A Novel Hemocyte-Specific Small Protein Participates in White Spot Syndrome Virus Infection via Binding to Viral Envelope Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals, Viral Challenge and Tissue Collection
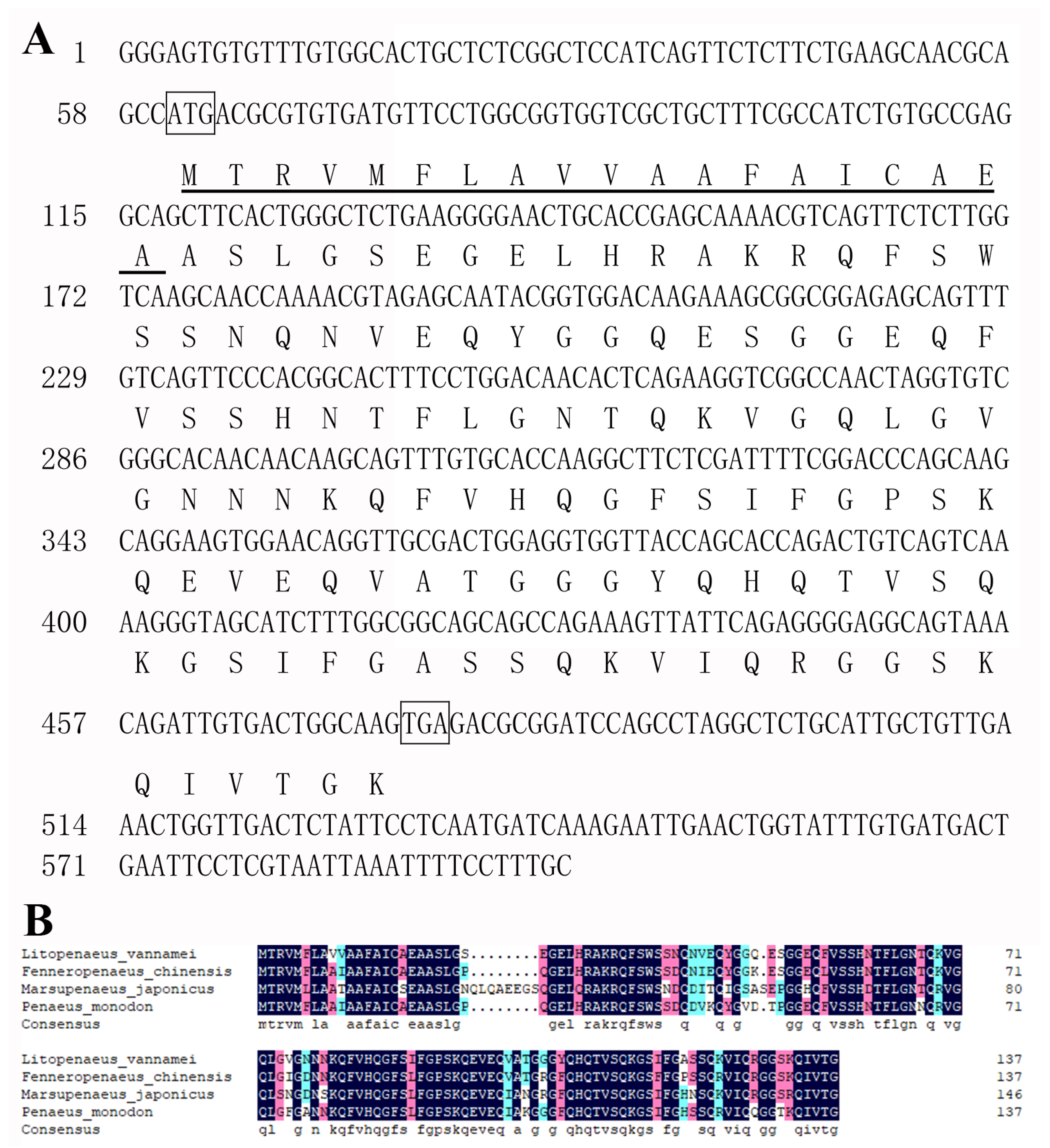
2.2. RNA Extraction, cDNA Synthesis, Cloning and Sequence Analysis of LvHSSP
2.3. Tissue Distribution and Detection of LvHSSP mRNA Expression
2.4. RNA Interference Assay
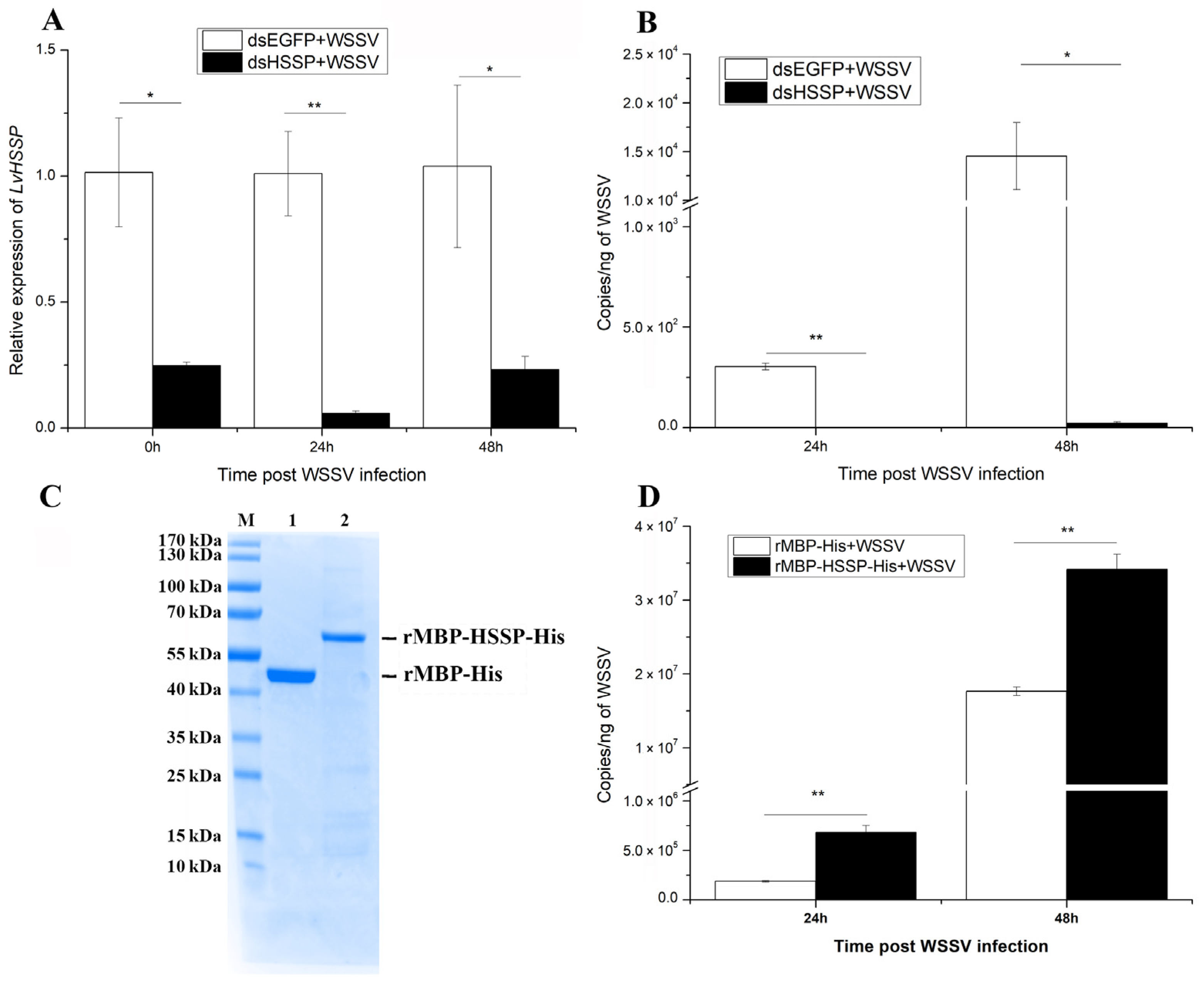
2.5. Recombinant Expression and Purification of LvHSSP
2.6. DNA Extraction and WSSV Load Quantification
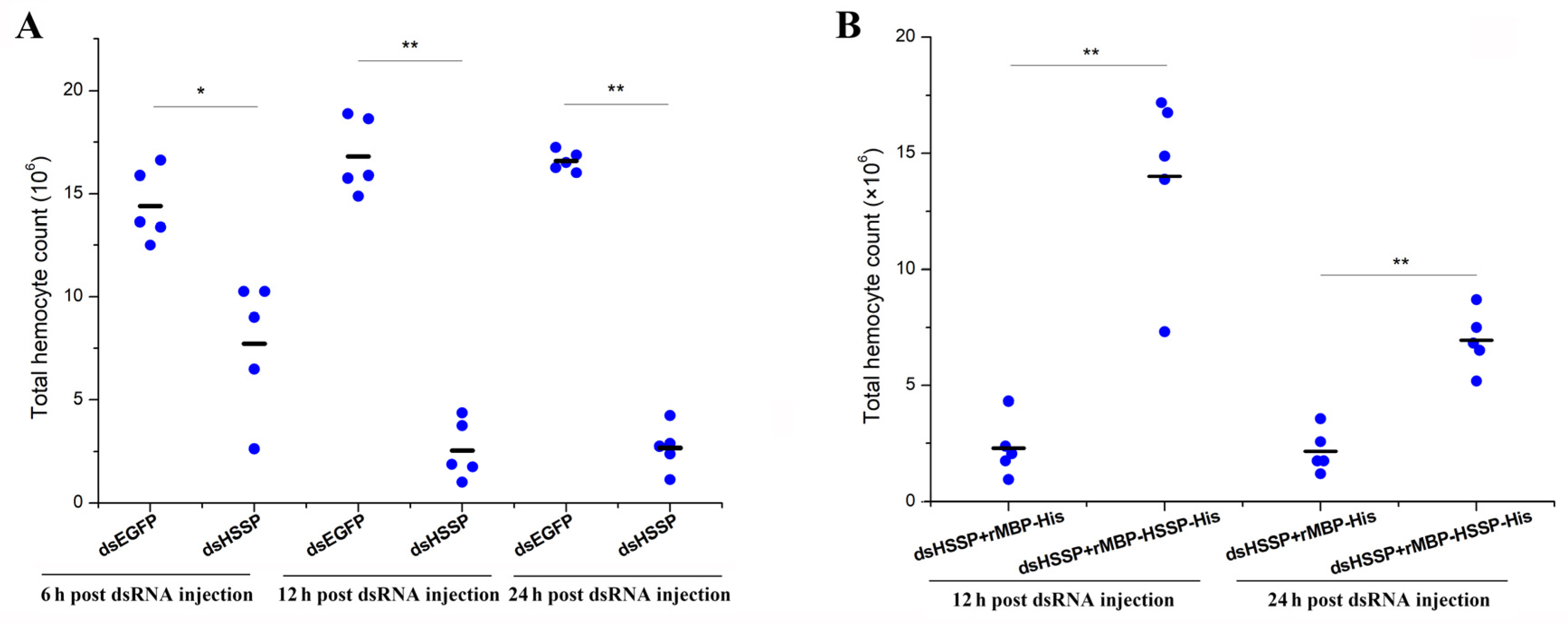
2.7. Total Hemocytes Counting
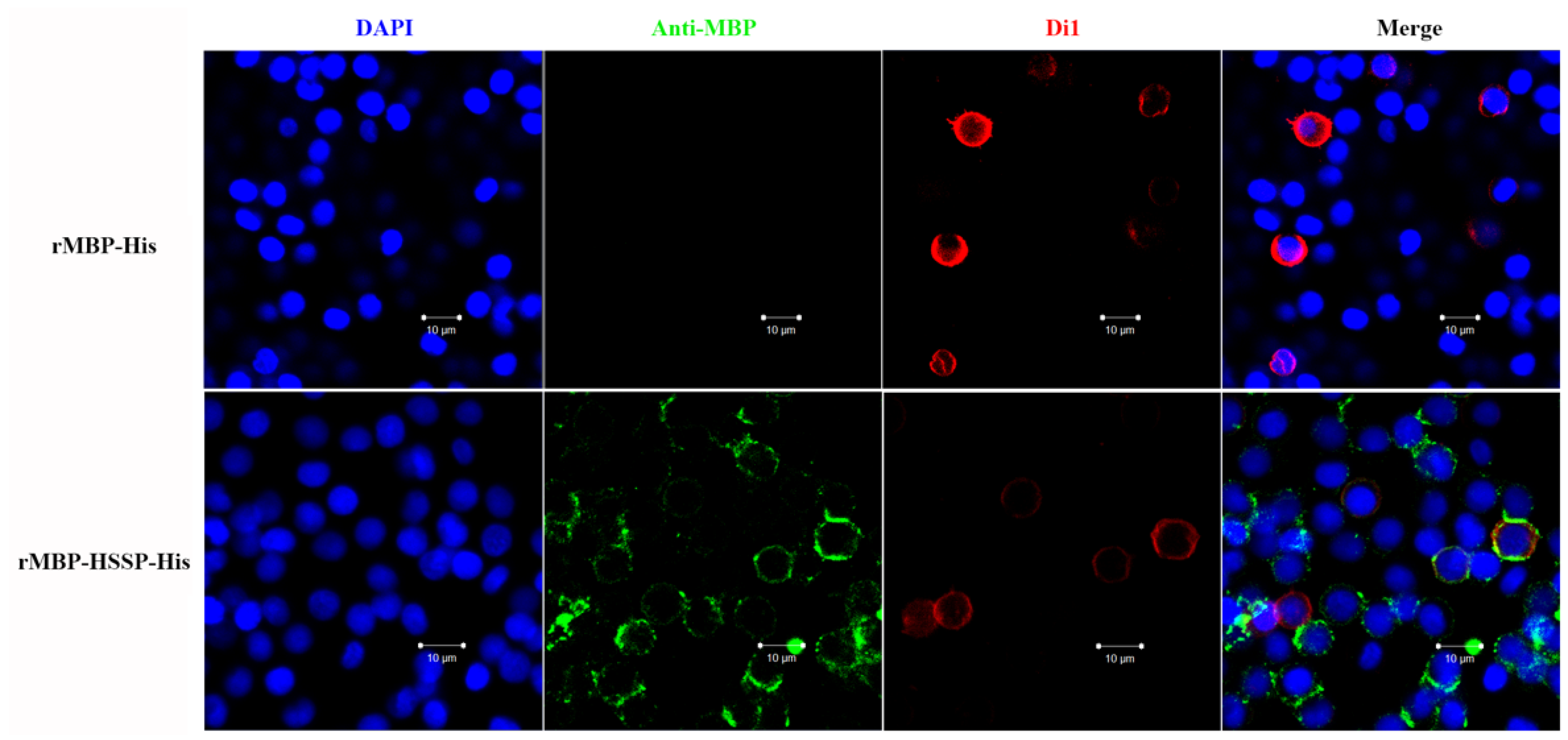
2.8. Immunocytochemical Assay
2.9. Cell Culture, Transfection, Co-Immunoprecipitation and Western Blot Assay
2.10. Statistical Analysis
3. Results
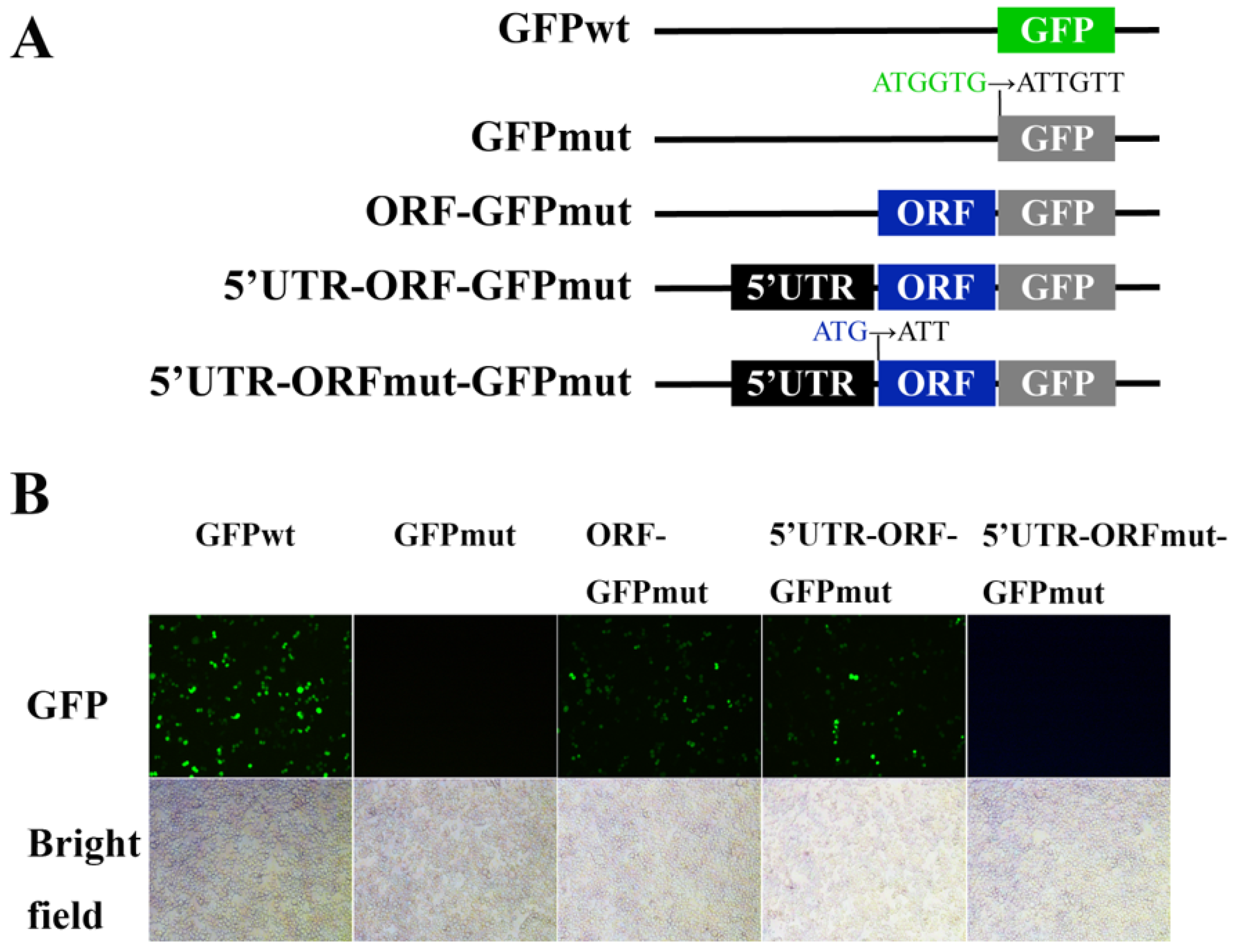
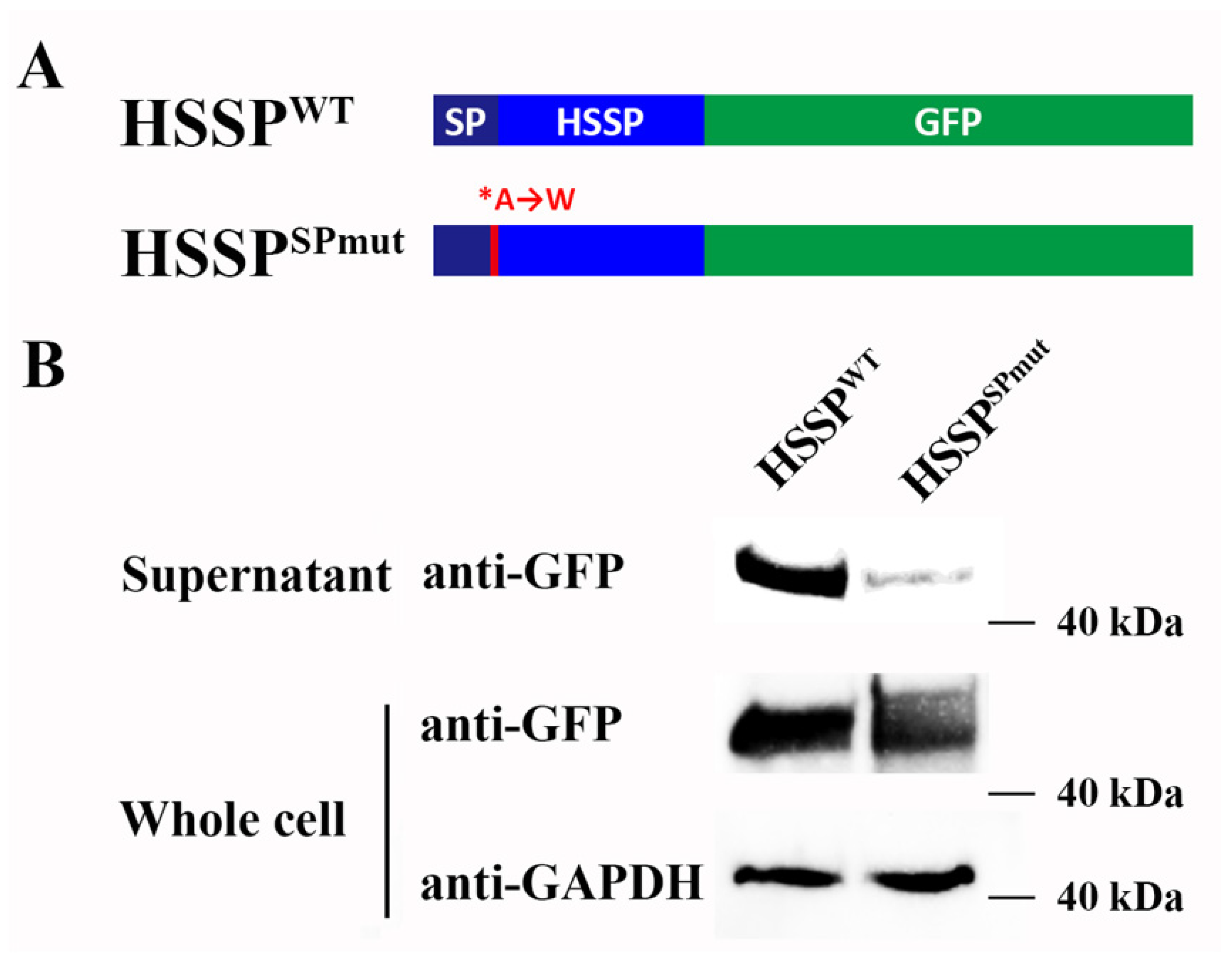
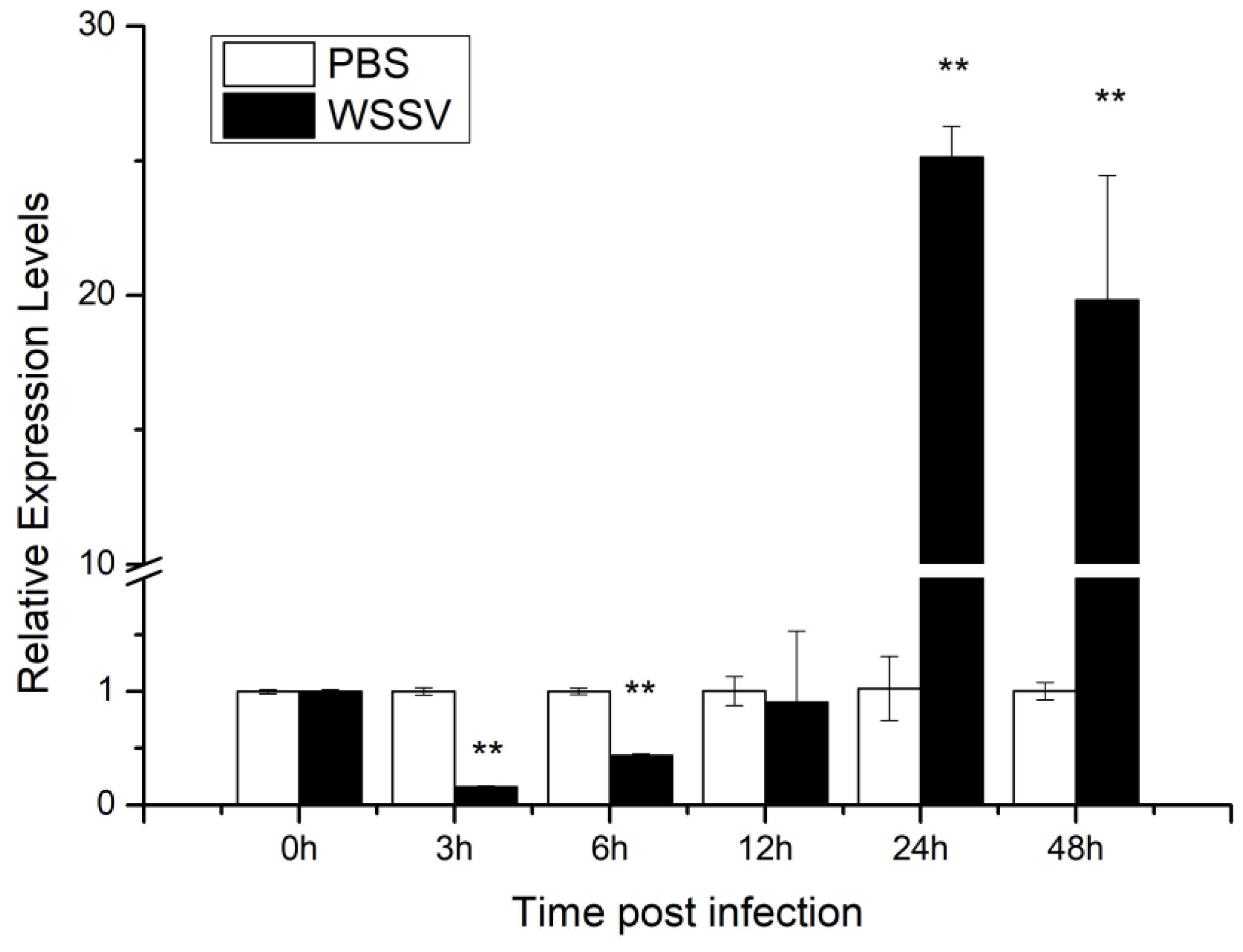
3.1. LvHSSP Is a Hemocyte-Specific Peptide and Responsive to WSSV Infection
3.2. LvHSSP Facilitates WSSV Proliferation in Shrimp
3.3. LvHSSP Interacts with VP26 of WSSV and Binds to Shrimp Hemocytes
3.4. LvHSSP Modulated the Number of Total Circulating Hemocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.H.; Xiang, J.H. Recent advances in researches on the innate immunity of shrimp in China. Dev. Comp. Immunol. 2013, 39, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiang, J. Signaling pathways regulating innate immune responses in shrimp. Fish Shellfish Immun. 