Equine Arteritis Virus in Monocytic Cells Suppresses Differentiation and Function of Dendritic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Generation of Equine Monocyte-Derived Dendritic Cells
2.2. Virus Strains
2.3. Virus Infection of Monocytes and MoDC
2.4. Virus Titration and Detection
2.5. Phenotypic Analysis of Infected Cells by Flow Cytometry and qPCR
2.6. Functional Assays
3. Results
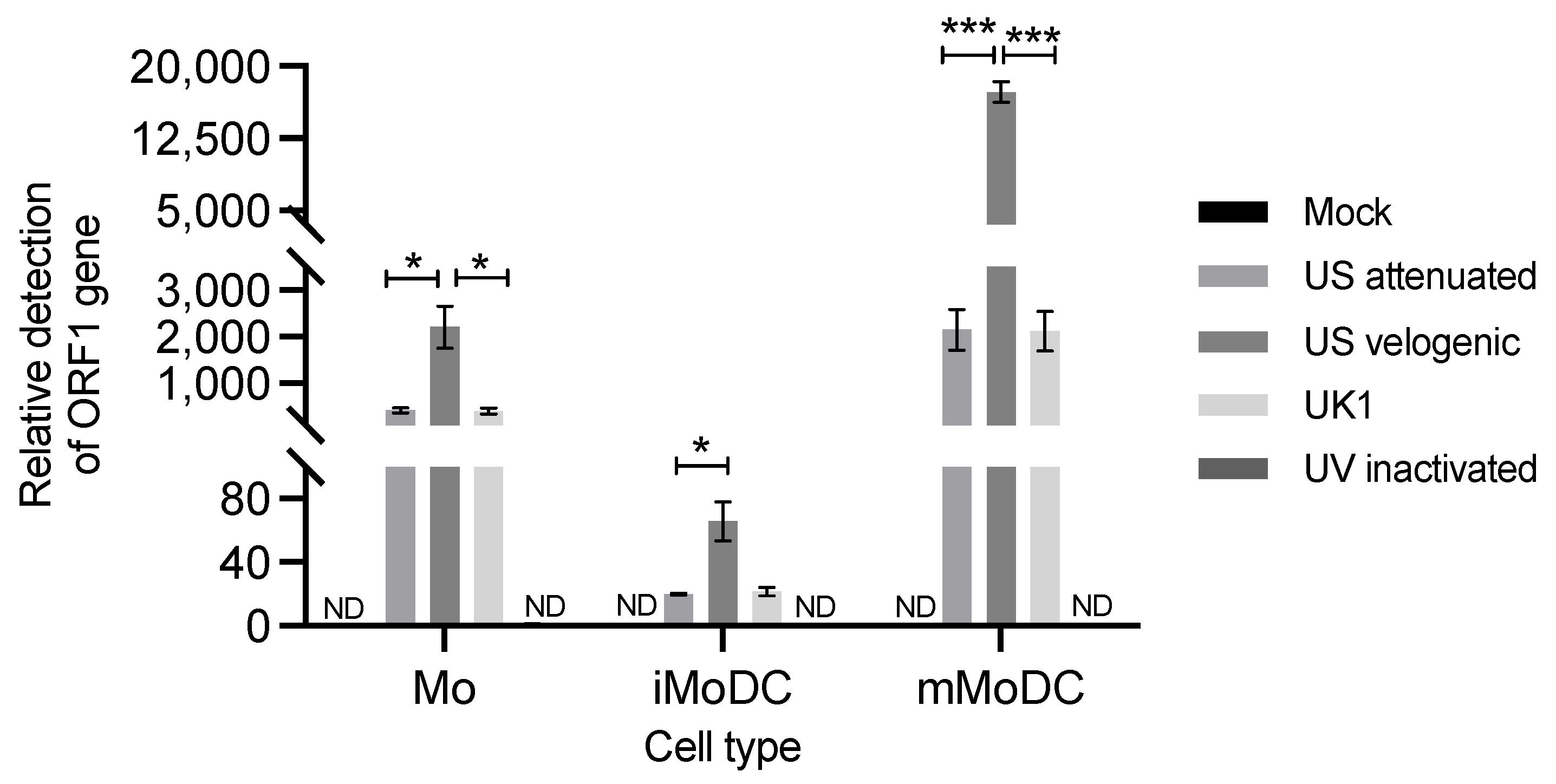
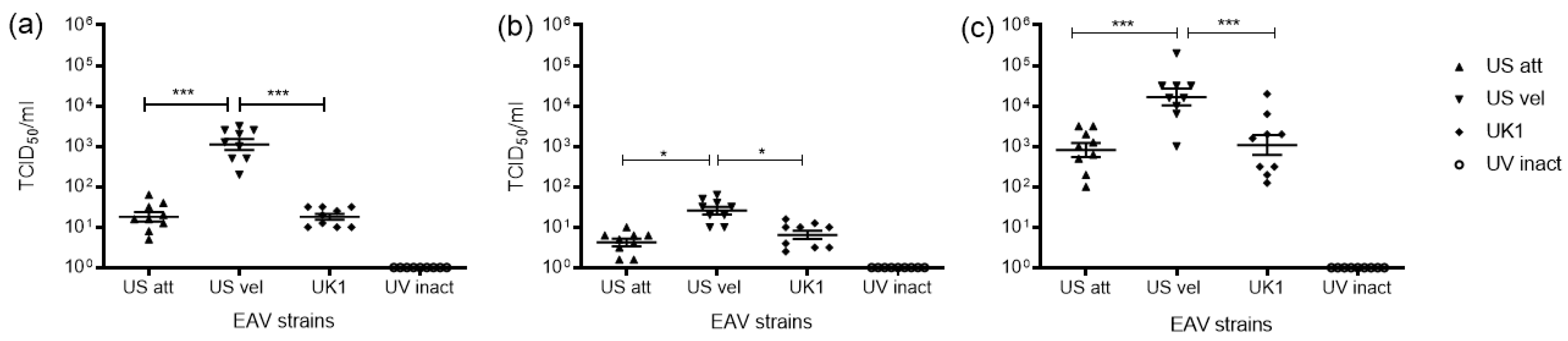
3.1. Replication of EAV in Equine Mo and MoDC
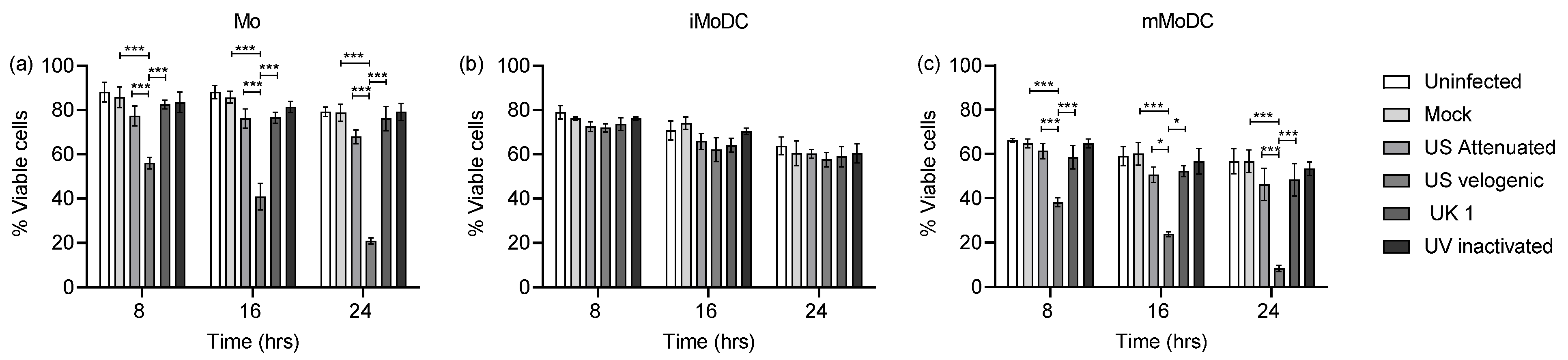
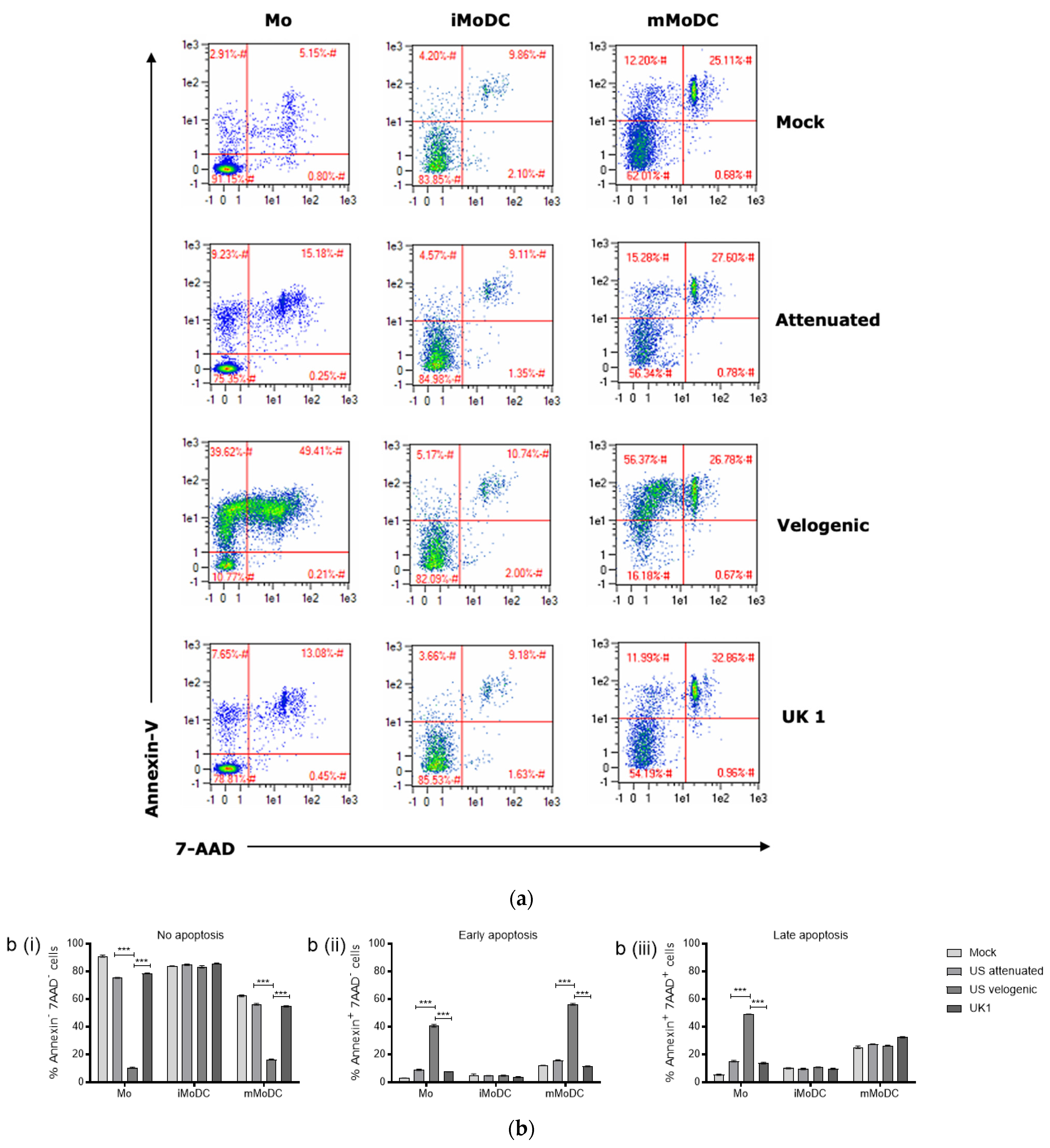
3.2. The Effect of EAV on Equine Mo and MoDC Viability
3.3. Phenotypic Changes in Mo and MoDC Induced by EAV Infection
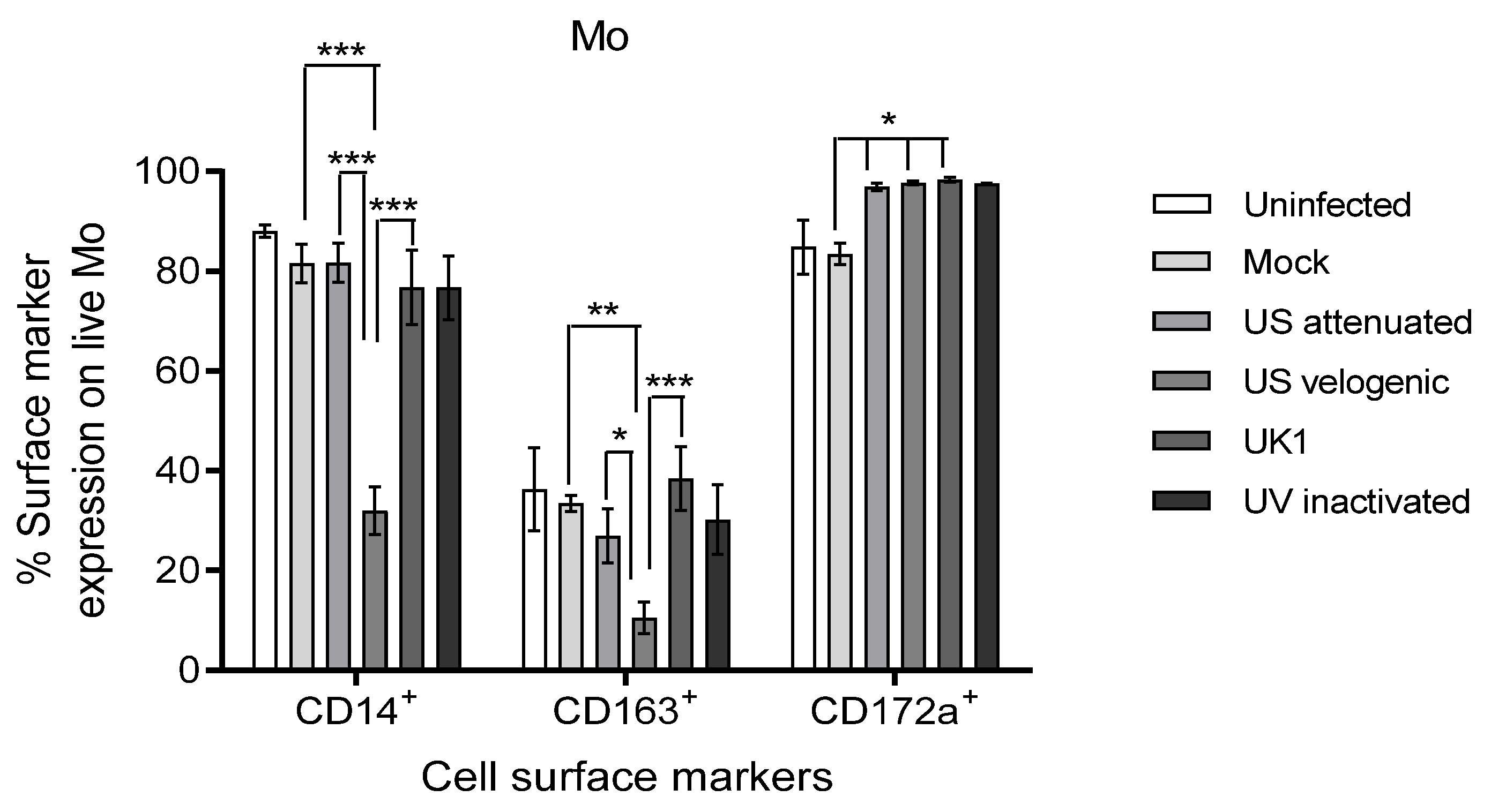
3.3.1. Effect of EAV Infection on Live Mo and MoDC
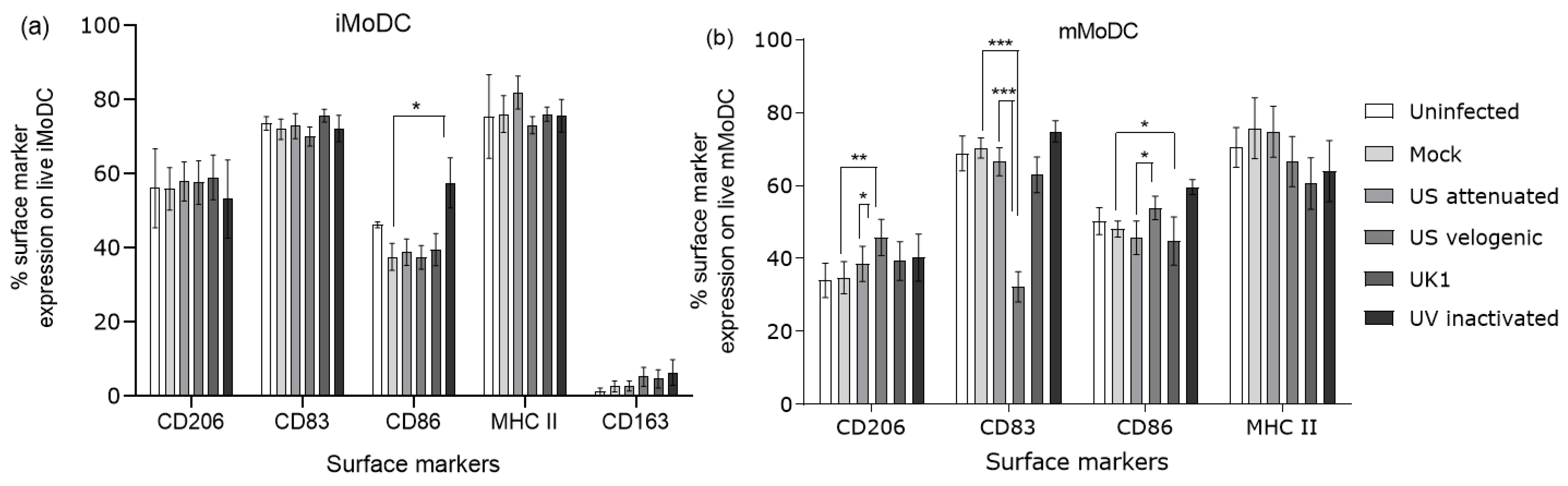
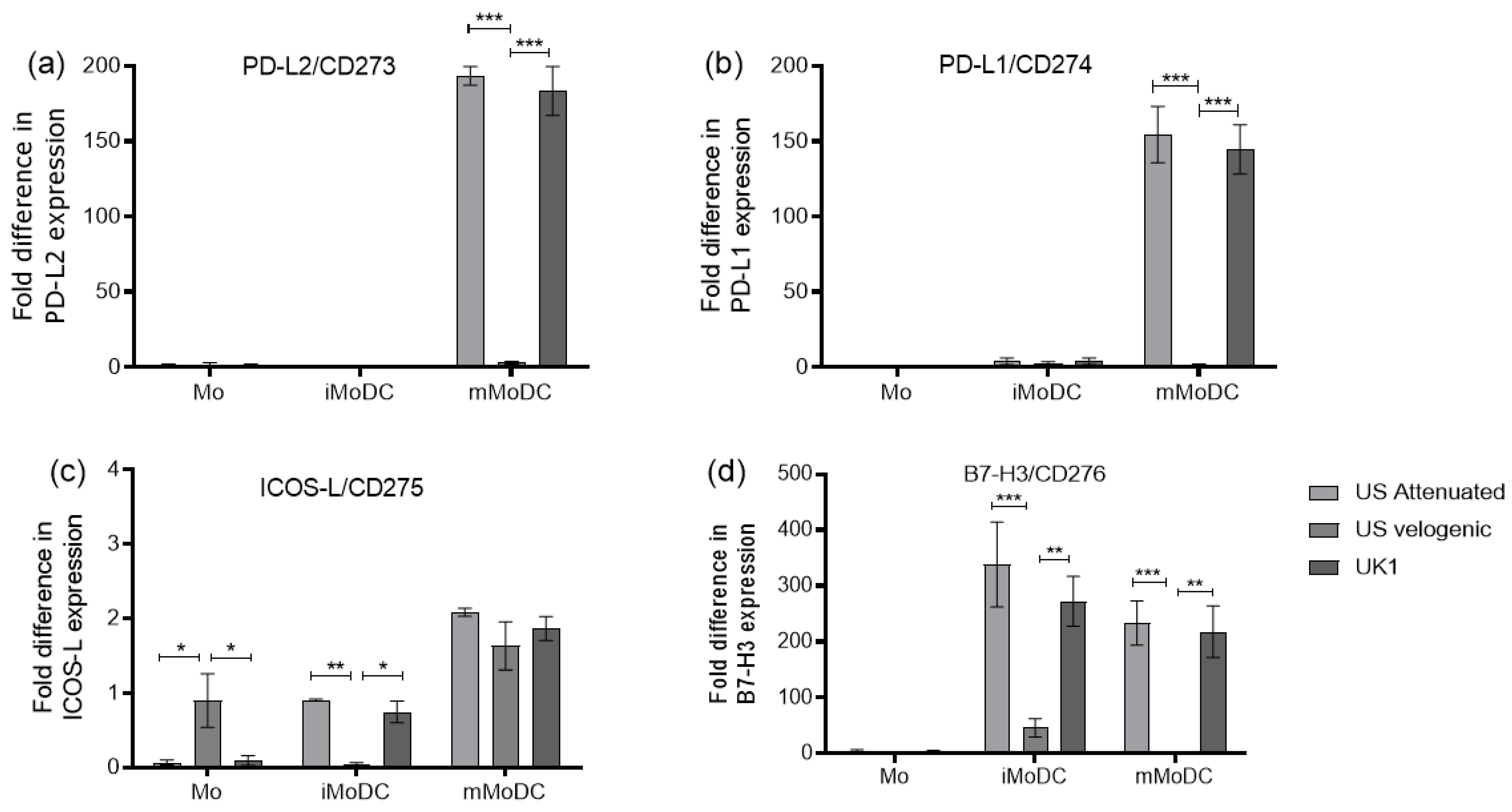
3.3.2. Expression of Costimulatory Molecules EAV Infected Mo and MoDC
3.4. Changes Induced by EAV Infection on the Function of Mo and MoDC
