Flaviviruses in AntiTumor Therapy
Abstract
:1. Introduction
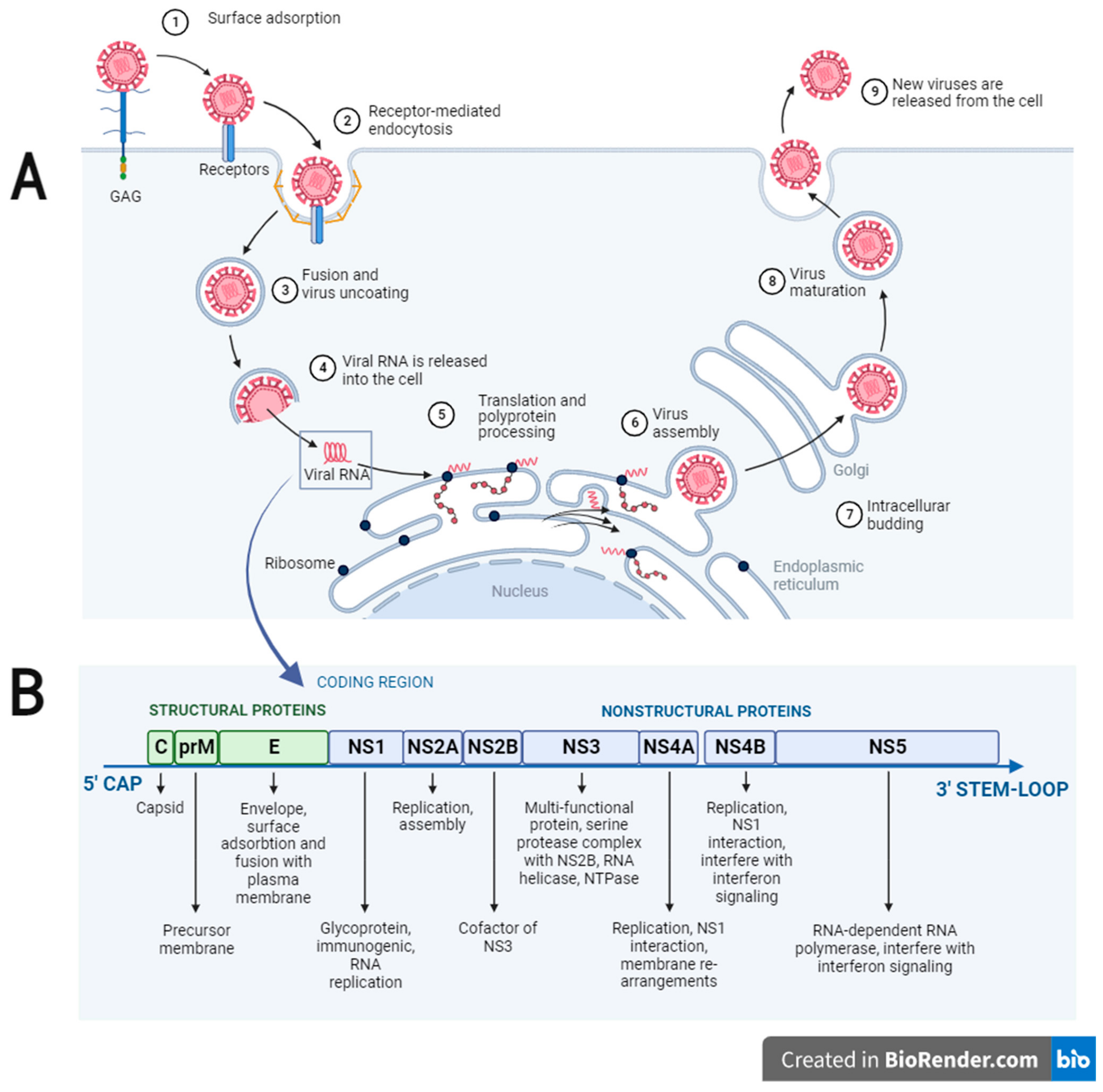
2. Description and Life Cycle of Flaviviruses
3. Flaviviruses as Oncolytic Viruses
- ➢
- The Dengvaxia® vaccine, built on this technology, has been licensed in 20 countries following successful preclinical trials in non-human primates and human clinical trials. Its safety is comparable to that of YFV-17D. However, DENV seronegative individuals who get vaccinated and later contract DENV might be at an increased risk of severe Dengue course due to antibody-dependent enhancement of infection [46,47,48]. Similarly, chimeric DENVax viruses containing the prM and E genes of DEN-1, DEN-3, and DEN-4 were successfully created [49]. This vaccine has completed preclinical testing on Macaca fascicularis, demonstrating promising safety, and is now undergoing phase 3 clinical trials [47]. Another technique to produce attenuated flavivirus strains involves deletions in the 3′-UTR [50];
- ➢
- Remarkably, this vaccine shows cased the most impressive safety record among all vaccines developed using this technology, even surpassing YFV-17D itself. Even after intracerebral administration in non-human primates, there were no indications of encephalitis. This vaccine has since been registered and is now in use [51,52];
- ➢
- ➢
- ChimeriVax-Zika, in comparison to the above vaccines, has only recently begun development and its positive safety profile can currently only be inferred from studies in mouse models [55]. However, a ChinZIKV vaccine was developed based on JEV LAV using a similar technique of replacing prM-E JEV genes with similar ZIKV genes. Tests in mice and rhesus macaque models demonstrated its safety, with no placental or fetal damage observed in pregnant mice infected with ZIKV after immunization with ChinZIKV [56].
