Effect of EGCG Extracted from Green Tea against Largemouth Bass Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Aptamer
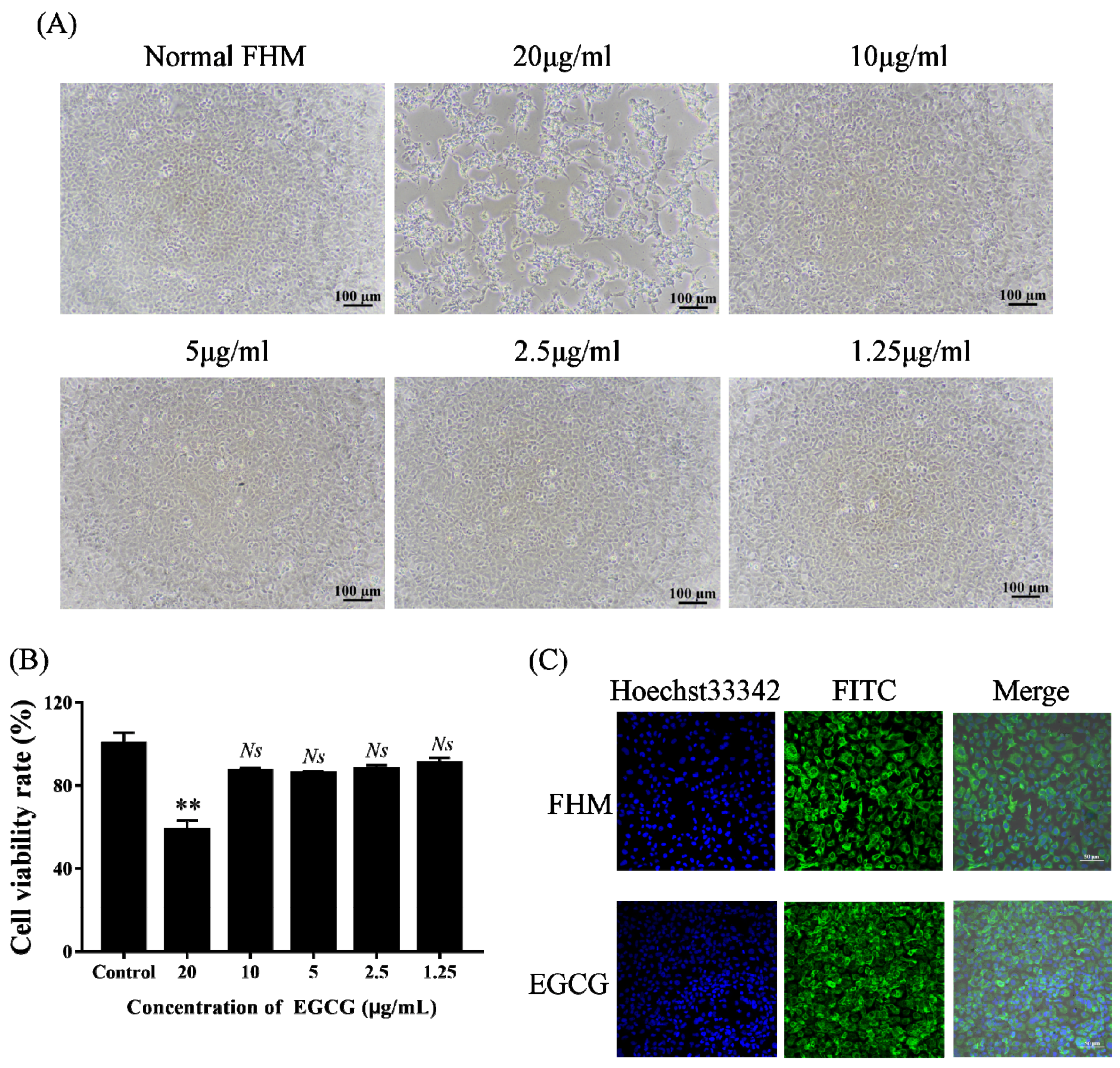
2.2. Cell Viability Assay to Identify the Safe Concentration of EGCG
2.3. Fluorescence Microscopy Observation of the Cytoskeleton at the Safe Concentration of EGCG
2.4. Gene Expression Measurement by RT-qPCR
2.5. FAM-LBVA1 Monitoring of LMBV Infection
2.6. Cy5-LMBV Monitoring of LMBV Infection
2.7. Analysis of the Antiviral Effect of EGCG against LMBV In Vitro
2.8. Analysis of the Anti-LMBV Mechanism of EGCG: Effects on Virus Particle Structure
2.9. Analysis of the Anti-LMBV Mechanism by Which EGCG Affects the Virus Infection Process
2.10. Quantification of Inhibitory Effects of EGCG against Absorption, Invasion, and Replication of LMBV
2.11. Antiviral Activity of EGCG against LMBV Infection In Vivo
2.12. Statistical Analysis
3. Results
3.1. Safe Concentration of EGCG
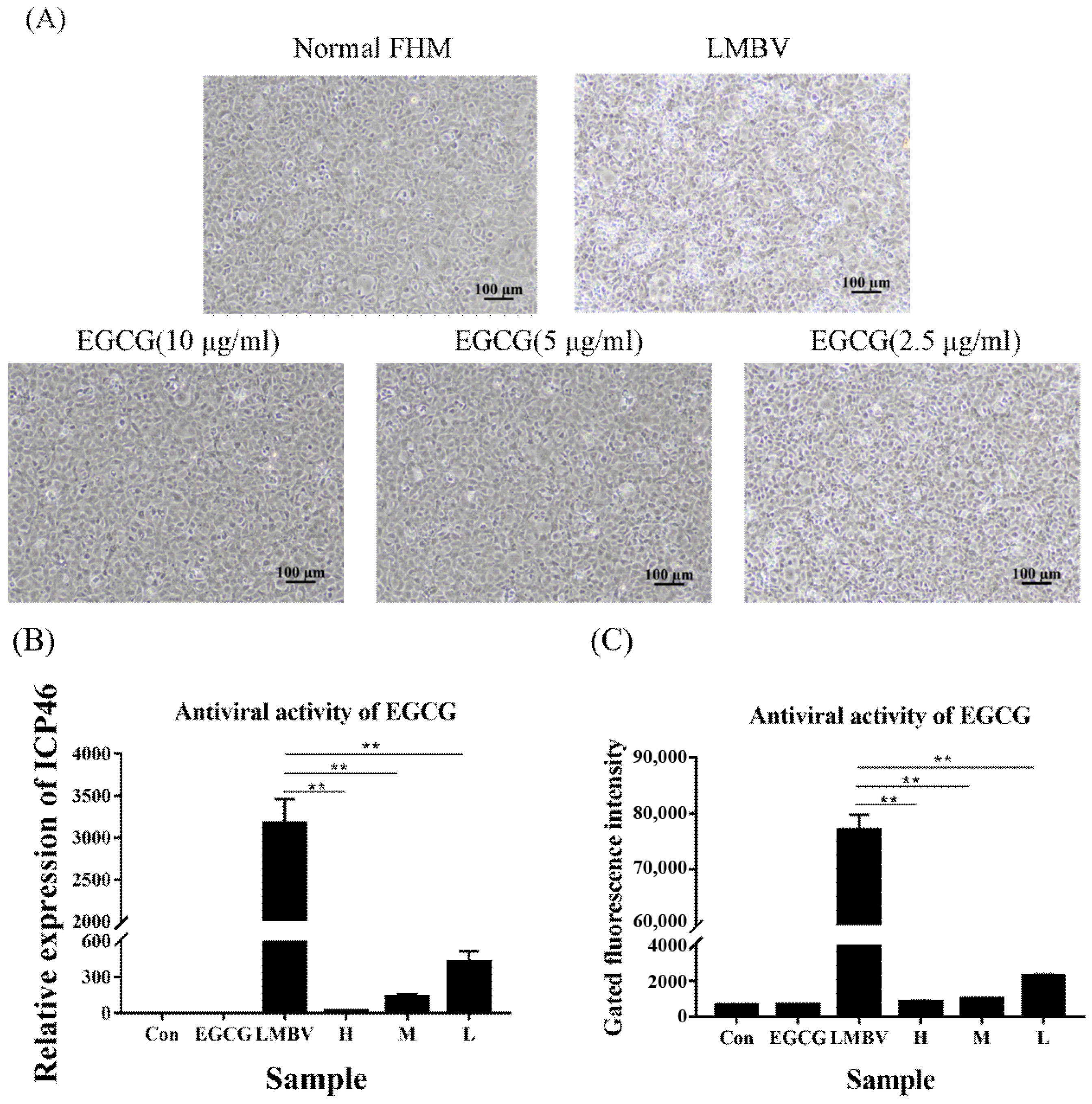
3.2. Antiviral Activity of EGCG
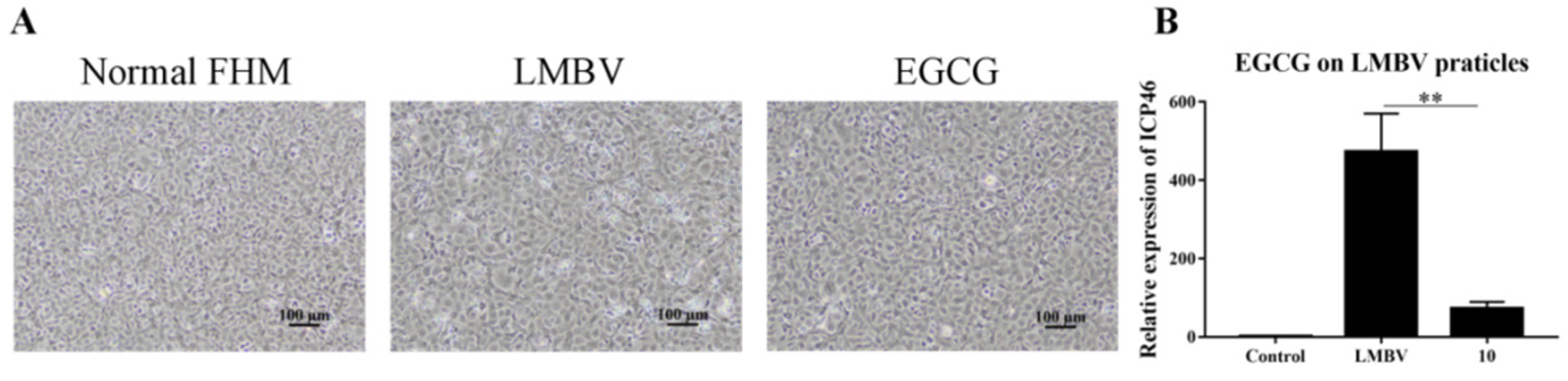
3.3. Anti-LMBV Mechanism of EGCG: EGCG Destroys Virus Particles
3.4. Anti-LMBV Mechanism of EGCG: EGCG Affects the LMBV Infection Process
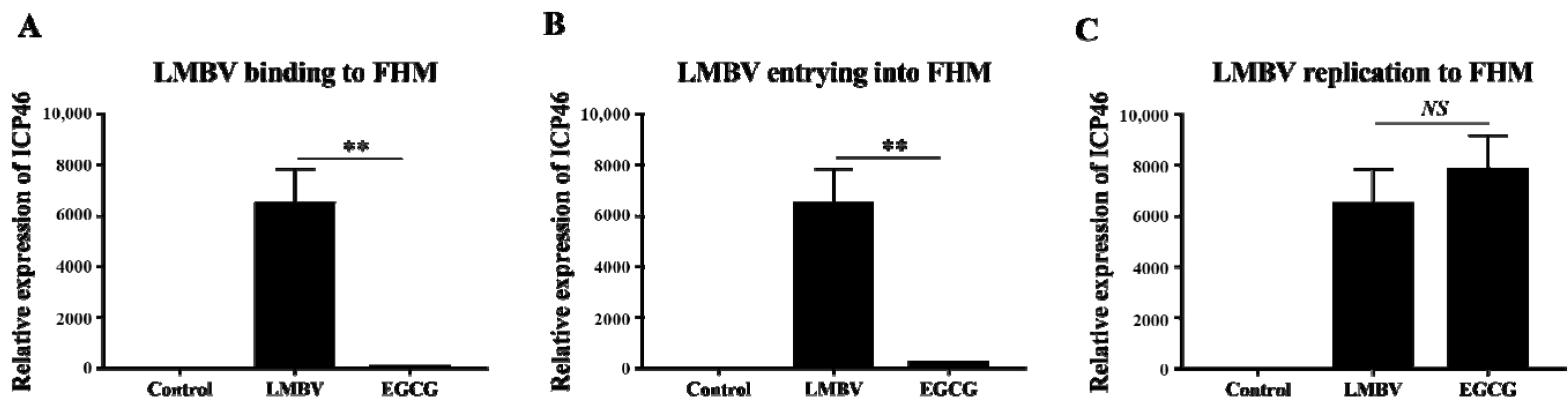
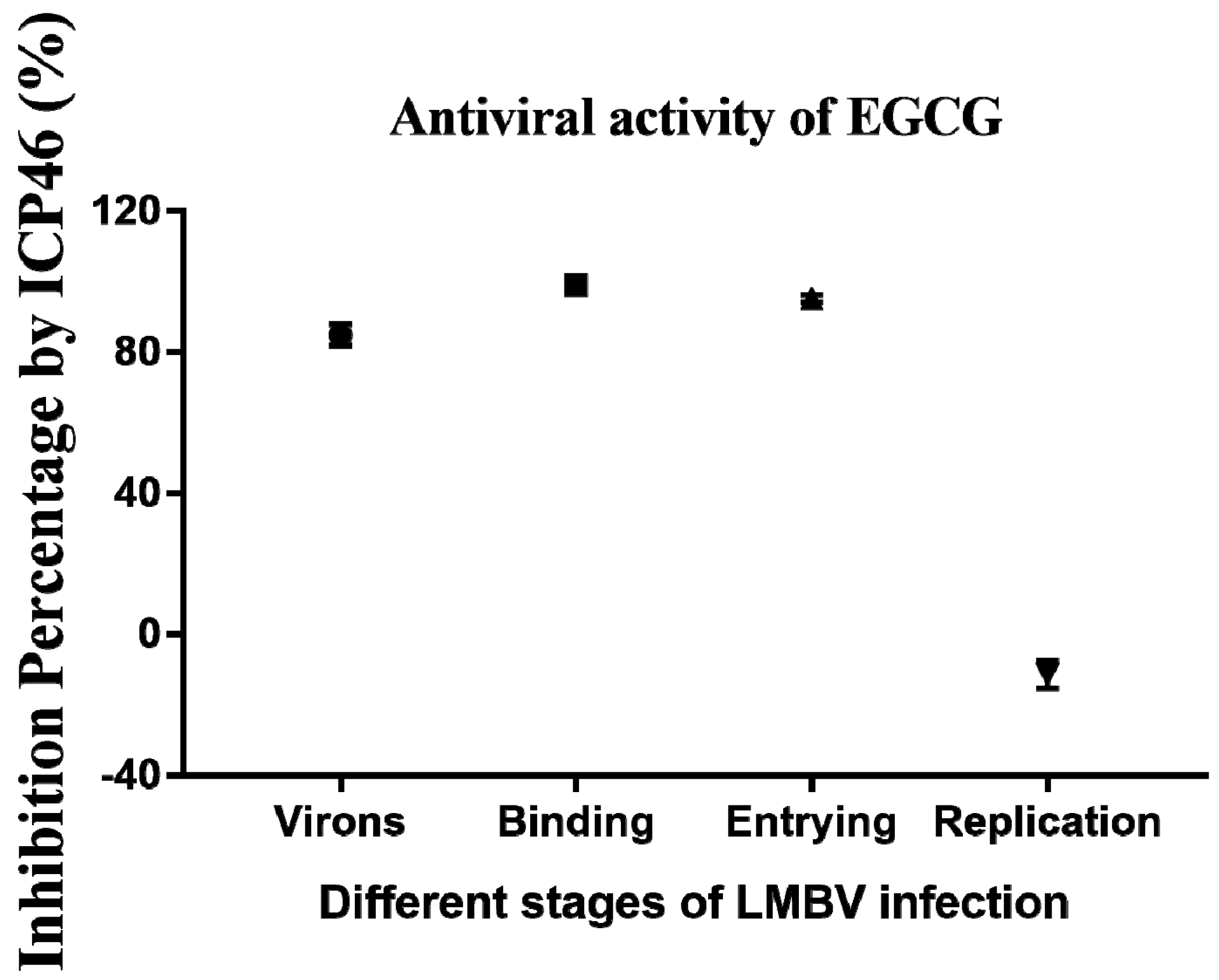
3.5. Inhibitory Effects of EGCG on Different Phases of LMBV Infection
3.6. Antiviral Activity of EGCG against LMBV Infection In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Yao, H.; Li, Y.H.; Xu, Y.J.; Ma, X.F.; Wang, H.P. Global diversity and genetic landscape of natural populations and hatchery stocks of largemouth bass micropterus salmoides across American and Asian regions. Sci. Rep. 2019, 9, 16697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.M. Antiviral Effect and Mechanism of Metformin against SGIV and Artemisinin and Their Derivatives against LMBV; Guangxi University for Nationalities: Nanning, China, 2022. [Google Scholar]
