Beet Curly Top Iran Virus Rep and V2 Suppress Post-Transcriptional Gene Silencing via Distinct Modes of Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Constructs for Transient Expression
2.2. Agroinfiltration
2.3. GFP Imaging and Quantification
2.4. Protein Extraction and Western Blot
2.5. RNA Extraction, RT-PCR, and Northern Blot
2.6. Rdr6 N. benthamiana Mutant Lines
2.7. Statistical Analysis
2.8. Phylogenetic Tree
3. Results
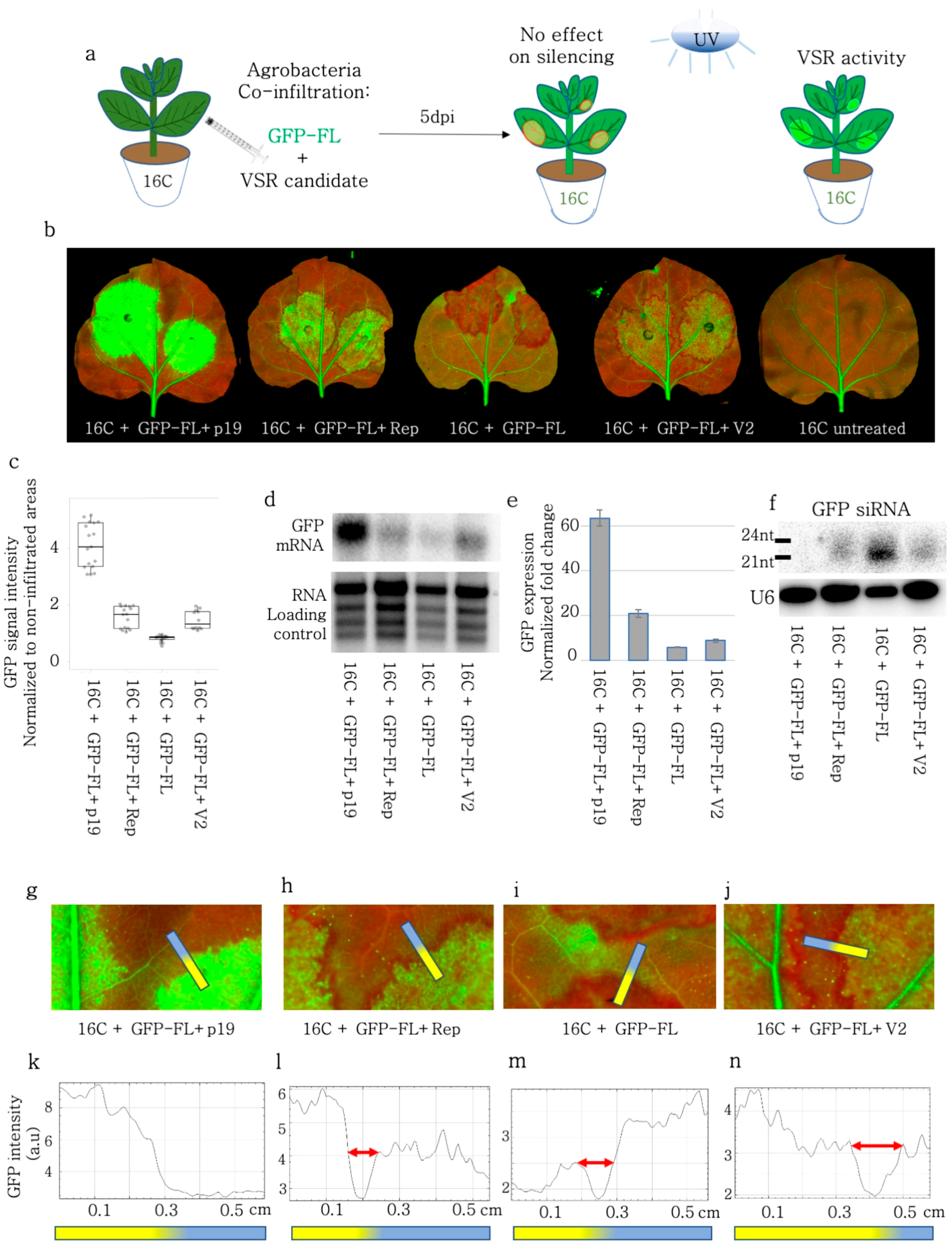
3.1. Phenotypic Analysis of Transient BCTIV Gene Expression and Silencing Inducers on N. benthamiana
3.2. BCTIV-Rep and -V2 Suppressed GFP-FL Induced Local PTGS in 16C N. benthamiana
3.3. Rep and V2 Did Not Interfere with the Spread of Silencing through Plasmodesmata
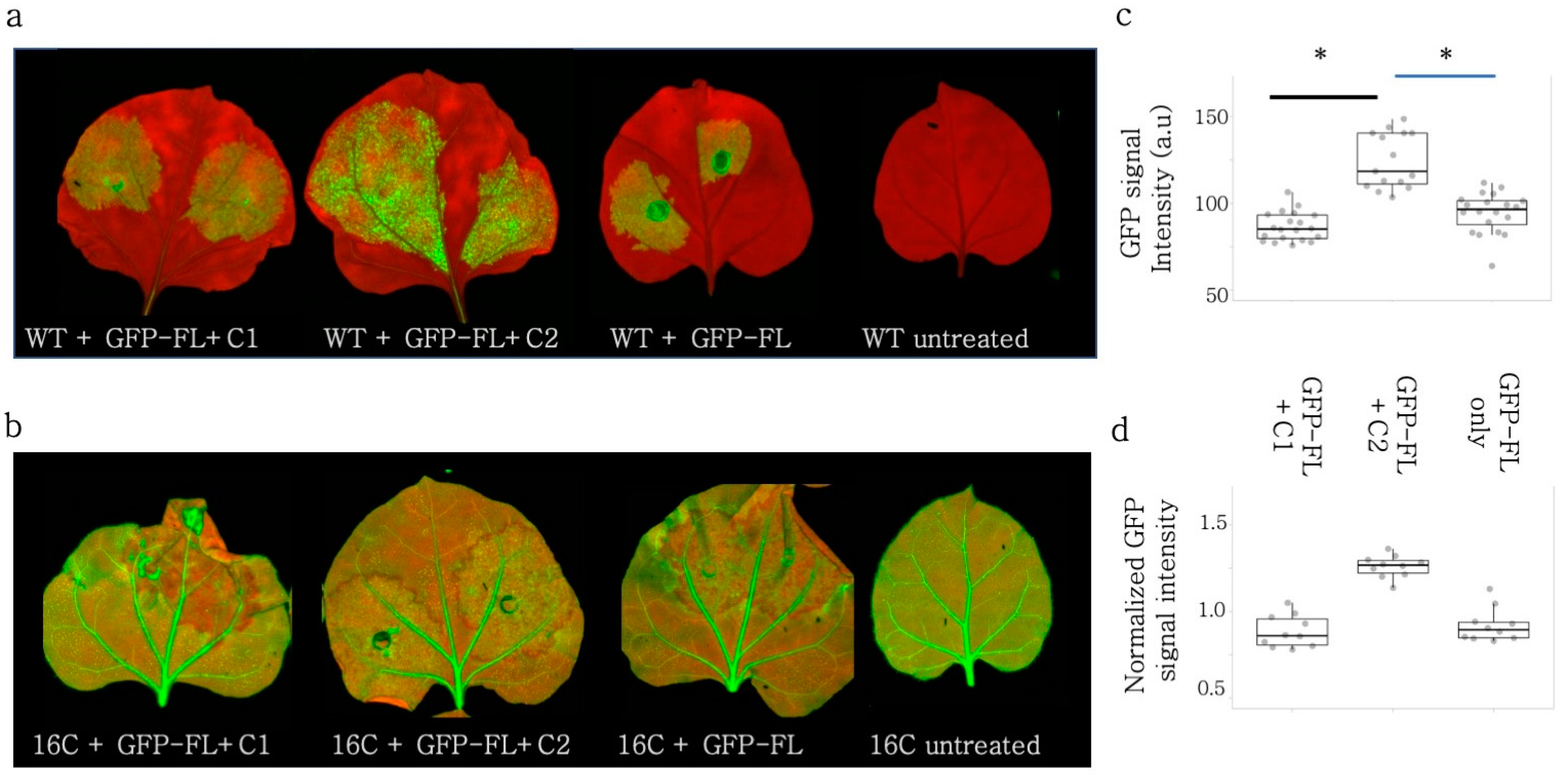
3.4. Rep and V2 Suppressed Self PTGS of GFP-FL in Wild-Type N. benthamiana
3.5. Rep but Not V2 Suppressed Local Silencing Induced by a 5′GFP in 16C N. benthamiana
3.6. PTGS Induced by a Hairpin Construct Targeting GFP Was Partly Repressed by Rep but Not by V2
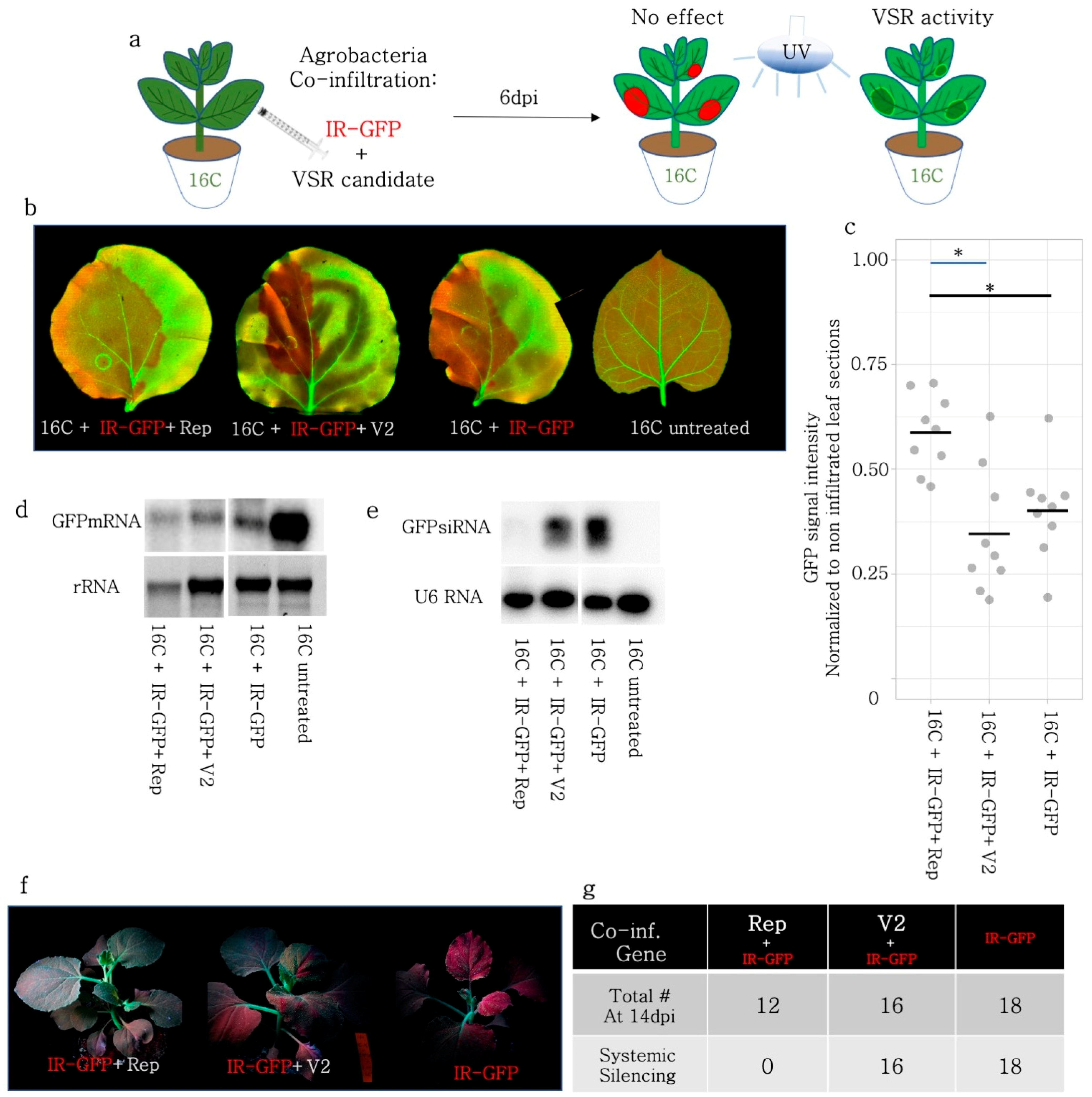
3.7. Rep but Not V2 Suppressed Systemic Silencing
3.8. Multiple Rep Domains Were Indispensable for VSR Activity
3.9. VSR Activity of Rep but Not of V2 Is RDR6 Dependent
3.10. Rep and V2 Lead to Changes in the Expression of RNAi Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yazdi, H.R.B.; Heydarnejad, J.; Massumi, H. Genome characterization and genetic diversity of beet curly top Iran virus: A geminivirus with a novel nonanucleotide. Virus Genes 2008, 36, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Heydarnejad, J.; Keyvani, N.; Razavinejad, S.; Massumi, H.; Varsani, A. Fulfilling Koch’s postulates for beet curly top Iran virus and proposal for consideration of new genus in the family Geminiviridae. Arch. Virol. 2013, 158, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Anabestani, A.; Behjatnia, S.A.A.; Izadpanah, K.; Tabein, S.; Accotto, G.P. Seed Transmission of Beet Curly Top Virus and Beet Curly Top Iran Virus in a Local Cultivar of Petunia in Iran. Viruses 2017, 9, 299. [Google Scholar] [CrossRef]