3.1. Zika Virus
3.2. Yellow Fever Virus
3.3. West Nile Virus
3.4. Japanese Encephalitis Virus
3.5. Dengue Virus
| Virus * | Strain | Combination Therapy | Tumor Type (Model) | Animal Model | Description | Reference |
|---|---|---|---|---|---|---|
| ZIKV | ZIKV-LAV | - | Glioblastoma xenografts (Orthotopic) | BALB/c mice | Safe for intracerebral administration; delayed tumor growth and increased survival rate; development of antitumor immunity | [23] |
| ZIKV-Dakar | - | Mouse glioma GL261 and CT-2A (Orthotopic) | C57BL/6, C57BL6/J mice | Increase in survival; tumor regression; activation of CD8+, myeloid cells; development of antitumor immunity | [22,69] | |
| ZIKV-Dakar | Anti-PD-1 | Mouse glioma CT-2A (Orthotopic) | C57BL6/J mice | Increased survival; tumor regression | [69] | |
| ZIKV-GZ01 ZIKV-FSS | - | Mouse glioma GL261 and CT-2A (orthotopic) | C57BL/6N mice | Suppression of tumor growth; increased survival; activation of CD4+ and CD8+, type 1 interferon signaling pathways; increased efficacy of immune checkpoint blockers | [74] | |
| ZIKVBR | - | Human medulloblastoma DAOY, USP13-MED, human rhabdoid tumor USP7-ATRT (orthotopic) | BALB/c mice | Increased survival rate; tumor regression | [71] | |
| - | CNS tumor (spontaneous) | Pit Bull, Boxer, Dachshund Dogs | Improvement of neurological symptoms; reduction in tumor volume | [72] | ||
| YFV | 17D-pOva | - | B16-Ova, B16F0 mouse melanoma, pulmonary metastasis | C57BL/6 mice | Delayed tumor growth; increased survival, reduced both the size and number of lung metastases; induction of Ova-specific CD8+ T cells | [81] |
| 17D | Anti-PD-1 Anti-CD137 | B16-OVA mouse melanoma, MC38 mouse colon carcinoma (subcutaneous) | C57BL/6 mice | Delayed tumor growth; increased survival; activation of IFN I, CD8+ | [42] | |
| WNV | WNV-poly(A) | - | Mouse melanoma B16F1 (subcutaneous) | C57BL/6 mice | Delayed tumor growth; decrease in tumor volume; CD8+ activation | [45] |
| - | Human melanoma A375, human ovarian carcinoma SKOV3, human hepatoma Huh7 (subcutaneous) | BALB/c-nu/nu mice | ||||
| KUN | - | CT26 mouse colon carcinoma, B16-OVA mouse melanoma (subcutaneous) | BALB/c, C57BL/6 mice | Regression of tumors and metastases; CD8+ induction; development of antitumor immunity | [88] | |
| KUN with E7 epitope | - | Tumor cell lines EL4.A2 and TC-1 (subcutaneous) | BALB/c mice | Development of antitumor immunity to herpes simplex virus 16 protein E7 | [89] | |
| JEV | JEV-LAV JEV-LAV | Anti-PD-1 | Mouse glioma GL261-luc (orthotopic) | C57BL/6J mice | Inhibition of tumor growth; reduction in tumor volume; increase in lifetime, CD8+ infiltration; increase in PDL-1 expression | [43] |
| TBEV | Far East | - | MCI mouse fibrosarcoma, C1300 mouse neuroblastoma, EO 771 mouse mammary adenocarcinoma, Ridgeway mouse osteogenic sarcoma, T241, and 180 mouse sarcomas | C57BL mice, outbred mice | Delayed tumor growth | [33,34] |
| SLE | - | - | Mouse Sarcoma 180(subcutaneous) | White swiss mice | Increased survival | [39] |
| Parton | - | Ehrlich’s tumor (intraperitoneal) | Albino mice | Tumor regression in 20% of cases | [40] | |
| DENV | TR1751 | - |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| CTLs | cytotoxic T lymphocytes |
| DENV | Dengue virus |
| GAG | glycosaminoglycans |
| G-CSF | granulocyte colony-stimulating factor |
| GSCs | glioblastoma stem cells |
| HSV-1 | herpes simplex virus type 1 |
| ICI | immune checkpoint inhibitors |
| IL | interleukin |
| INF | interferon |
| JEV | Japanese encephalitis virus |
| KUN | Kunjin virus |
| NSCs | neural stem cells |
| OV | oncolytic virus |
| OVA | chicken ovalbumin |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed cell death-ligand 1 |