- Zhang, X.L.; Cui, L.; Li, S.; Liu, X.; Han, X.; Jiang, K. China Fishery Statistical Yearbook; China Agricultural Press: Beijing, China, 2021. [Google Scholar]
- Huang, X.; Wang, W.; Huang, Y.; Xu, L.; Qin, Q. Involvement of the PI3K and ERK signaling pathways in largemouth bass virus-induced apoptosis and viral replication. Fish Shellfish Immunol. 2014, 41, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Guo, Z.R.; Ma, R.; Qiu, D.K.; Wang, G.X.; Zhu, B. Protective immunity of largemouth bass immunized with immersed DNA vaccine against largemouth bass ulcerative syndrome virus. Fish Shellfish Immunol. 2020, 107 (Pt A), 269–276. [Google Scholar] [CrossRef]
- Dong, H.X.; Zeng, W.W. Research Progress on Largemouth Bass Ranavirus Disease. Chin. J. Virol. 2022, 38, 746–756. [Google Scholar]
- Yi, W.Y.; Zhang, X.; Zeng, K.; Xie, D.F.; Song, C.; Kachon, T.; Liu, Z.J.; Zhou, T.H.; Li, W. Construction of a DNA vaccine and its protective effect on largemouth bass (Micropterus salmoides) challenged with largemouth bass virus (LMBV). Fish Shellfish Immunol. 2020, 106, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Li, P.F.; Yu, Q.; Zhou, L.L.; Dong, D.X.; Wei, S.N.; Ya, H.Z.; Chen, B.; Qin, Q.W. Probing and characterizing the high specific sequences of ssDNA aptamer against SGIV-infected cells. Virus Res. 2018, 246, 46–54. [Google Scholar] [CrossRef]
- Li, P.F.; Huang, S.S.; Xiao, S.Y.; Xu, Y.H.; Wei, X.X.; Xiao, J.; Guo, Z.B.; Yu, Q.; Liu, M.Z. Antiviral activities of green tea components against grouper iridovirus infection in vitro and in vivo. Viruses 2022, 14, 1227. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Xie, G.Y.; Chen, X.J. Research progress on the antiviral effect and mechanism of tea polyphenols. Chin. J. Prev. Vet. Med. 2021, 43, 672–678. [Google Scholar]