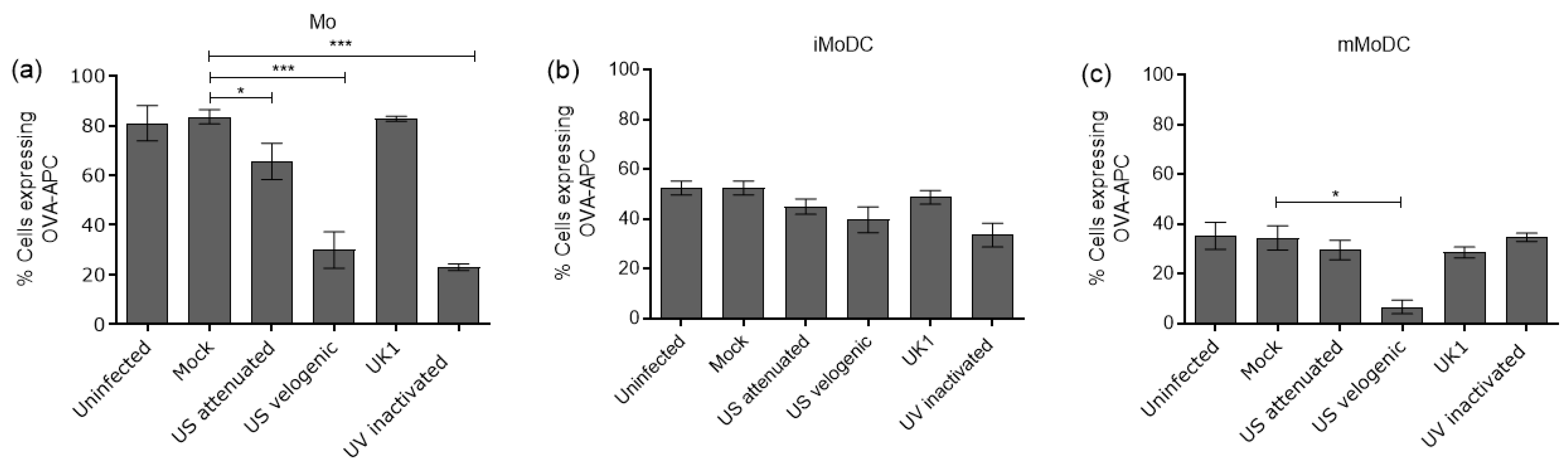
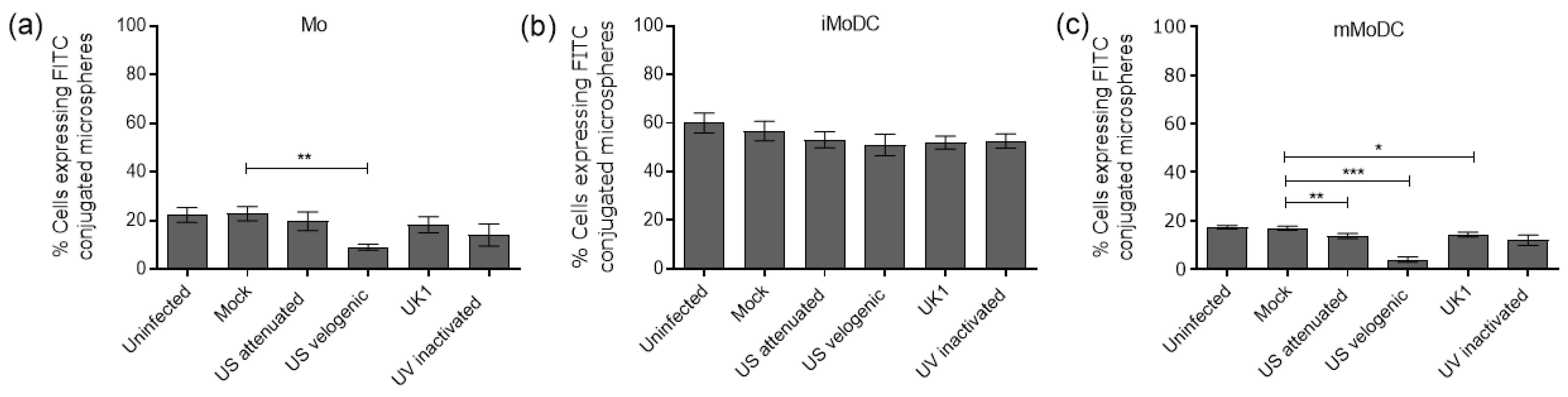
3.4.1. The Impact of EAV on the Endocytic and Phagocytic Capacity of Mo and MoDC
3.4.2. The Ability of Infected MoDC to Stimulate T Cells
3.5. The Impact of EAV Infection on Differentiation and Activation
3.5.1. Differentiation of EAV Infected Mo to iMoDC
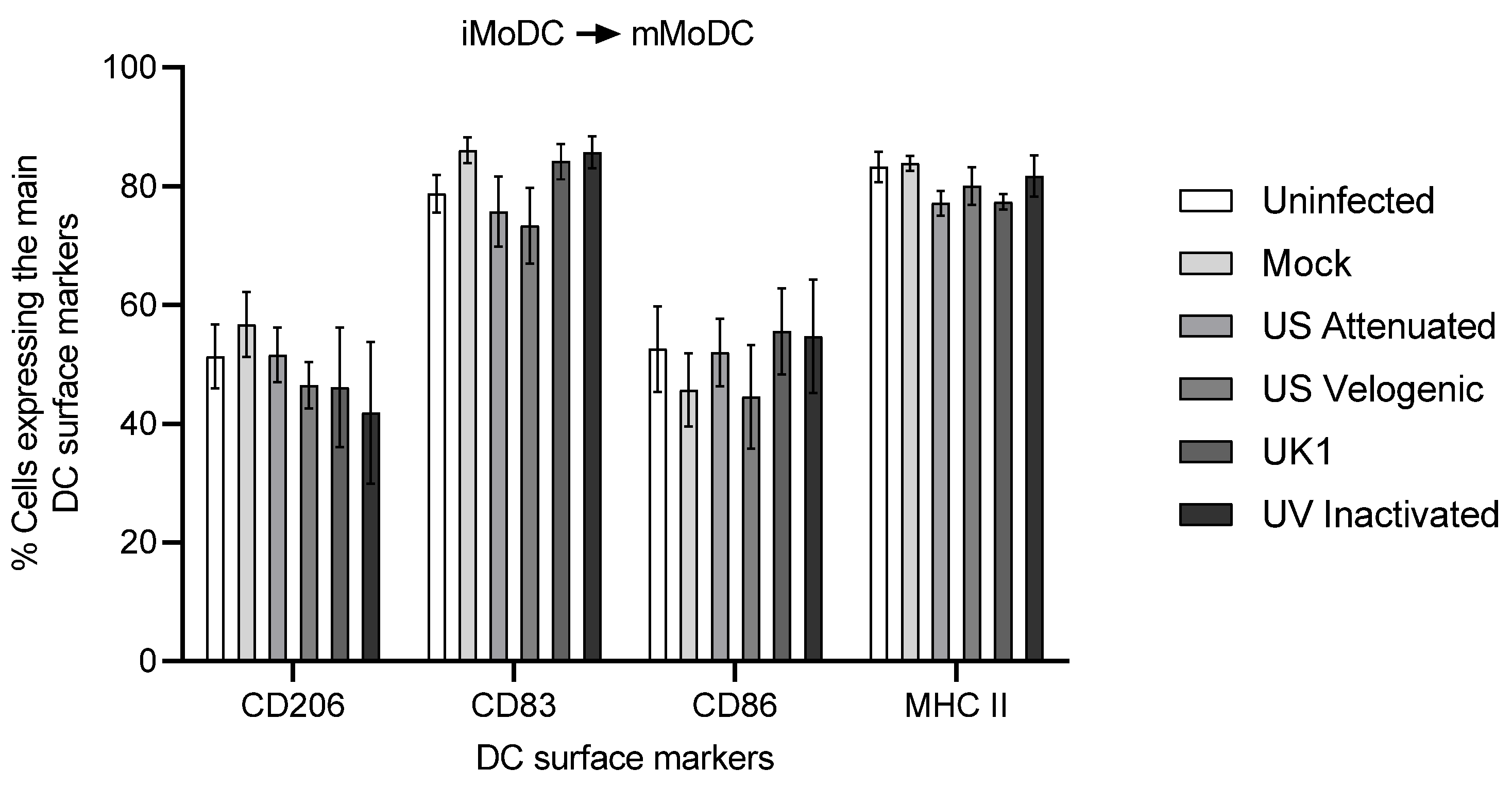
3.5.2. Activation of EAV Infected iMoDC
3.6. Modulation of Cytokine Expression in Infected Mo and MoDC
4. Discussion
4.1. Replication of EAV in Mo and MoDC
4.2. Modulation of DCs by EAV Infection
4.3. EAV in Monocytic Cells Suppressed Their Differentiation into DC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, U.B.; Zhang, J.; Go, Y.Y.; MacLachlan, N.J. Experiences with infectious cDNA clones of equine arteritis virus: Lessons learned and insights gained. Virology 2014, 462–463, 388–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbach, F.; Westcott, D.G.; McGowan, S.L.; Grierson, S.S.; Frossard, J.P.; Choudhury, B. Re-emergence