| SLE | St. Louis encephalitis virus |
| TAA | tumor-associated antigens |
| TBEV | Tick-borne encephalitis virus |
| TILs | tumor infiltrating lymphocytes |
| WNV | West Nile virus |
| YFV | Yellow Fever Virus |
| ZIKV | Zika virus |
References
- Zhang, S.; Rabkin, S.D. The Discovery and Development of Oncolytic Viruses: Are They the Future of Cancer Immunotherapy? Expert Opin. Drug Discov. 2021, 16, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Vandeborne, L.; Pantziarka, P.; Van Nuffel, A.M.T.; Bouche, G. Repurposing Infectious Diseases Vaccines against Cancer. Front. Oncol. 2021, 11, 688755. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xie, D.; Yang, L. Engineering Strategies to Enhance Oncolytic Viruses in Cancer Immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Rezaei, R.; Singaravelu, R.; Pelin, A.; Boulton, S.; Petryk, J.; Onsu, K.A.; Martin, N.T.; Hoskin, V.; Ghahremani, M.; et al. Synthetic virology approaches to improve the safety and efficacy of oncolytic virus therapies. Nat. Commun. 2023, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, C.; Xiong, Z.; Chen, M.; Li, Z.; Tao, T.; Liu, X. Development and application of oncolytic viruses as the nemesis of tumor cells. Front. Microbiol. 2023, 14, 1188526. [Google Scholar] [CrossRef]
- Lauer, U.M.; Beil, J. Oncolytic viruses: Challenges and considerations in an evolving clinical landscape. Future Oncol. 2022, 18, 2713–2732. [Google Scholar] [CrossRef]
- Ji, Q.; Wu, Y.; Albers, A.; Fang, M.; Qian, X. Strategies for Advanced Oncolytic Virotherapy: Current Technology Innovations and Clinical Approaches. Pharmaceutics 2022, 14, 1811. [Google Scholar] [CrossRef]
- Kolyasnikova, N.M.; Pestov, N.B.; Sanchez-Pimentel, J.M.; Barlev, N.A.; Ishmukhametov, A.A. Anti-Cancer Virotherapy in Russia: Lessons from the Past, Current Challenges and Prospects for the Future. Curr. Pharm. Biotechnol. 2022, 24, 266–278. [Google Scholar] [CrossRef]
- Chen, K.; Wang, S.; Chen, B.; Sun, F.; Li, N. Gene therapy in solid tumors: Trends in trials in China and beyond. Drug Discov. Today 2021, 26, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Nettelbeck, D.M.; Leber, M.F.; Altomonte, J.; Angelova, A.; Beil, J.; Berchtold, S.; Delic, M.; Eberle, J.; Ehrhardt, A.; Engeland, C.E.; et al. Virotherapy in Germany—Recent Activities in Virus Engineering, Preclinical Development, and Clinical Studies. Viruses 2021, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Fukuhara, H.; Todo, T. Oncolytic virus therapy in Japan: Progress in clinical trials and future perspectives. Jpn. J. Clin. Oncol. 2019, 49, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zolaly, M.A.; Mahallawi, W.; Khawaji, Z.Y.; Alahmadi, M.A. The Clinical Advances of Oncolytic Viruses in Cancer Immunotherapy. Cureus 2023, 15, e40742. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Bell, J.C.; Engeland, C.E.; McFadden, G. Advances in oncolytic virotherapy. Commun. Med. 2022, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jou, T.H.-T.; Hsin, J.; Wang, Z.; Huang, K.; Ye, J.; Yin, H.; Xing, Y. Talimogene Laherparepvec (T-VEC): A Review of the Recent Advances in Cancer Therapy. J. Clin. Med. 2023, 12, 1098. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Wei, S.P.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Ylösmäki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Zika Virus Has Oncolytic Activity against Glioblastoma Stem Cells. J. Exp. Med. 2017, 214, 2843–2857. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Ye, Q.; Ma, F.; Zhu, Q.; Wu, Y.; Shan, C.; Xie, X.; Li, D.; Zhan, X.; et al. Treatment of Human Glioblastoma with a Live Attenuated Zika Virus Vaccine Candidate. mBio 2018, 9, e01683-18. [Google Scholar] [CrossRef] [PubMed]