- Patrick, M.; Kevin, M.D. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 222–230. [Google Scholar]
- Sahoo, M.; Jena, L.; Rath, S.N.; Kumar, S. Identification of suitable natural inhibitor against influenza A (H1N1) neuraminidase protein by molecular docking. Genom. Inform. 2016, 14, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Kuzuhara, T.; Iwai, Y.; Takahashi, H.; Takahashi, H.; Hatakeyama, D.; Echigo, N. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. 2009, 1, RRN1052. [Google Scholar] [CrossRef] [PubMed]
- Jae, M.S.; Kwang, H.L.; Baik, L.S. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar]
- Chen, W.J.; Wan, S.Q. Overview of the pharmacodynamic study of tea polyphenols. Chin. Tradit. Herb. Drugs 1993, 24, 493–499. [Google Scholar]
- Xu, J.; Gan, Z.X.; Song, S.H.; Yang, X.J.; Zheng, W.M. Research progress on antiviral effect of green tea extract. Chin. Agric. Sci. Bull. 2009, 25, 79–82. [Google Scholar]
- Knox, Y.M.; Suzutani, T.; Yosida, I.; Azuma, M. Anti-influenza virus activity of crude extract of Ribes nigrum L. Phytother. Res. 2003, 17, 120–122. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Ru, G.; Xu, Y.; Lu, L. EGCG: Potential application as a protective agent against grass carp reovirus in aquaculture. J. Fish Dis. 2018, 41, 1259–1267. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Honda, M.; Ikigai, H.; Hara, Y.; Shimamura, T. Inhibitory effects of (-)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2002, 53, 19–34. [Google Scholar] [CrossRef]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouillé, Y.; Séron, K. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef]