2013, 34, 973–980. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Huang, J. Impact of Vibrio parahaemolyticus and white spot syndrome virus (WSSV) co-infection on survival of penaeid shrimp Litopenaeus vannamei. Chin. J. Oceanol. Limn. 2016, 34, 1278–1286. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Cy, H.; Ch, W.; Hc, C.; Lo, C. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Organ. 1995, 23, 165–173. [Google Scholar] [CrossRef]
- Li, C.Z.; Wang, S.; He, J.G. The Two NF-kappa B Pathways Regulating Bacterial and WSSV Infection of Shrimp. Front. Immunol. 2019, 10, 26. [Google Scholar]
- Watson, A.; Agius, J.; Ackerly, D.; Beddoe, T.; Helbig, K. The Role of Anti-Viral Effector Molecules in Mollusc Hemolymph. Biomolecules 2022, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.F.; Wang, J.X. The antimicrobial peptides of the immune response of shrimp. ISI-Invert. Surviv. J. 2008, 5, 162–179. [Google Scholar]
- Li, S.; Li, F. The Anti-lipopolysaccharide Factors in Crustaceans. Subcell Biochem 2020, 94, 63–80. [Google Scholar]
- Rosa, R.D.; Barracco, M.A. Antimicrobial peptides in crustaceans. ISI-Invert. Surviv. J. 2010, 7, 262–284. [Google Scholar]
- Maningas, M.B.; Kondo, H.; Hirono, I.; Saito-Taki, T.; Aoki, T. Essential function of transglutaminase and clotting protein in shrimp immunity. Mol. Immunol. 2008, 45, 1269–1275. [Google Scholar] [CrossRef]