- Trus, I.; Berube, N.; Jiang, P.; Rak, J.; Gerdts, V.; Karniychuk, U. Zika Virus with Increased CpG Dinucleotide Frequencies Shows Oncolytic Activity in Glioblastoma Stem Cells. Viruses 2020, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Barrows, N.J.; Campos, R.K.; Liao, K.-C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.-C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef] [PubMed]
- Maramorosch, K.; Chambers, T.J.; Monath, T.P.; Shatkin, A.J.; Murphy, F.A. (Eds.) The Flaviviruses: Structure, Replication and Evolution; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 978-0-08-049381-7. [Google Scholar]
- Anwar, M.N.; Akhtar, R.; Abid, M.; Khan, S.A.; Rehman, Z.U.; Tayyub, M.; Malik, M.I.; Shahzad, M.K.; Mubeen, H.; Qadir, M.S.; et al. The interactions of flaviviruses with cellular receptors: Implications for virus entry. Virology 2022, 568, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Apte-Sengupta, S.; Sirohi, D.; Kuhn, R.J. Coupling of Replication and Assembly in Flaviviruses. Curr. Opin. Virol. 2014, 9, 134–142. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [CrossRef]
- Kamhi, E.; Joo, E.J.; Dordick, J.S.; Linhardt, R.J. Glycosaminoglycans in Infectious Disease. Biol. Rev. 2013, 88, 928–943. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2014, 6, 69–88. [Google Scholar] [CrossRef]
- Dobler, P.D.G.; Erber, D.W.; Bröker, D.M.; Schmit, P.D.H.-J. The TBE Book, 2nd ed.; Global Health Press: Singapore, 2019; ISBN 9789811409141. [Google Scholar]
- Moore, A.E. Inhibition of growth of five transplantable mouse tumors by the virus of Russian Far East encephalitis. Cancer 1951, 4, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.E. The destructive effect of the virus of russian far east encephalitis on the transplantable mouse sarcoma 180. Cancer 1949, 2, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Levkovich, E.N.; Karpovich, L.G. Comparative study of the viruses of the tick encephalitis group in HeLa cell cultures. Vopr. Virusol. 1960, 5, 30–39. [Google Scholar] [PubMed]
- Levkovich, E.N.; Sergeeva, G.I. Inhibitory action of viruses of the tick-borne encephalitis complex differing in neurovirulence on mouse tumors in vivo. Bull. Exp. Biol. Med. 1967, 64, 885–887. [Google Scholar] [CrossRef]
- Růžek, D.; Vancová, M.; Tesařová, M.; Ahantarig, A.; Kopecký, J.; Grubhoffer, L. Morphological changes in human neural cells following tick-borne encephalitis virus infection. J. Gen. Virol. 2009, 90, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, G.I.; Levkovich, E.N. A study of the features of reproduction in tumor cells in vitro and in vivo of different viruses of the tick-borne encephalitis complex, possessing various degress of neurovirulence. Vopr. Virusol. 1966, 11, 539–545. [Google Scholar] [PubMed]
- Buckley, S.M.; Buckley, J.J.; Snipes, A.E. The effect of St. Louis encephalitis virus on transplantable Crocker mouse sarcoma 180. Cancer 1951, 4, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Mettler, N.E.; Clarke, D.H.; Casals, J. Virus inoculation in mice bearing Ehrlich ascitic tumors: Antigen production and tumor regression. Infect. Immun. 1982, 37, 23–27. [Google Scholar] [CrossRef]
- Webb, H.E.; Wetherley-Mein, G.; Smith, C.E.; McMahon, D. Leukaemia and neoplastic processes treated with Langat and Kyasanur Forest disease viruses: A clinical and laboratory study of 28 patients. Br. Med. J. 1966, 1, 258–266. [Google Scholar] [CrossRef]