- Taheri, H.; Izadpanah, K.; Behjatnia, S.A.A. Criculifer hematocepts, the vector of the beet curly top Iran virus. Phytopathology. 2012, 48, 141, (In Persian, Abstract in English). [Google Scholar]
- Teixeira, R.M.; Ferreira, M.A.; Raimundo, G.A.S.; Fontes, E.P.B. Geminiviral Triggers and Suppressors of Plant Antiviral Immunity. Microorganisms 2021, 9, 775. [Google Scholar] [CrossRef]
- Kumar, R.V. Plant Antiviral Immunity against Geminiviruses and Viral Counter-Defense for Survival. Front. Microbiol. 2019, 10, 1460. [Google Scholar] [CrossRef]
- Ruhel, R.; Chakraborty, S. Multifunctional roles of geminivirus encoded replication initiator protein. VirusDisease 2019, 30, 66–73. [Google Scholar] [CrossRef]
- Kotlizky, G.; Boulton, M.I.; Pitaksutheepong, C.; Davies, J.W.; Epel, B.L. Intracellular and Intercellular Movement of Maize Streak Geminivirus V1 and V2 Proteins Transiently Expressed as Green Fluorescent Protein Fusions. Virology 2000, 274, 32–38. [Google Scholar] [CrossRef]
- Bozorgi, N.; Heydarnejad, J.; Kamali, M.; Massumi, H. Splicing features in the expression of the complementary-sense genes of Beet curly top Iran virus. Virus Genes 2017, 53, 323–327. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Briddon, R.W.; Idris, A.; Martin, D.P.; Moriones, E.; Navas-Castillo, J.; Rivera-Bustamante, R.; Roumagnac, P.; Varsani, A.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Geminiviridae. J. Gen. Virol. 2017, 98, 131–133. [Google Scholar] [CrossRef]
- Hanley-Bowdoin, L.; Bejarano, E.R.; Robertson, D.; Mansoor, S. Geminiviruses: Masters at redirecting and reprogramming plant processes. Nat. Rev. Microbiol. 2013, 11, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Vanitharani, R.; Chellappan, P.; Fauquet, C.M. Geminiviruses and RNA silencing. Trends Plant Sci. 2005, 10, 144–151. [Google Scholar] [CrossRef]
- Bisaro, D.M. Silencing suppression by geminivirus proteins. Virology 2006, 344, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Han, Y.-W.; Fujii, H.; Aizawa, S.; Nishino, T.; Ishikawa, M. Cooperative recruitment of RDR6 by SGS3 and SDE5 during small interfering RNA amplification in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102885118. [Google Scholar] [CrossRef] [PubMed]