- Maningas, M.B.B.; Kondo, H.; Hirono, I. Molecular mechanisms of the shrimp clotting system. Fish Shellfish Immunol. 2013, 34, 968–972. [Google Scholar] [CrossRef]
- Soetrisno, C.K. Non-clotting haemolymph of WSSV-infected shrimp: Is it a factor in infection processes? Indones. Aquac. J. 2009, 4, 109–119. [Google Scholar] [CrossRef]
- Sahul Hameed, A.S.; Sarathi, M.; Sudhakaran, R.; Balasubramanian, G.; Syed Musthaq, S. Quantitative assessment of apoptotic hemocytes in white spot syndrome virus (WSSV)-infected penaeid shrimp, Penaeus monodon and Penaeus indicus, by flow cytometric analysis. Aquaculture 2006, 256, 111–120. [Google Scholar] [CrossRef]
- Sellos, D.; Lemoine, S.; Van Wormhoudt, A. Molecular cloning of hemocyanin cDNA from Penaeus vannamei (Crustacea, Decapoda): Structure, evolution and physiological aspects 1EBI sequence accession number: X82502.1. FEBS Lett. 1997, 407, 153–158. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Qin, Q. Antiviral properties of hemocyanin isolated from shrimp Penaeus monodon. Antivir. Res. 2004, 61, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Li, F.; Zhang, M.; Yang, H.; Luo, T.; Xu, X. Difference between hemocyanin subunits from shrimp Penaeus japonicus in anti-WSSV defense. Dev. Comp. Immunol. 2008, 32, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Aweya, J.J.; Wang, F.; Yao, D.; Zhong, M.; Chen, J.; Li, S.; Zhang, Y. Litopenaeus vannamei attenuates white spot syndrome virus replication by specific antiviral peptides generated from hemocyanin. Dev. Comp. Immunol. 2019, 91, 50–61. [Google Scholar] [CrossRef]