- Aznar, M.A.; Molina, C.; Teijeira, A.; Rodriguez, I.; Azpilikueta, A.; Garasa, S.; Sanchez-Paulete, A.R.; Cordeiro, L.; Etxeberria, I.; Alvarez, M.; et al. Repurposing the yellow fever vaccine for intratumoral immunotherapy. EMBO Mol. Med. 2020, 12, e10375. [Google Scholar] [CrossRef]
- Qi, Z.; Zhao, J.; Li, Y.; Zhang, B.; Hu, S.; Chen, Y.; Ma, J.; Shu, Y.; Wang, Y.; Cheng, P. Live-attenuated Japanese encephalitis virus inhibits glioblastoma growth and elicits potent antitumor immunity. Front Immunol. 2022, 14, 982180. [Google Scholar] [CrossRef] [PubMed]
- Wood, H. Neuro-oncology: A new role for Zika virus in glioblastoma therapy? Nat. Rev. Neurol. 2017, 13, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, Y.-Y.; Zhang, Q.-Y.; Zhang, Y.-N.; Li, N.; Zhang, Z.-R.; Zhan, S.-L.; Gao, L.; Deng, C.-L.; Li, X.-D.; et al. Attenuated WNV-poly(A) exerts a broad-spectrum oncolytic effect by selective virus replication and CD8+ T cell-dependent immune response. Biomed. Pharmacother. 2023, 158, 114094. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Yoon, I.-K. A Review of Dengvaxia®: Development to Deployment. Hum. Vaccines Immunother. 2019, 15, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Torres-Flores, J.M.; Reyes-Sandoval, A.; Salazar, M.I. Dengue Vaccines: An Update. BioDrugs 2022, 36, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Guirakhoo, F.; Pugachev, K.; Zhang, Z.; Myers, G.; Levenbook, I.; Draper, K.; Lang, J.; Ocran, S.; Mitchell, F.; Parsons, M.; et al. Safety and Efficacy of Chimeric Yellow Fever-Dengue Virus Tetravalent Vaccine Formulations in Nonhuman Primates. J. Virol. 2004, 78, 4761–4775. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Huang, C.Y.-H.; Kinney, R.M.; Stinchcomb, D.T. Development of DENVax: A chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine 2011, 29, 7251–7260. [Google Scholar] [CrossRef]
- Durbin, A.P.; Kirkpatrick, B.D.; Pierce, K.K.; Schmidt, A.C.; Whitehead, S.S. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 2011, 29, 7242–7250. [Google Scholar] [CrossRef]
- Satchidanandam, V. Japanese Encephalitis Vaccines. Curr. Treat. Options Infect. Dis. 2020, 12, 375–386. [Google Scholar] [CrossRef]
- Chin, R.; Torresi, J. Japanese B Encephalitis: An Overview of the Disease and Use of Chimerivax-JE as a Preventative Vaccine. Infect. Dis. Ther. 2013, 2, 145–158. [Google Scholar] [CrossRef]
- Arroyo, J.; Miller, C.; Catalan, J.; Myers, G.A.; Ratterree, M.S.; Trent, D.W.; Monath, T.P. ChimeriVax-West Nile Virus Live-Attenuated Vaccine: Preclinical Evaluation of Safety, Immunogenicity, and Efficacy. J. Virol. 2004, 78, 12497–12507. [Google Scholar] [CrossRef]
- Kaiser, J.A.; Barrett, A.D.T. Twenty Years of Progress Toward West Nile Virus Vaccine Development. Viruses 2019, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Giel-Moloney, M.; Goncalvez, A.P.; Catalan, J.; Lecouturier, V.; Girerd-Chambaz, Y.; Diza, F.; Maldonado-Arocho, F.; Gomila, R.C.; Bernard, M.-C.; Oomen, R.; et al. Chimeric Yellow Fever 17D-Zika Virus (ChimeriVax-Zika) as a Live-Attenuated Zika Virus Vaccine. Sci. Rep. 2018, 8, 13206. [Google Scholar] [CrossRef]
- Li, X.-F.; Dong, H.-L.; Wang, H.-J.; Huang, X.-Y.; Qiu, Y.-F.; Ji, X.; Ye, Q.; Li, C.; Liu, Y.; Deng, Y.-Q.; et al. Development of a Chimeric Zika Vaccine Using a Licensed Live-Attenuated Flavivirus Vaccine as Backbone. Nat. Commun. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Barros-Aragão, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; de Sousa, V.L.; Souza, A.S.; et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef]
- Wells, M.F.; Salick, M.R.; Wiskow, O.; Ho, D.J.; Worringer, K.A.; Ihry, R.J.; Kommineni, S.; Bilican, B.; Klim, J.R.; Hill, E.J.; et al. Genetic Ablation of AXL Does Not Protect Human Neural Progenitor Cells and Cerebral Organoids from Zika Virus Infection. Cell Stem Cell 2016, 19, 703–708. [Google Scholar] [CrossRef]