- Uslu, V.V.; Dalakouras, A.; Steffens, V.A.; Krczal, G.; Wassenegger, M. High-pressure sprayed siRNAs influence the efficiency but not the profile of transitive silencing. Plant J. 2022, 109, 1199–1212. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Iwakawa, H.-O.; Pan, Y.; Tang, X.; Ling-Hu, Q.; Liu, Y.; Sheng, S.; Feng, L.; Zhang, H.; et al. Plant 22-nt siRNAs mediate translational repression and stress adaptation. Nature 2020, 581, 89–93. [Google Scholar] [CrossRef]
- Boutet, S.; Vazquez, F.; Liu, J.; Béclin, C.; Fagard, M.; Gratias, A.; Morel, J.B.; Crété, P.; Chen, X.; Vaucheret, H. Arabidopsis HEN1: A genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr. Biol. CB 2003, 13, 843–848. [Google Scholar] [CrossRef]
- Jin, L.; Chen, M.; Xiang, M.; Guo, Z. RNAi-Based Antiviral Innate Immunity in Plants. Viruses 2022, 14, 432. [Google Scholar] [CrossRef]
- Ghosh, D.; Chakraborty, S. Impact of viral silencing suppressors on plant viral synergism: A global agro-economic concern. Appl. Microbiol. Biotechnol. 2021, 105, 6301–6313. [Google Scholar] [CrossRef]
- Zhang, X.; Du, P.; Lu, L.; Xiao, Q.; Wang, W.; Cao, X.; Ren, B.; Wei, C.; Li, Y. Contrasting effects of HC-Pro and 2b viral suppressors from Sugarcane mosaic virus and Tomato aspermy cucumovirus on the accumulation of siRNAs. Virology 2008, 374, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Jamous, R.M.; Boonrod, K.; Fuellgrabe, M.W.; Ali-Shtayeh, M.S.; Krczal, G.; Wassenegger, M. The helper component-proteinase of the Zucchini yellow mosaic virus inhibits the Hua Enhancer 1 methyltransferase activity in vitro. J. Gen. Virol. 2011, 92, 2222–2226. [Google Scholar] [CrossRef]
- Sanobar, N.; Lin, P.-C.; Pan, Z.-J.; Fang, R.-Y.; Tjita, V.; Chen, F.-F.; Wang, H.-C.; Tsai, H.-L.; Wu, S.-H.; Shen, T.-L.; et al. Investigating the Viral Suppressor HC-Pro Inhibiting Small RNA Methylation through Functional Comparison of HEN1 in Angiosperm and Bryophyte. Viruses 2021, 13, 1837. [Google Scholar] [CrossRef] [PubMed]
- Voinnet, O.; Rivas, S.; Mestre, P.; Baulcombe, D. Retracted: An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003, 33, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Malinina, L.; Patel, D.J. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 2003, 426, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zuo, D.; Jiang, X.; Li, S.; Chen, J.; Jiang, L.; Zhou, X.; Jiang, T. Identification of Strawberry vein banding virus encoded P6 as an RNA silencing suppressor. Virology 2018, 520, 103–110. [Google Scholar] [CrossRef]