of a genetic outlier strain of equine arteritis virus: Impact on phylogeny. Virus Res. 2015, 202, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Timoney, P.J.; Shuck, K.M.; Seoul, G.; Go, Y.Y.; Lu, Z.; Powell, D.G.; Meade, B.J.; Balasuriya, U.B.R. Molecular epidemiology and genetic characterization of equine arteritis virus isolates associated with the 2006–2007 multi-state disease occurrence in the USA. J. Gen. Virol. 2010, 91, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Pronost, S.; Pitel, P.H.; Miszczak, F.; Legrand, L.; Marcillaud-Pitel, C.; Hamon, M.; Tapprest, J.; Balasuriya, U.B.; Freymuth, F.; Fortier, G. Description of the first recorded major occurrence of equine viral arteritis in France. Equine Vet. J. 2010, 42, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Barrandeguy, M. EVA outbreak in Argentina. Equine Dis. Q. 2010, 19, 3–4. [Google Scholar]
- Newton, R.; Cruz, D.V.M. Current and future approaches to EAV control. Equine Q. Dis. Surveill. Rep. 2010, 6, 13–14. [Google Scholar]
- Moore, B.D.; Balasuriya, U.B.; Hedges, J.F.; MacLachlan, N.J. Growth characteristics of a highly virulent, a moderately virulent, and an avirulent strain of equine arteritis virus in primary equine endothelial cells are predictive of their virulence to horses. Virology 2002, 298, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.D.; Balasuriya, U.B.; Watson, J.L.; Bosio, C.M.; MacKay, R.J.; MacLachlan, N.J. Virulent and avirulent strains of equine arteritis virus induce different quantities of TNF-alpha and other proinflammatory cytokines in alveolar and blood-derived equine macrophages. Virology 2003, 314, 662–670. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Cheong, C.; Matos, I.; Choi, J.H.; Dandamudi, D.B.; Shrestha, E.; Longhi, M.P.; Jeffrey, K.L.; Anthony, R.M.; Kluger, C.; Nchinda, G.; et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143, 416–429. [Google Scholar] [CrossRef]