- Altevogt, P.; Sammar, M.; Hüser, L.; Kristiansen, G. Novel insights into the function of CD24: A driving force in cancer. Int. J. Cancer 2021, 148, 546–559. [Google Scholar] [CrossRef]
- Mazar, J.; Li, Y.; Rosado, A.; Phelan, P.; Kedarinath, K.; Parks, G.D.; Alexander, K.A.; Westmoreland, T.J. Zika virus as an oncolytic treatment of human neuroblastoma cells requires CD24. PLoS ONE 2018, 13, e0200358. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Y.; Huang, J.; Feng, Y.; Zhang, Z.; Zhong, K.; Chen, Y.; Wang, Z.; Huang, C.; Yang, H.; et al. Zika virus NS5 protein inhibits cell growth and invasion of glioma. Biochem. Biophys. Res. Commun. 2019, 516, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.A.; Norris, P.S.; Ware, C.F. To kill or be killed: Viral evasion of apoptosis. Nat. Immunol. 2002, 3, 1013–1018. [Google Scholar] [CrossRef]
- Mlera, L.; Melik, W.; Bloom, M.E. The role of viral persistence in flavivirus biology. Pathog. Dis. 2014, 71, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Lemos de Matos, A.; Franco, L.S.; McFadden, G. Oncolytic Viruses and the Immune System: The Dynamic Duo. Mol. Ther. Methods Clin. Dev. 2020, 17, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Mazzoccoli, L.; Jash, A.; Govero, J.; Bais, S.S.; Hu, T.; Fontes-Garfias, C.R.; Shan, C.; Okada, H.; Shresta, S.; et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. J. Clin. Investig. Insight 2021, 6, e144619. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef]
- Kaid, C.; Goulart, E.; Caires-Júnior, L.C.; Araujo, B.H.S.; Soares-Schanoski, A.; Bueno, H.M.S.; Telles-Silva, K.A.; Astray, R.M.; Assoni, A.F.; Júnior, A.F.R.; et al. Zika Virus Selectively Kills Aggressive Human Embryonal CNS Tumor Cells In Vitro and In Vivo. Cancer Res. 2018, 78, 3363–3374. [Google Scholar] [CrossRef]
- Kaid, C.; Madi, R.A.D.S.; Astray, R.; Goulart, E.; Caires-Junior, L.C.; Mitsugi, T.G.; Moreno, A.C.R.; Castro-Amarante, M.F.; Pereira, L.R.; Porchia, B.F.M.M.; et al. Safety, Tumor Reduction, and Clinical Impact of Zika Virus Injection in Dogs with Advanced-Stage Brain Tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 1276–1286. [Google Scholar] [CrossRef]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Wettersten, H.I.; Beck, S.; Clark, A.E.; Wu, Q.; et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin αvβ5 Axis. Cell Stem Cell 2020, 26, 187–204.e10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, C.; Chen, Q.; Shang, J.; Liu, Z.; Guo, Y.; Li, C.; Wang, H.; Ye, Q.; Li, X.; et al. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol. Ther. Oncolytics 2022, 24, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fontela, C.; Macip, S.; Martínez-Sobrido, L.; Brown, L.; Ashour, J.; García-Sastre, A.; Lee, S.W.; Aaronson, S.A. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 2008, 205, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, P.; Aksenova, V.; Petrova, V.; Tentler, D.; Orlova, D.; Smirnov, S.; Gurzhiy, V.; Okorokov, A.L.; Garabadzhiu, A.; Melino, G.; et al. Discovery of Novel Isatin-Based p53 Inducers. ACS Med. Chem. Lett. 2015, 6, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Daks, A.; Petukhov, A.; Fedorova, O.; Shuvalov, O.; Merkulov, V.; Vasileva, E.; Antonov, A.; Barlev, N.A. E3 ubiquitin ligase Pirh2 enhances tumorigenic properties of human non-small cell lung carcinoma cells. Genes Cancer 2016, 7, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Lezina, L.; Aksenova, V.; Fedorova, O.; Malikova, D.; Shuvalov, O.; Antonov, A.V.; Tentler, D.; Garabadgiu, A.V.; Melino, G.; Barlev, N.A. KMT Set7/9 affects genotoxic stress response via the Mdm2 axis. Oncotarget 2015, 6, 25843–25855. [Google Scholar] [CrossRef] [PubMed]