- Lu, R.; Folimonov, A.; Shintaku, M.; Li, W.-X.; Falk, B.W.; Dawson, W.O.; Ding, S.-W. Three distinct suppressors of RNA silencing encoded by a 20-kb viral RNA genome. Proc. Natl. Acad. Sci. USA 2004, 101, 15742–15747. [Google Scholar] [CrossRef]
- Haas, G.; Azevedo, J.; Moissiard, G.; Geldreich, A.; Himber, C.; Bureau, M.; Fukuhara, T.; Keller, M.; Voinnet, O. Nuclear import of CaMV P6 is required for infection and suppression of the RNA silencing factor DRB4. EMBO J. 2008, 27, 2102–2112. [Google Scholar] [CrossRef]
- Iki, T.; Tschopp, M.-A.; Voinnet, O. Biochemical and genetic functional dissection of the P38 viral suppressor of RNA silencing. RNA 2017, 23, 639–654. [Google Scholar] [CrossRef]
- Giner, A.; Lakatos, L.; García-Chapa, M.; López-Moya, J.J.; Burgyán, J. Viral protein inhibits RISC activity by argonaute binding through conserved WG/GW motifs. PLoS Pathog. 2010, 6, e1000996. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Wang, L.; Wang, X. Replication-associated proteins encoded by Wheat dwarf virus act as RNA silencing suppressors. Virus Res. 2014, 190, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, M.; Hou, H.; Mei, Y.; Qian, Y.; Zhou, X. Identification of an RNA silencing suppressor encoded by a mastrevirus. J. Gen. Virol. 2014, 95, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Lauter, A.; Bassler, A.; Krczal, G.; Wassenegger, M. Transient expression of intron-containing transgenes generates non-spliced aberrant pre-mRNAs that are processed into siRNAs. Planta 2019, 249, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Briddon, R.W.; Dalakouras, A.; Krczal, G.; Wassenegger, M. Functional Analysis of Cotton Leaf Curl Kokhran Virus/Cotton Leaf Curl Multan Betasatellite RNA Silencing Suppressors. Biology 2015, 4, 697–714. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Eini, O.; Koolivand, D.; Varrelmann, M. The Rep and C1 of Beet curly top Iran virus represent pathogenicity factors and induce hypersensitive response in Nicotiana benthamiana plants. Virus Genes 2022, 58, 550–559. [Google Scholar] [CrossRef]