- Chen, C.; Qiu, H.; Gong, J.; Liu, Q.; Xiao, H.; Chen, X.W.; Sun, B.L.; Yang, R.G. (-)-Epigallocatechin-3-gallate inhibits the replication cycle of hepatitis C virus. Arch. Virol. 2012, 157, 1301–1312. [Google Scholar] [CrossRef]
- Wu, D.D.; Qu, C.; Zhang, W.F.; Luo, F.; Wang, X.K.; Hou, W. Antiviral Effect of Epigallocatechin gallate on respiratory syncytial virus. Med. J. Wuhan Univ. 2016, 37, 177–182. [Google Scholar]
- Wu, Q.Q. Antiviral Activities of Procyanidin and EGCG against Infections of Porcine Reproductive and Respire; South China Agricultural University: Guangzhou, China, 2016. [Google Scholar]
- He, X.R.; Gao, B.; Zhou, L.; Xiong, S.D. Green Tea Polyphenol Epigallocatechin-3-gallate-Alleviated Coxsackievirus B3-induced Myocarditis Through Inhibiting Viral Replication but Not Through Inhibiting Inflammatory Responses. J. Cardiovasc. Pharmacol. 2017, 69, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Qiu, S.Y.; Ban, S.D.; Luo, X.Y.; Wang, X.D. Research progress on bioactivity of epigallocatechin gallate in green tea. China Brew. 2019, 38, 12–18. [Google Scholar]
- Xu, J.; Wang, J.; Deng, F.; Hu, Z.H.; Wang, H.L. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antivir. Res. 2007, 78, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.B.; Zhang, Y.; Xu, H.; Chen, X.W.; Chen, S.Y. Inhibition of the replication of hepatitis B virus in vitro by pu-erh tea extracts. J. Agric. Food Chem. 2011, 59, 9927–9934. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, Y.Q.; Guo, Z.W.; Zhou, X.H.; Lu, M.D.; Xue, T.C.; Gao, B. ERK1/2-HNF4α axis is involved in epigallocatechin-3-gallate inhibition of HBV replication. ACTA Pharmacol. Sin. 2019, 41, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, M.M.; Liu, M.Z.; Huang, S.S.; Wang, G.X.; Wang, T.X.; Li, P.F. Selection and characterization of ssDNA aptamers targeting Largemouth Bass virus infected cells with antiviral activities. Front. Microbiol. 2021, 12, 785318. [Google Scholar] [CrossRef] [PubMed]
