A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell, Virus, and Plaque Assay
2.2. Mouse Strains
2.3. MHV-3 and SARS-CoV-2 Infections
2.4. Treatment with Zi
2.5. Tissue Collection
2.6. Hematological Evaluation
2.7. Histopathology
2.8. Cytokine Assay
2.9. Respiratory Mechanics
2.10. ECG Recording
2.11. Flow Cytometry
2.12. Statistical Analysis
3. Results
3.1. Treatment with Zi Improves Survival Rates of MHV-3-Infected Mice Regardless a Control of Viremia
3.2. Zi Treatment Prevent Lung Tissue Damage Regardless of Viral Load Control
3.3. Zi Reduces the Severity of Cardiopulmonary Complications during MHV-3 Infection
3.4. Zi Treatment Was Associated with an Increased Expansion of IL-10 Producing Neutrophils in the Lungs of MHV-3 Infected-Mice
3.5. Treatment with Zi Promotes the Increasing Treg Cells Producing IL-10 into Lung Tissue during MHV-3 Infection
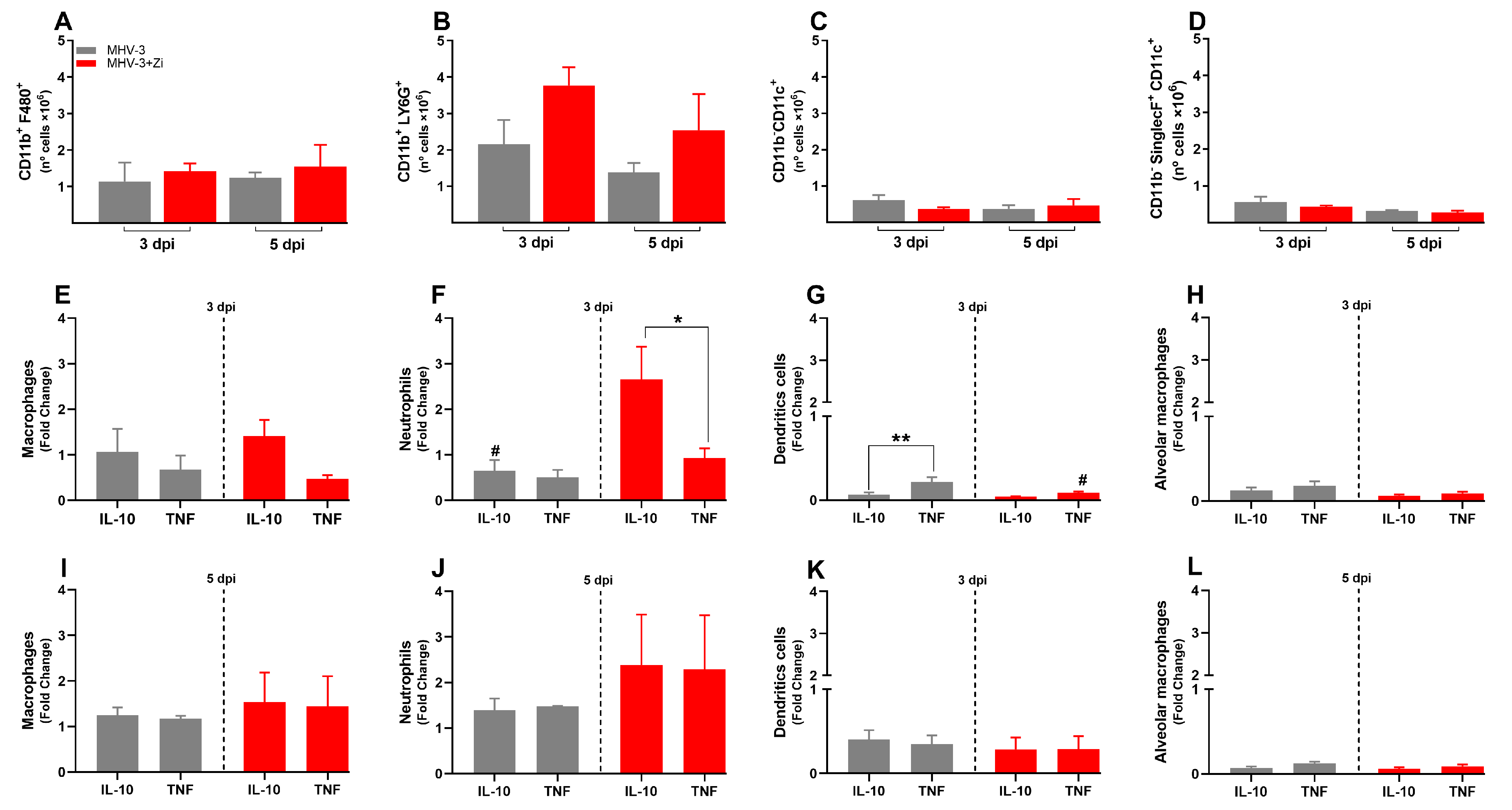
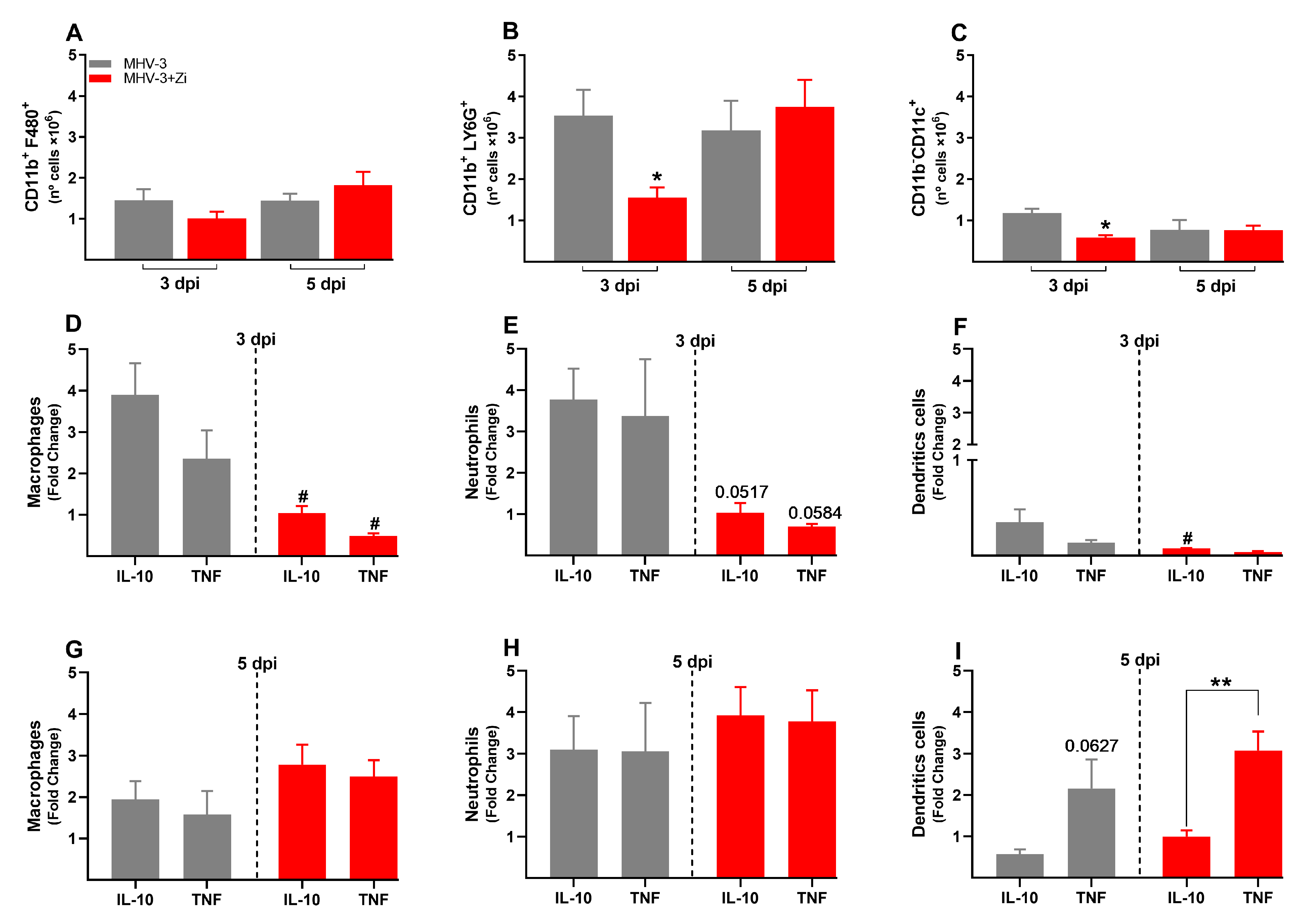
3.6. Zi Treatment Reduced the Number of Innate Immune Cells in the Spleen during MHV-3 Infection
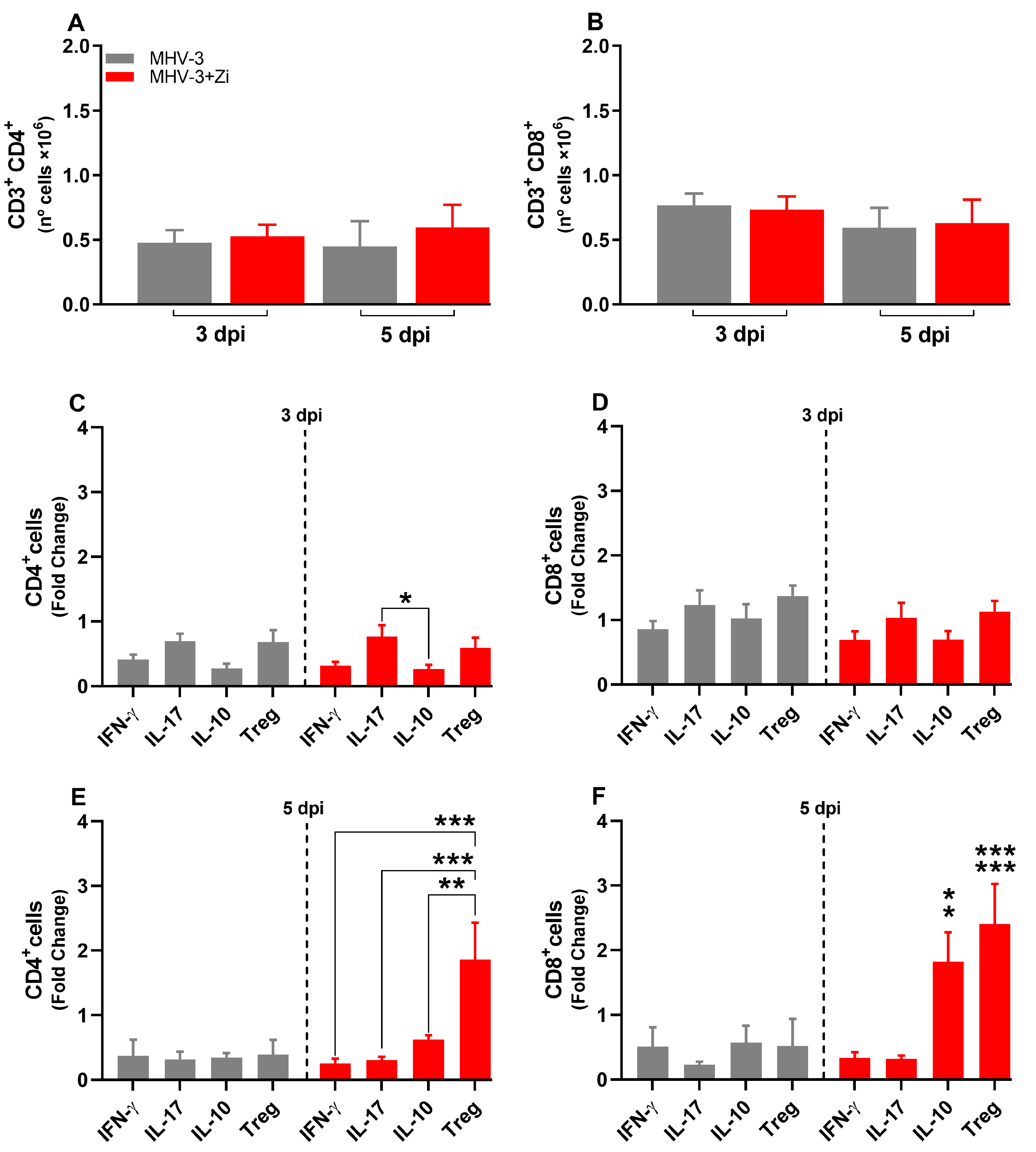