- Ludman, M.; Fátyol, K. The virological model plant, Nicotiana benthamiana expresses a single functional RDR6 homeolog. Virology 2019, 537, 143–148. [Google Scholar] [CrossRef]
- Eini, O.; Sahraei, G.E.; Ali, S.; Behjatnia, A. Molecular characterization and construction of an infectious clone of a pepper isolate of Beet curly top Iran virus. Mol. Biol. Res. Commun. 2016, 5, 101. [Google Scholar]
- Teng, K.; Chen, H.; Lai, J.; Zhang, Z.; Fang, Y.; Xia, R.; Zhou, X.; Guo, H.; Xie, Q. Involvement of C4 Protein of Beet Severe Curly Top Virus (Family Geminiviridae) in Virus Movement. PLoS ONE 2010, 5, e11280. [Google Scholar] [CrossRef]
- Himber, C.; Dunoyer, P.; Moissiard, G.; Ritzenthaler, C.; Voinnet, O. Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J. 2003, 22, 4523–4533. [Google Scholar] [CrossRef]
- Dunoyer, P.; Himber, C.; Voinnet, O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat. Genet. 2005, 37, 1356–1360. [Google Scholar] [CrossRef]
- Kalantidis, K.; Schumacher, H.T.; Alexiadis, T.; Helm, J.M. RNA silencing movement in plants. Biol. Cell 2008, 100, 13–26. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Molnar, A.; Baulcombe, D.C. Intercellular and systemic movement of RNA silencing signals. EMBO J. 2011, 30, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-W.; Lee, J.S.; Davarpanah, S.J.; Jeon, J.-H.; Park, Y.-I.; Liu, J.R.; Jeong, W.J. Host-dependent suppression of RNA silencing mediated by the viral suppressor p19 in potato. Planta 2011, 234, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Guo, J.; Zhang, X.; Meulia, T.; Paul, P.; Madden, L.V.; Li, D.; Qu, F. Random Plant Viral Variants Attain Temporal Advantages During Systemic Infections and in Turn Resist other Variants of the Same Virus. Sci. Rep. 2015, 5, 15346. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.P.; Rodríguez-Negrete, E.A.; Morilla, G.; Wang, L.; Lozano-Durán, R.; Castillo, A.G.; Bejarano, E.R. V2 from a curtovirus is a suppressor of post-transcriptional gene silencing. J. Gen. Virol. 2017, 98, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.V.; Sahu, P.P.; Prasad, M.; Praveen, S.; Pappu, H.R. Geminiviruses and Plant Hosts: A Closer Examination of the Molecular Arms Race. Viruses 2017, 9, 256. [Google Scholar] [CrossRef]
