricular fibrillation: 5 | Death: 60 No clinical severity described WHO, 2009 Related to gravity | n: 13 Comorbidities: hypertension; diabetes mellitus; chronic kidney disease; cardiovascular |
| Lee et al., 2022 [66] Travel Medicine and Infectious Disease | Taiwan | Observational prospective descriptive study | n: 163 Median age: 72 yrs M: 25/F: 16 | Troponin I: 82 | Death: 21 Severe dengue with s-294 WHO, 2009 Related to gravity | n: 41 Comorbidities: hypertension; diabetes mellitus, kidney disease, ischemic heart disease |
| Wei et al., 2022 [43] PLos NTD | Taiwan | Population-based observation study Follow up: by 28 days | n: 65.906 Age: (Yrs) 0–39: 30 40–59: 237 ≥60: 977 | Heart failure: 195 | Dengue fever Secondary Dengue: not reported WHO: not reported | n: 844 Comorbidities: hypertension, diabetes mellitus, dyslipidemia Not related to gravity |
| Gupta et al., 2022 [44] Tropical Doctor | India | Observational prospective descriptive study Follow up: 1–22 days | n: 150 M: 100/F: 50 Mean age: 36 yrs | Myocarditis: 41 Sinus bradycardia: 11 Sinus tachycardia: 33 CK-MB: 43 Troponin I: 31 | DF without warning: 41 DF with warning: 47 Severe dengue: 62 WHO, 2009 | n: 41 Associated with dengue severity Comorbidities: not reported |
| CHIKUNGUNYA | ||||||
| Obeyesekere et al., 1973 [32] Amer Heart Jour. | Sri Lanka | Observational study, prospective | n: 35 M: 17/F: 18 Age: 5–58 yrs | Cardiomegaly: 26 Cardiac arrhythmia: 25 | Death: 3 (2-heart failure). | n: 26 Comorbidities: not reported |
| Lemant et al., 2008 [77] Crit Care Med | Reunion | Observational study, prospective | n: 33 Median age: 62 yrs M: 17/F: 16 | Myocarditis: 1 | Death: 16 Atypical forms: 19 (hepatitis, encephalopathy, shock, myocarditis) | n: 1 (myocarditis) Comorbidities: diabetes mellitus, cardiac failure Related to gravity |
| Rollé et al., 2016 [74] Emerging Infectious Diseases | Guadalupe | Prospective observational/ sample for convenience | n: 110 Median age: 71 yrs M: 62/F: 48 | Cardio-circulatory failure: 22 | Death: 14 (severe sepsis) Atypical forms: 34 Severe forms: 32 Non severe forms: 44 | n: 38 Cardiac manifestations (10 non severe; 28 severe CHIKV) Comorbidities: DM; chronic heart disease; chronic renal disease Related to gravity |
| Bonifay et al., 2018 [78] PLoS ONE | French Guiana | Retrospective descriptive Follow up: median-5 days | n: 96 Median age-57 years | Cardio-respiratory failure (acute respiratory failure = 4, acute heart failure = 2 | Death: 01 Common CHIKV-68 Atypical CHIKV-23 Severe CHIKV-5 | n: 2 Comorbidities: fever, arthralgia headache, myalgia Related to gravity |
| Sharp et al., 2021 [75] Clinical Infectious Diseases | Puerto Rico | Retrospective descriptive sample for convenience | n: 30-fatal cases Median age: 61 yrs M: 19/F: 11 | Cardiac arrhythmias: 11 Myocarditis: 1 Myocardial infarct: 4 | Death: 30 Not reported | n: 16 Cardiac arrhythmias: 11 Myocarditis: 1 Myocardial infarct: 4 Comorbidities: 27 (DM; obesity, hypertension, coronary artery disease, asthma) |
| Lima et al., 2021 [76] Clinical Infectious Diseases | Brazil | Retrospective descriptive sample for convenience | n: 68 fatal cases Median age: 51 yrs F: 37/M: 31 | Cardiac arrest: 23 Myocarditis: 15 | Death: 68 Not reported | n: 38 Heart and/or respiratory failure were the most frequent causes of death. Comorbidities: hypertension; diabetes Related to gravity |
| ZIKA | ||||||
| Cavalcanti at al., 2017 [80] PLoS One | Brazil | Observational retrospective study Follow up: not reported | n: 103 Mean age: 58 days | Clinical cardiologic- congenital heart disease - Ostium secundum: 5 - Small apical muscular ventricular septal defect.: 8 | Clinical cardiologic-dyspnea | n: 14 Comorbities: not |
| Orofino et al., 2020 [79] Rev Inst Med Trop São Paulo | Brazil | Prospective observational study | n: 15 Median age: 16 months F: 6/M: 9 | Not reported | Congenital Zika virus syndrome Severe microcephaly | The findings in the 24-h Holter monitoring suggest that infants with in utero exposure to ZIKV and severe ### CZS are at higher risk of #### SIDS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicacio, J.M.; Gomes, O.V.; Carmo, R.F.d.; Nunes, S.L.P.; Rocha, J.R.C.F.; Souza, C.D.F.d.; Franca, R.F.d.O.; Khouri, R.; Barral-Netto, M.; Armstrong, A.d.C. Heart Disease and Arboviruses: A Systematic Review and Meta-Analysis. Viruses 2022, 14, 1988. https://doi.org/10.3390/v14091988
Nicacio JM, Gomes OV, Carmo RFd, Nunes SLP, Rocha JRCF, Souza CDFd, Franca RFdO, Khouri R, Barral-Netto M, Armstrong AdC. Heart Disease and Arboviruses: A Systematic Review and Meta-Analysis. Viruses. 2022; 14(9):1988. https://doi.org/10.3390/v14091988
Chicago/Turabian StyleNicacio, Jandir Mendonça, Orlando Vieira Gomes, Rodrigo Feliciano do Carmo, Sávio Luiz Pereira Nunes, José Roberto Coelho Ferreira Rocha, Carlos Dornels Freire de Souza, Rafael Freitas de Oliveira Franca, Ricardo Khouri, Manoel Barral-Netto, and Anderson da Costa Armstrong. 2022. "Heart Disease and Arboviruses: A Systematic Review and Meta-Analysis" Viruses 14, no. 9: 1988. https://doi.org/10.3390/v14091988
APA StyleNicacio, J. M., Gomes, O. V., Carmo, R. F. d., Nunes, S. L. P., Rocha, J. R. C. F., Souza, C. D. F. d., Franca, R. F. d. O., Khouri, R., Barral-Netto, M., & Armstrong, A. d. C. (2022). Heart Disease and Arboviruses: A Systematic Review and Meta-Analysis. Viruses, 14(9), 1988. https://doi.org/10.3390/v14091988