- Mauel, S.; Steinbach, F.; Ludwig, H. Monocyte-derived dendritic cells from horses differ from dendritic cells of humans and mice. Immunology 2006, 117, 463–473. [Google Scholar] [CrossRef]
- Moyo, N.A.; Marchi, E.; Steinbach, F. Differentiation and activation of equine monocyte-derived dendritic cells are not correlated with CD206 or CD83 expression. Immunology 2013, 139, 472–483. [Google Scholar] [CrossRef]
- Steinbach, F.; Bischoff, S.; Freund, H.; Metzner-Flemisch, S.; Ibrahim, S.; Walter, J.; Wilke, I.; Mauel, S. Clinical application of dendritic cells and interleukin-2 and tools to study activated T cells in horses--first results and implications for quality control. Vet. Immunol. Immunopathol. 2009, 128, 16–23. [Google Scholar] [CrossRef]
- Bryans, J.T.; Crowe, M.E.; Doll, E.R.; McCollum, W.H. Isolation of a filterable agent causing arteritis of horses and abortion by mares; its differentiation from the equine abortion (influenza) virus. Cornell Vet. 1957, 47, 3–41. [Google Scholar]
- McCollum, W.H.; Timoney, P.J. Experimental observation on the virulence of isolates of equine arteritis virus. In Proceedings of the Eighth International Conference Equine Infectious Disease, Dubai, United Arab Emirates, 23–26 March 1998; pp. 558–559. [Google Scholar]
- Westcott, D.; Wescott, D.G.; Mildenberg, Z.; Bellaiche, M.; McGowan, S.L.; Grierson, S.S.; Choudhury, B.; Steinbach, F. Evidence for the circulation of equine encephalosis virus in Israel since 2001. PLoS ONE 2013, 8, e70532. [Google Scholar] [CrossRef]
- Westcott, D.G.; Hannant, D.; Tearle, J.; Mittelholzer, C.; Baule, C.; Drew, T.W.; Belak, S.; Paton, D.J. Differences in pathogenicity between closely related biological clones of equine arteritis virus. In Proceedings of the Sixth International Symposium on Positive Strand RNA Viruses, Paris, France, 28 May–2 June 2001. [Google Scholar]
- Drew, T.W. Equine Viral Arteritis: Virulence and Immunity. MAFF SE750, Final Report. Available online: https://randd.defra.gov.uk/Document.aspx?Document=SE0750_2240_FRP.doc (accessed on 13 June 2022).