- Parent, J.-S.; Jauvion, V.; Bouché, N.; Béclin, C.; Hachet, M.; Zytnicki, M.; Vaucheret, H. Post-transcriptional gene silencing triggered by sense transgenes involves uncapped antisense RNA and differs from silencing intentionally triggered by antisense transgenes. Nucleic Acids Res. 2015, 43, 8464–8475. [Google Scholar] [CrossRef]
- Voinnet, O. Revisiting small RNA movement in plants. Nat. Rev. Mol. Cell Biol. 2022, 23, 163–164. [Google Scholar] [CrossRef]
- Kasschau, K.D.; Carrington, J.C. A Counterdefensive Strategy of Plant Viruses: Suppression of Posttranscriptional Gene Silencing. Cell 1998, 95, 461–470. [Google Scholar] [CrossRef]
- Rodríguez-Negrete, E.; Lozano-Durán, R.; Piedra-Aguilera, A.; Cruzado, L.; Bejarano, E.R.; Castillo, A.G. Geminivirus Rep protein interferes with the plant DNA methylation machinery and suppresses transcriptional gene silencing. New Phytol. 2013, 199, 464–475. [Google Scholar] [CrossRef]
- Jones, L.; Ratcliff, F.; Baulcombe, D.C. RNA-directed transcriptional gene silencing in plants can be inherited independently of the RNA trigger and requires Met1 for maintenance. Curr. Biol. 2001, 11, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Bahari, A.; Castillo, A.G.; Safaie, N.; Bejarano, E.R.; Luna, A.P.; Shams-Bakhsh, M. Functional Analysis of V2 Protein of Beet Curly Top Iran Virus. Plants 2022, 11, 3351. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ikegami, M.; Kon, T. Identification of the virulence factors and suppressors of posttranscriptional gene silencing encoded by Ageratum yellow vein virus, a monopartite begomovirus. Virus Res. 2010, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Harmoko, R.; Fanata WI, D.; Yoo, J.Y.; Ko, K.S.; Rim, Y.G.; Uddin, M.N.; Siswoyo, T.A.; Lee, S.S.; Kim, D.Y.; Lee, S.Y.; et al. RNA-Dependent RNA Polymerase 6 Is Required for Efficient hpRNA-Induced Gene Silencing in Plants. Mol. Cells 2013, 35, 202. [Google Scholar] [CrossRef]