- Ndozangue-Touriguine, O.; Hamelin, J.; Bréard, J. Cytoskeleton and apoptosis. Biochem. Pharmacol. 2008, 76, 11–18. [Google Scholar] [CrossRef]
- Chong, C.M.; Low, C.F. Synthetic antibody: Prospects in aquaculture biosecurity. Fish Shellfish Immunol. 2019, 86, 361–367. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.Z.; Wei, S.N.; Xiao, H.H.; Wu, S.T.; Ke, K.; Huang, X.H.; Qin, Q.W.; Li, P.F. Identification of major capsid protein as a potential biomarker of grouper iridovirus-infected cells using aptamers selected by SELEX. Front. Microbiol. 2019, 10, 2684. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.; Li, S.; Zhan, J.; Ho, C. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of Tea Components with Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.H.; Tang, Y.Z.; Zhou, X.M.; Xie, G.; Su, Y.B.; He, Z.H.; Chen, J.X. Study on Anti-influenza Virus Effect of Tea Polyphenols in vitro and in vivo. J. Tea Sci. 2010, 30, 302–308. [Google Scholar]
- Xie, H.J.; Liu, N.; Ding, W.; Zhao, F.; Shi, C.X.; Xu, P.P.; Lai, X.P.; Zhang, F.X. Antiviral Effect of Tea Catechin EGCG on Influenza Virus FM1 Strain in Vitro. J. Guangzhou Univ. Tradit. Chin. Med. 2012, 29, 172–175+224. [Google Scholar]
- Xu, J.; Gu, W.Z.; Li, C.Y.; Li, X.; Xing, G.Z.; Li, Y.; Song, Y.H.; Zheng, W.M. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016, 70, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.M.; Jia, J.H.; Rohan, L.; Corbo, C.; Di, M.V.; Jenkins, E.C.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Li, P.F.; Zhou, L.L.; Ni, S.W.; Xu, M.; Yu, Y.P.; Cai, J.; Wei, S.N.; Qin, Q.W. Establishment and characterization of a novel cell line from the brain of golden pompano (Trachinotus ovatus). Vitr. Cell. Dev. Biol.-Anim. 2016, 52, 410–418. [Google Scholar] [CrossRef]