- Mittenberg, A.G. Role of proteasomes in transcription and their regulation by covalent modifications. Front. Biosci. 2008, 13, 7184–7192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO position on the use of fractional doses—June 2017, addendum to vaccines and vaccination against yellow fever WHO: Position paper—June 2013. Vaccine 2017, 35, 5751–5752. [Google Scholar] [CrossRef]
- McAllister, A.; Arbetman, A.E.; Mandl, S.; Peña-Rossi, C.; Andino, R. Recombinant Yellow Fever Viruses Are Effective Therapeutic Vaccines for Treatment of Murine Experimental Solid Tumors and Pulmonary Metastases. J. Virol. 2000, 74, 9197–9205. [Google Scholar] [CrossRef]
- Southam, C.M.; Moore, A.E. West Nile, Ilheus, and Bunyamwera Virus Infections in Man. Am. J. Trop. Med. 1951, 31, 724–741. [Google Scholar] [CrossRef]
- Southam, C.M.; Moore, A.E. Clinical studies of viruses as antineoplastic agents, with particular reference to egypt 101 virus. ACS J. 1952, 5, 1025–1034. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Li, N.; Zhang, Q.-Y.; Liu, J.; Zhan, S.-L.; Gao, L.; Zeng, X.-Y.; Yu, F.; Zhang, H.-Q.; Li, X.-D.; et al. Rational design of West Nile virus vaccine through large replacement of 3′ UTR with internal poly(A). EMBO Mol. Med. 2021, 13, e14108. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Scherret, J.H.; Mackenzie, J.S. Kunjin virus: An Australian variant of West Nile? Ann. N. Y. Acad. Sci. 2001, 951, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.J.; Liu, W.J.; Wang, X.J.; Linedale, R.; Jacobs, M.; Davidson, A.; Le, T.T.T.; Anraku, I.; Suhrbier, A.; Shi, P.-Y.; et al. Tetracycline-inducible packaging cell line for production of flavivirus replicon particles. J. Virol. 2004, 78, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Khromykh, A.A.; Varnavski, A.N.; Westaway, E.G. Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 1998, 72, 5967–5977. [Google Scholar] [CrossRef] [PubMed]
- Hoang-Le, D.; Smeenk, L.; Anraku, I.; Pijlman, G.P.; Wang, X.J.; de Vrij, J.; Liu, W.J.; Le, T.T.; Schroder, W.A.; Khromykh, A.A.; et al. A Kunjin replicon vector encoding granulocyte macrophage colony-stimulating factor for intra-tumoral gene therapy. Gene Ther. 2009, 16, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Herd, K.A.; Harvey, T.; Khromykh, A.A.; Tindle, R.W. Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour. Virology 2004, 319, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.J.; Anraku, I.; Linedale, R.; Harrich, D.; Mackenzie, J.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicon vectors for human immunodeficiency virus vaccine development. J. Virol. 2003, 77, 7796–7803. [Google Scholar] [CrossRef]
- Yang, M.-R.; Lee, S.R.; Oh, W.; Lee, E.-W.; Yeh, J.-Y.; Nah, J.-J.; Joo, Y.-S.; Shin, J.; Lee, H.-W.; Pyo, S.; et al. West Nile virus capsid protein induces p53-mediated apoptosis via the sequestration of HDM2 to the nucleolus. Cell. Microbiol. 2007, 10, 165–176. [Google Scholar] [CrossRef]
- Bulatov, E.; Sayarova, R.; Mingaleeva, R.; Miftakhova, R.; Gomzikova, M.; Ignatyev, Y.; Petukhov, A.; Davidovich, P.; Rizvanov, A.; Barlev, N.A. Isatin-Schiff base-copper (II) complex induces cell death in p53-positive tumors. Cell Death Discov. 2018, 4, 103. [Google Scholar] [CrossRef]