- Vaistij, F.E.; Jones, L. Compromised Virus-Induced Gene Silencing in RDR6-Deficient Plants. Plant Physiol. 2009, 149, 1399–1407. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Mourrain, P.; Béclin, C.; Elmayan, T.; Feuerbach, F.; Godon, C.; Morel, J.-B.; Jouette, D.; Lacombe, A.-M.; Nikic, S.; Picault, N.; et al. Arabidopsis SGS2 and SGS3 Genes Are Required for Posttranscriptional Gene Silencing and Natural Virus Resistance. Cell 2000, 101, 533–542. [Google Scholar] [CrossRef]
- Taochy, C.; Yu, A.; Bouché, N.; Bouteiller, N.; Elmayan, T.; Dressel, U.; Carroll, B.J.; Vaucheret, H. Post-transcriptional gene silencing triggers dispensable DNA methylation in gene body in Arabidopsis. Nucleic Acids Res. 2019, 47, 9104–9114. [Google Scholar] [CrossRef]
- Martín-Hernández, A.M.; Baulcombe, D.C. Tobacco Rattle Virus 16-Kilodalton Protein Encodes a Suppressor of RNA Silencing That Allows Transient Viral Entry in Meristems. J. Virol. 2008, 82, 4064–4071. [Google Scholar] [CrossRef]
- Vargason, J.M.; Szittya, G.; Burgyán, J.; Hall, T.M. Size Selective Recognition of siRNA by an RNA Silencing Suppressor. Cell 2003, 115, 799–811. [Google Scholar] [CrossRef]
- Silhavy, D.; Molnár, A.; Lucioli, A.; Szittya, G.; Hornyik, C.; Tavazza, M.; Burgyán, J. A viral protein suppresses RNA silencing and binds silencing-generated, 21- to 25-nucleotide double-stranded RNAs. EMBO J. 2002, 21, 3070–3080. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, S.; Eini, O.; Baßler, A.; Hanke, A.; Yildirim, Z.; Wassenegger, M.; Krczal, G.; Uslu, V.V. Beet Curly Top Iran Virus Rep and V2 Suppress Post-Transcriptional Gene Silencing via Distinct Modes of Action. Viruses 2023, 15, 1996. https://doi.org/10.3390/v15101996
Ebrahimi S, Eini O, Baßler A, Hanke A, Yildirim Z, Wassenegger M, Krczal G, Uslu VV. Beet Curly Top Iran Virus Rep and V2 Suppress Post-Transcriptional Gene Silencing via Distinct Modes of Action. Viruses. 2023; 15(10):1996. https://doi.org/10.3390/v15101996
Chicago/Turabian StyleEbrahimi, Saeideh, Omid Eini, Alexandra Baßler, Arvid Hanke, Zeynep Yildirim, Michael Wassenegger, Gabi Krczal, and Veli Vural Uslu. 2023. "Beet Curly Top Iran Virus Rep and V2 Suppress Post-Transcriptional Gene Silencing via Distinct Modes of Action" Viruses 15, no. 10: 1996. https://doi.org/10.3390/v15101996