- Liu, S.; Aweya, J.J.; Zheng, L.; Wang, F.; Zheng, Z.; Zhong, M.; Lun, J.; Zhang, Y. A Litopenaeus vannamei Hemocyanin-Derived Antimicrobial Peptide (Peptide B11) Attenuates Cancer Cells’ Proliferation. Molecules 2018, 23, 3202. [Google Scholar] [CrossRef]
- Liu, S.; Aweya, J.J.; Zheng, L.; Zheng, Z.; Huang, H.; Wang, F.; Yao, D.; Ou, T.; Zhang, Y. LvHemB1, a novel cationic antimicrobial peptide derived from the hemocyanin of Litopenaeus vannamei, induces cancer cell death by targeting mitochondrial voltage-dependent anion channel 1. Cell Biol. Toxicol. 2022, 38, 87–110. [Google Scholar] [CrossRef]
- Zhao, B.-R.; Zheng, Y.; Gao, J.; Wang, X.-W. Maturation of an Antimicrobial Peptide Inhibits Aeromonas hydrophila Infection in Crayfish. J. Immunol. 2020, 204, 487–497. [Google Scholar] [CrossRef]
- Diao, M.-Q.; Li, C.; Xu, J.-D.; Zhao, X.-F.; Wang, J.-X. RPS27, a sORF-Encoded Polypeptide, Functions Antivirally by Activating the NF-κB Pathway and Interacting With Viral Envelope Proteins in Shrimp. Front. Immunol. 2019, 10, 2763. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Sun, Y.; Li, S.; Gao, Y.; Yu, Y.; Liu, C.; Wang, Q.; Lv, X.; Zhang, X.; et al. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 2019, 10, 356. [Google Scholar] [CrossRef]