- Wood, J.L.; Chirnside, E.D.; Mumford, J.A.; Higgins, A.J. First recorded outbreak of equine viral arteritis in the United Kingdom. Vet. Rec 1995, 136, 381–385. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn-Schmiedebergs Arch. Für Exp. Pathol. Und Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Spinsanti, G.; Silvestrelli, M.; Supplizi, A.V. Exercise induced stress in horses: Selection of the most stable reference genes for quantitative RT-PCR normalization. BMC Mol. Biol. 2008, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Cheng, A.; Wang, M.; Zhang, S.; Zhu, D.; Jia, R.; Luo, Q.; Chen, Z.; Chen, X. Establishment of real-time quantitative reverse transcription polymerase chain reaction assay for transcriptional analysis of duck enteritis virus UL55 gene. Virol. J. 2011, 8, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schuler, G.; Steinman, R.M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J. Exp. Med. 1985, 161, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Cavatorta, D.J.; Erb, H.N.; Flaminio, M.J. Ex vivo generation of mature equine monocyte-derived dendritic cells. Vet. Immunol. Immunopathol. 2009, 131, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Colmenero, P.; Purath, U.; Teixeira de Matos, C.; Hueber, W.; Klareskog, L.; Tarner, I.H.; Engleman, E.G.; Soderstrom, K. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood 2007, 110, 2484–2493. [Google Scholar] [CrossRef] [Green Version]
- Quah, B.J.; Warren, H.S.; Parish, C.R. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2007, 2, 2049–2056. [Google Scholar] [CrossRef]
- Snijder, E.J.; Meulenberg, J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998, 79 Pt 5, 961–979. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [Green Version]
- Filin, I.Y.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Recent Advances in Experimental Dendritic Cell Vaccines for Cancer. Front. Oncol. 2021, 11, 730824. [Google Scholar] [CrossRef]
- Go, Y.Y.; Zhang, J.; Timoney, P.J.; Cook, R.F.; Horohov, D.W.; Balasuriya, U.B. Complex interactions between the major and minor envelope proteins of equine arteritis virus determine its tropism for equine CD3+ T lymphocytes and CD14+ monocytes. J. Virol. 2010, 84, 4898–4911. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.G.; Slade, D.E.; Shields, S.L.; Jolie, R.; Mannan, R.M.; Ankenbauer, R.G.; Welch, S.K. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J. Virol. 2007, 81, 7371–7379. [Google Scholar] [CrossRef] [Green Version]
- Burkard, C.; Lillico, S.G.; Reid, E.; Jackson, B.; Mileham, A.J.; Ait-Ali, T.; Whitelaw, C.B.; Archibald, A.L. Precision engineering for PRRSV resistance in pigs: Macrophages from genome edited pigs lacking CD163 SRCR5 domain are fully resistant to both PRRSV genotypes while maintaining biological function. PLoS Pathog. 2017, 13, e1006206. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Wei, Z.; Zevenhoven-Dobbe, J.C.; Liu, R.; Tong, G.; Snijder, E.J.; Yuan, S. Arterivirus minor envelope proteins are a major determinant of viral tropism in cell culture. J. Virol. 2012, 86, 3701–3712. [Google Scholar] [CrossRef]
- Sarkar, S.; Chelvarajan, L.; Go, Y.Y.; Cook, F.; Artiushin, S.; Mondal, S.; Anderson, K.; Eberth, J.; Timoney, P.J.; Kalbfleisch, T.S.; et al. Equine Arteritis Virus Uses Equine CXCL16 as an Entry Receptor. J. Virol. 2016, 90, 3366–3384. [Google Scholar] [CrossRef] [Green Version]
- Costers, S.; Lefebvre, D.J.; Delputte, P.L.; Nauwynck, H.J. Porcine reproductive and respiratory syndrome virus modulates apoptosis during replication in alveolar macrophages. Arch. Virol. 2008, 153, 1453–1465. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 2002, 14, 420–431. [Google Scholar] [CrossRef]
- Wollenberg, A.; Mommaas, M.; Oppel, T.; Schottdorf, E.M.; Gunther, S.; Moderer, M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J. Investig. Derm. 2002, 118, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Schaer, D.J.; Schaer, C.A.; Buehler, P.W.; Boykins, R.A.; Schoedon, G.; Alayash, A.I.; Schaffner, A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 2006, 107, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Kitchens, R.L.; Wang, P.; Munford, R.S. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J. Immunol. 1998, 161, 5534–5545. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, M.; Rosorius, O.; Kratzer, F.; Bevec, D.; Kuhnt, C.; Steinkasserer, A.; Schuler, G.; Hauber, J. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J. Exp. Med. 2000, 191, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Lechmann, M.; Krooshoop, D.J.; Dudziak, D.; Kremmer, E.; Kuhnt, C.; Figdor, C.G.; Schuler, G.; Steinkasserer, A. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J. Exp. Med. 2001, 194, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.; Kindsvogel, W.; Xu, W.; Henderson, K.; Schlutsmeyer, S.; Whitmore, T.E.; Kuestner, R.; Garrigues, U.; Birks, C.; Roraback, J.; et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol 2003, 4, 63–68. [Google Scholar] [CrossRef]