- Tovar, C.; Graves, B.; Packman, K.; Filipovic, Z.; Xia, B.H.M.; Tardell, C.; Garrido, R.; Lee, E.; Kolinsky, K.; To, K.-H.; et al. MDM2 Small-Molecule Antagonist RG7112 Activates p53 Signaling and Regresses Human Tumors in Preclinical Cancer Models. Cancer Res. 2013, 73, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine 2010, 28, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Goldufsky, J.W.; Daniels, P.; Williams, M.D.; Gupta, K.; Lyday, B.; Chen, T.; Singh, G.; Zloza, A.; Marzo, A.L. Attenuated Dengue Virus PV001-DV Induces Oncolytic Cell Death and Potent Anti-Tumor Immunity. bioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Thongtan, T.; Panyim, S.; Smith, D.R. Apoptosis in dengue virus infected liver cell lines HepG2 and Hep3B. J. Med. Virol. 2004, 72, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, X.; Li, B.; Huang, L.; Wang, X.; He, B.; Xie, H.; Wu, Q.; Chen, L. Identification and Validation of Ferroptosis-Related Genes in Patients Infected with Dengue Virus: Implication in the Pathogenesis of DENV. Virus Genes 2023, 59, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Turtle, L.; Solomon, T. Chapter 26—Japanese encephalitis virus infection. In Handbook of Clinical Neurology; Tselis, A.C., Booss, J., Eds.; Neurovirology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 561–576. [Google Scholar]
- Vasconcelos, P.F.d.C.; Quaresma, J.A.S. Yellow Fever. In Infectious Tropical Diseases and One Health in Latin America; Mehlhorn, H., Heukelbach, J., Eds.; Parasitology Research Monographs; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–17. ISBN 978-3-030-99712-0. [Google Scholar]
- Wong, J.M.; Adams, L.E.; Durbin, A.P.; Muñoz-Jordán, J.L.; Poehling, K.A.; Sánchez-González, L.M.; Volkman, H.R.; Paz-Bailey, G. Dengue: A Growing Problem With New Interventions. Pediatrics 2022, 149, e2021055522. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, H. Diagnosis of tick-borne encephalitis. Vaccine 2003, 21, S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Yokoda, R.; Nagalo, B.M.; Vernon, B.; Oklu, R.; Albadawi, H.; DeLeon, T.T.; Zhou, Y.; Egan, J.B.; Duda, D.G.; Borad, M.J. Oncolytic virus delivery: From nano-pharmacodynamics to enhanced oncolytic effect. Oncolytic Virotherapy 2017, 6, 39–49. [Google Scholar] [CrossRef]
- Jayawardena, N.; Poirier, J.T.; Burga, L.N.; Bostina, M. Virus–Receptor Interactions and Virus Neutralization: Insights for Oncolytic Virus Development. Oncolytic Virotherapy 2020, 9, 1–15. [Google Scholar] [CrossRef]
- Barrett, A.D.T. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. npj Vaccines 2018, 3, 24. [Google Scholar] [CrossRef]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef]
- Monath, T.P. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev. Vaccines 2012, 11, 427–448. [Google Scholar] [CrossRef]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarenko, A.S.; Vorovitch, M.F.; Biryukova, Y.K.; Pestov, N.B.; Orlova, E.A.; Barlev, N.A.; Kolyasnikova, N.M.; Ishmukhametov, A.A. Flaviviruses in AntiTumor Therapy. Viruses 2023, 15, 1973. https://doi.org/10.3390/v15101973
Nazarenko AS, Vorovitch MF, Biryukova YK, Pestov NB, Orlova EA, Barlev NA, Kolyasnikova NM, Ishmukhametov AA. Flaviviruses in AntiTumor Therapy. Viruses. 2023; 15(10):1973. https://doi.org/10.3390/v15101973
Chicago/Turabian StyleNazarenko, Alina S., Mikhail F. Vorovitch, Yulia K. Biryukova, Nikolay B. Pestov, Ekaterina A. Orlova, Nickolai A. Barlev, Nadezhda M. Kolyasnikova, and Aydar A. Ishmukhametov. 2023. "Flaviviruses in AntiTumor Therapy" Viruses 15, no. 10: 1973. https://doi.org/10.3390/v15101973
APA StyleNazarenko, A. S., Vorovitch, M. F., Biryukova, Y. K., Pestov, N. B., Orlova, E. A., Barlev, N. A., Kolyasnikova, N. M., & Ishmukhametov, A. A. (2023). Flaviviruses in AntiTumor Therapy. Viruses, 15(10), 1973. https://doi.org/10.3390/v15101973