- Rodriguez, J.; Boulo, V.; Mialhe, E.; Bachere, E. Characterisation of shrimp haemocytes and plasma components by monoclonal antibodies. J. Cell Sci. 1995, 108, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, F.; Xiang, J. Analysis on the dynamic changes of the amount of WSSV in Chinese shrimp Fenneropenaeus chinensis during infection. Aquaculture 2013, 376, 124–132. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Zhang, X.; Xiang, J.; Li, F. Isolation and transcriptome analysis of three subpopulations of shrimp hemocytes reveals the underlying mechanism of their immune functions. Dev. Comp. Immunol. 2020, 108, 103689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Li, S.H.; Li, F.H.; Xie, S.J.; Xiang, J.H. Identification and function analysis of a novel vascular endothelial growth factor, LvVEGF3, in the Pacific whiteleg shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2016, 63, 111–120. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Sun, M.; Yu, Y.; Zhang, X.; Xiang, J.; Li, F. A newly identified NLR-like gene participates in bacteria and virus infection possibly through regulating hemocytes apoptosis in shrimp. Dev. Comp. Immunol. 2022, 132, 104395. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Zhang, H.; Zhang, R.; Li, H.; Wang, W.; Wang, L.; Wang, H.; Qiu, L.; Song, L. CgIL17-5, an ancient inflammatory cytokine in Crassostrea gigas exhibiting the heterogeneity functions compared with vertebrate interleukin17 molecules. Dev. Comp. Immunol. 2015, 53, 339–348. [Google Scholar] [CrossRef]