- Ghiotto, M.; Gauthier, L.; Serriari, N.; Pastor, S.; Truneh, A.; Nunes, J.A.; Olive, D. PD-L1 and PD-L2 differ in their molecular mechanisms of interaction with PD-1. Int. Immunol. 2010, 22, 651–660. [Google Scholar] [CrossRef]
- Hong, J.J.; Amancha, P.K.; Rogers, K.; Ansari, A.A.; Villinger, F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS ONE 2013, 8, e60186. [Google Scholar] [CrossRef] [Green Version]
- Miron, M.; Kumar, B.V.; Meng, W.; Granot, T.; Carpenter, D.J.; Senda, T.; Chen, D.; Rosenfeld, A.M.; Zhang, B.; Lerner, H.; et al. Human Lymph Nodes Maintain TCF-1(hi) Memory T Cells with High Functional Potential and Clonal Diversity throughout Life. J. Immunol. 2018, 201, 2132–2140. [Google Scholar] [CrossRef] [Green Version]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Prasad, D.V.; Nguyen, T.; Li, Z.; Yang, Y.; Duong, J.; Wang, Y.; Dong, C. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004, 173, 2500–2506. [Google Scholar] [CrossRef] [Green Version]
- Kontos, F.; Michelakos, T.; Kurokawa, T.; Sadagopan, A.; Schwab, J.H.; Ferrone, C.R.; Ferrone, S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin. Cancer Res. 2021, 27, 1227–1235. [Google Scholar] [CrossRef]
- Castillo-Olivares, J.; Wieringa, R.; Bakonyi, T.; de Vries, A.A.; Davis-Poynter, N.J.; Rottier, P.J. Generation of a candidate live marker vaccine for equine arteritis virus by deletion of the major virus neutralization domain. J. Virol. 2003, 77, 8470–8480. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, K.; Witte, E.; Proesch, S.; Schulze-Tanzil, G.; Nasilowska, K.; Thilo, J.; Asadullah, K.; Sterry, W.; Volk, H.D.; et al. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J. Leukoc. Biol. 2008, 83, 1181–1193. [Google Scholar] [CrossRef]
- Brooks, D.G.; Walsh, K.B.; Elsaesser, H.; Oldstone, M.B. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 3018–3023. [Google Scholar] [CrossRef] [Green Version]
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef]
- Osterlund, P.; Veckman, V.; Siren, J.; Klucher, K.M.; Hiscott, J.; Matikainen, S.; Julkunen, I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J. Virol. 2005, 79, 9608–9617. [Google Scholar] [CrossRef] [Green Version]
- Melchjorsen, J.; Siren, J.; Julkunen, I.; Paludan, S.R.; Matikainen, S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J. Gen. Virol. 2006, 87 Pt 5, 1099–1108. [Google Scholar] [CrossRef]
- Gredmark, S.; Soderberg-Naucler, C. Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J. Virol. 2003, 77, 10943–10956. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Gibbs, J.S.; Lu, X.; Brooke, C.B.; Roy, D.; Modlin, R.L.; Bennink, J.R.; Yewdell, J.W. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood 2012, 119, 3128–3131. [Google Scholar] [CrossRef] [Green Version]
- Moutaftsi, M.; Mehl, A.M.; Borysiewicz, L.K.; Tabi, Z. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 2002, 99, 2913–2921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Li, Y.; Ye, J.; Yang, X.; Chen, L.; Liu, X.; Chen, H. Japanese encephalitis Virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells. Virol. J. 2011, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, L.J.; Wang, J.J.; Shaio, M.F.; Kao, C.L.; Chang, D.M.; Han, S.W.; Lai, J.H. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 2001, 166, 1499–1506. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer and Probe Sequence 5′ →3′ | Source |
|---|---|---|
| EAV ORF1 | TAGCCATTGAAGAGGCAAGT a | in house |
| GGCAAAAGTTTTAACCAGCAb | ||
| 6FAM- GACCACGCGTCTGCTAAGCG- BBQ c | ||
| IL-29 | GGCAGGTTCCAATCTCTGTC a | in house |
| CAGCGTCAGGTGTAGCTCAG b | ||
| 6FAM- TCTTCCCCATGACCAGAGAC- BHQ-1 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyo, N.A.; Westcott, D.; Simmonds, R.; Steinbach, F. Equine Arteritis Virus in Monocytic Cells Suppresses Differentiation and Function of Dendritic Cells. Viruses 2023, 15, 255. https://doi.org/10.3390/v15010255
Moyo NA, Westcott D, Simmonds R, Steinbach F. Equine Arteritis Virus in Monocytic Cells Suppresses Differentiation and Function of Dendritic Cells. Viruses. 2023; 15(1):255. https://doi.org/10.3390/v15010255
Chicago/Turabian StyleMoyo, Nathifa A., Dave Westcott, Rachel Simmonds, and Falko Steinbach. 2023. "Equine Arteritis Virus in Monocytic Cells Suppresses Differentiation and Function of Dendritic Cells" Viruses 15, no. 1: 255. https://doi.org/10.3390/v15010255
APA StyleMoyo, N. A., Westcott, D., Simmonds, R., & Steinbach, F. (2023). Equine Arteritis Virus in Monocytic Cells Suppresses Differentiation and Function of Dendritic Cells. Viruses, 15(1), 255. https://doi.org/10.3390/v15010255