- Wang, S.W.; Huang, X.H.; Huang, Y.H.; Hao, X.; Xu, H.J.; Cai, M.J.; Wang, H.D.; Qin, Q.W. Entry of a novel marine DNA virus, Singapore grouper iridovirus, into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner. J. Virol. 2014, 88, 13047–13063. [Google Scholar] [CrossRef] [Green Version]
- Eira, J.; Silva, C.; Sousa, M.; Liz, M. The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog. Neurobiol. 2016, 141, 61–82. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Y.; Shen, Y.F.; Wang, G.X.; Zhu, B. Evaluation on antiviral activity of coumarin derivatives against spring viraemia of carp virus in epithelioma papulosum cyprini cells. Antivir. Res. 2017, 144, 173–185. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.Z.; Wu, S.T.; Wei, X.X.; Xiao, H.H.; Yi, Y.; Cheng, H.; Wang, S.W.; Zhang, Q.; Qin, Q.W.; et al. Specific aptamer-based probe for analyzing biomarker MCP entry into Singapore grouper iridovirus-infected host cells via clathrin-mediated endocytosis. Front. Microbiol. 2020, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 1993, 21, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyo, M.; Takao, M.; Satoru, F.; Hitoshi, O. Inhibitory effects of epigallocatechin gallate on the propagation of bovine coronavirus in Madin-Darby bovine kidney cells. Anim. Sci. J. 2005, 76, 507–512. [Google Scholar]