- Sun, M.Z.; Wang, L.L.; Jiang, S.; Liu, R.; Zhao, D.P.; Chen, H.; Song, X.R.; Song, L.S. CpG ODNs induced autophagy via reactive oxygen species (ROS) in Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 2015, 52, 1–9. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Liu, W.-J.; Lee, C.-C.; Chou, T.-L.; Lee, Y.-T.; Wu, T.-S.; Huang, J.-Y.; Huang, W.-T.; Lee, T.-L.; Kou, G.-H. A 3D model of the membrane protein complex formed by the white spot syndrome virus structural proteins. PLoS ONE 2010, 5, e10718. [Google Scholar] [CrossRef]
- Pauli, A.; Norris, M.L.; Valen, E.; Chew, G.-L.; Gagnon, J.A.; Zimmerman, S.; Mitchell, A.; Ma, J.; Dubrulle, J.; Reyon, D.; et al. Toddler: An Embryonic Signal That Promotes Cell Movement via Apelin Receptors. Science 2014, 343, 1248636. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Z.; Chen, M.; Chen, D.; Gao, X.-C.; Zhu, S.; Huang, H.; Hu, M.; Zhu, H.; Yan, G.-R. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol. Cell 2017, 68, 171–184.e6. [Google Scholar] [CrossRef]
- Xing, J.; Liu, H.; Jiang, W.; Wang, L. LncRNA-Encoded Peptide: Functions and Predicting Methods. Front. Oncol. 2020, 10, 622294. [Google Scholar] [CrossRef]
- Van Etten, J. Lesser known large dsDNA viruses. Preface. Curr. Top. Microbiol. Immunol. 2009, 328, 5–7. [Google Scholar]
- Yang, F.; Li, S.; Li, F.; Xiang, J. A cuticle protein from the Pacific white shrimp Litopenaeus vannamei involved in WSSV infection. Dev. Comp. Immunol. 2018, 81, 303–311. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Li, S.; Xiang, J.; Li, F. A novel cuticle protein involved in WSSV infection to the Pacific white shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 2020, 102, 103491. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-C.; Liu, Q.-H. LvCPG2 facilitated WSSV infection by interaction with VP26 and VP28. Fish Shellfish Immun. 2021, 118, 313–320. [Google Scholar] [CrossRef]
- Sánchez-Paz, A. White spot syndrome virus: An overview on an emergent concern. Vet. Res. 2010, 41, 43. [Google Scholar] [CrossRef]
- Tang, X.; Wu, J.; Sivaraman, J.; Hew, C.L. Crystal structures of major envelope proteins VP26 and VP28 from white spot syndrome virus shed light on their evolutionary relationship. J. Virol. 2007, 81, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, F. Interaction of white spot syndrome virus VP26 protein with actin. Virology 2005, 336, 93–99. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, Q.-H.; Huang, J. Multiple proteins of White spot syndrome virus involved in recognition of β-integrin. J. Biosci. 2014, 39, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J. Selectin synthesis and inflammation. Trends Biochem. Sci. 1996, 21, 65–69. [Google Scholar] [CrossRef]
- Johansson, M.W.; Söderhäll, K. Isolation and purification of a cell adhesion factor from crayfish blood cells. J. Cell Biol. 1988, 106, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.N.; Schneider, D.S. Evidence for specificity and memory in the insect innate immune response. In Insect Immunology; Beckage, N.E., Ed.; Academic Press: San Diego, CA, USA, 2008; pp. 97–127. [Google Scholar]