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Epigallocatechin gallate, the main component of tea polyphenol, binds to CD4 and interferes with gp120 binding. J. Allergy Clin. Immunol. 2003, 112, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Lu, H.; Zhao, Q.; He, Y.X.; Niu, J.K.; Debnath, A.K.; Wu, S.G.; Jiang, S.B. Theaflavin deri-vatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. BBA—Gen. Subj. 2005, 1723, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Tan, S.Y.; Jin, H.; Qiu, J.Y.; Mao, Q.C.; Li, R.M.; Xia, C.L.; Jiang, Z.H.; Jiang, S.B.; et al. A natural theaflavins preparation inhibits HIV-1 infection by targeting the entry step: Potential applications for preventing HIV-1 infection. Fitoterapia 2012, 83, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Murali, A.; Singh, S.K.; Giri, R. Epigallocateching-allate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. Int. J. Biol. Macromol. 2017, 104, 1046–1054. [Google Scholar] [CrossRef]
- Ciesek, S.; von, H.T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar] [CrossRef]



| Primer | Sequences |
|---|---|
| qICP46-F | 5′-CAACTGCAGACTGGTCCTGA-3′ |
| qICP46-R | 5′-AAAGCCTGTTGAGGAGACGA-3′ |
| β-actin-F | 5′-TCTTCCAGCCATCCTTCCTTGG-3′ |
| β-actin-R | 5′-CTGCATACGGTCAGCAATGCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Liu, M.; Yu, Q.; Huang, S.; Han, S.; Shi, J.; Wei, H.; Zou, J.; Li, P. Effect of EGCG Extracted from Green Tea against Largemouth Bass Virus Infection. Viruses 2023, 15, 151. https://doi.org/10.3390/v15010151
Cheng Y, Liu M, Yu Q, Huang S, Han S, Shi J, Wei H, Zou J, Li P. Effect of EGCG Extracted from Green Tea against Largemouth Bass Virus Infection. Viruses. 2023; 15(1):151. https://doi.org/10.3390/v15010151
Chicago/Turabian StyleCheng, Yuan, Mingzhu Liu, Qing Yu, Shuaishuai Huang, Shuyu Han, Jingu Shi, Hongling Wei, Jianwei Zou, and Pengfei Li. 2023. "Effect of EGCG Extracted from Green Tea against Largemouth Bass Virus Infection" Viruses 15, no. 1: 151. https://doi.org/10.3390/v15010151