- Cui, C.; Tang, X.; Xing, J.; Sheng, X.; Chi, H.; Zhan, W. Single-cell RNA-seq uncovered hemocyte functional subtypes and their differentiational characteristics and connectivity with morphological subpopulations in Litopenaeus vannamei. Front. Immunol. 2022, 13, 980021. [Google Scholar] [CrossRef]
- Ng, Y.S.; Lee, D.Y.; Liu, C.H.; Tung, C.Y.; He, S.T.; Wang, H.C. White Spot Syndrome Virus Triggers a Glycolytic Pathway in Shrimp Immune Cells (Hemocytes) to Benefit Its Replication. Front. Immunol. 2022, 13, 901111. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Li, S.; Yu, Y.; Zhang, X.; Li, F. A Novel Hemocyte-Specific Small Protein Participates in White Spot Syndrome Virus Infection via Binding to Viral Envelope Protein. Viruses 2023, 15, 227. https://doi.org/10.3390/v15010227
Sun M, Li S, Yu Y, Zhang X, Li F. A Novel Hemocyte-Specific Small Protein Participates in White Spot Syndrome Virus Infection via Binding to Viral Envelope Protein. Viruses. 2023; 15(1):227. https://doi.org/10.3390/v15010227
Chicago/Turabian StyleSun, Mingzhe, Shihao Li, Yang Yu, Xiaojun Zhang, and Fuhua Li. 2023. "A Novel Hemocyte-Specific Small Protein Participates in White Spot Syndrome Virus Infection via Binding to Viral Envelope Protein" Viruses 15, no. 1: 227. https://doi.org/10.3390/v15010227
APA StyleSun, M., Li, S., Yu, Y., Zhang, X., & Li, F. (2023). A Novel Hemocyte-Specific Small Protein Participates in White Spot Syndrome Virus Infection via Binding to Viral Envelope Protein. Viruses, 15(1), 227. https://doi.org/10.3390/v15010227