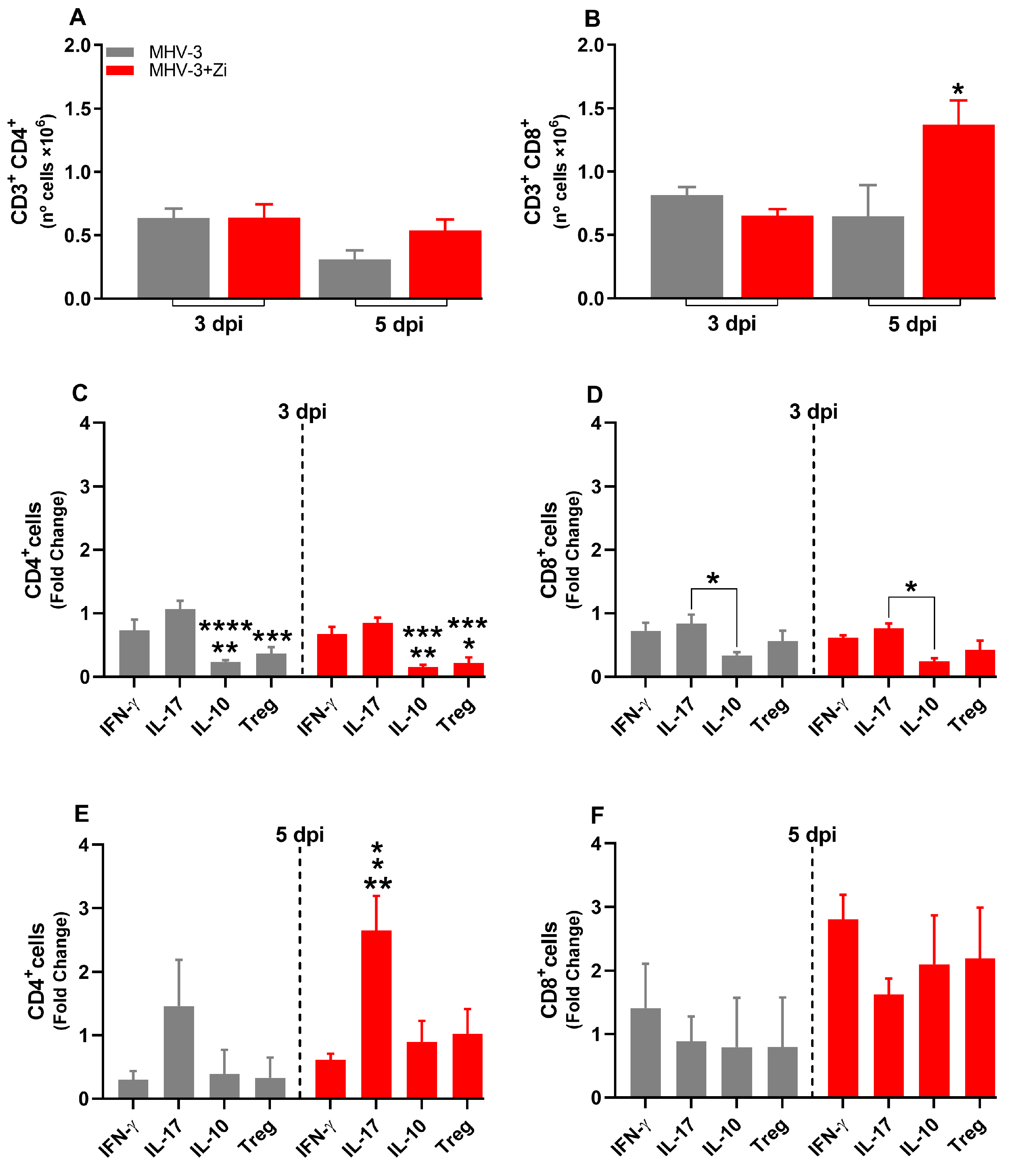
3.7. Zi Treatment Modulates the Expansion/Generation of T Cells in the Spleen during MHV-3 Infection
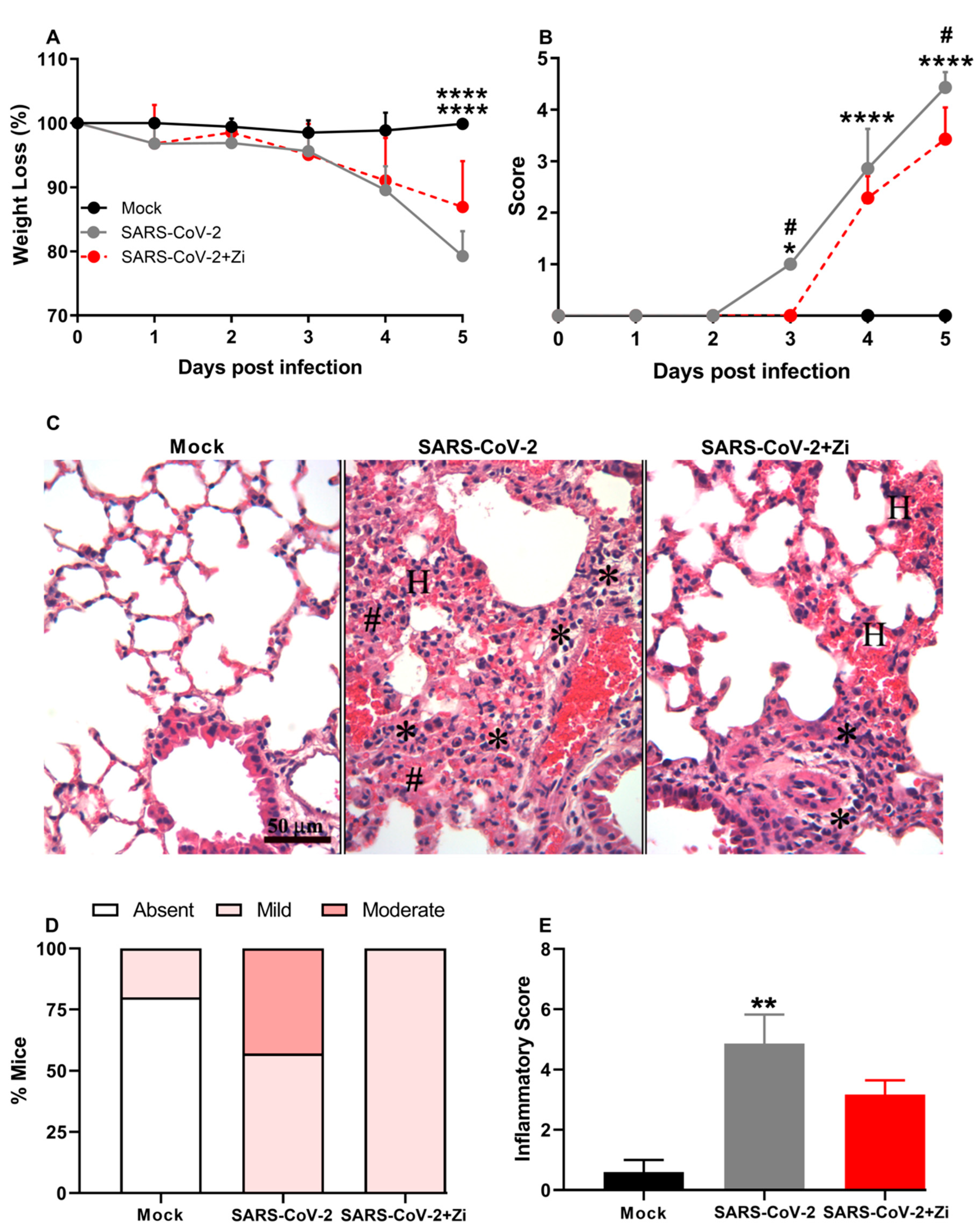
3.8. Zi Improves Clinical Parameters and Protects hK18ACE2 Mice from Lung Injury Induced by SARS-CoV-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Arish, M.; Qian, W.; Narasimhan, H.; Sun, J. COVID-19 immunopathology: From acute diseases to chronic sequelae. J. Med. Virol. 2023, 95, e28122. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Kalle, A.M.; Reddanna, P. Managing SARS-CoV2 Infections through Resolution of Inflammation by Eicosanoids: A Review. J. Inflamm. Res. 2022, 15, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Wang, R.; Feng, Z.; Zhang, J.; Yang, H.; Tan, Y.; Wang, H.; Wang, C.; Liu, L.; et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 2021, 12, 2506. [Google Scholar] [CrossRef]
- Citron, F.; Perelli, L.; Deem, A.K.; Genovese, G.; Viale, A. Leukotrienes, a potential target for COVID-19. Prostaglandins Leukot Essent Fat. Acids 2020, 161, 102174. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- Yan, B.; Chu, H.; Yang, D.; Sze, K.-H.; Lai, P.-M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.-T.; Chan, J.F.-W.; et al. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses 2019, 11, 73. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2021, 52, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- AAyola-Serrano, N.C.; Roy, N.; Fathah, Z.; Anwar, M.M.; Singh, B.; Ammar, N.; Sah, R.; Elba, A.; Utt, R.S.; Pecho-Silva, S.; et al. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflamm. Res. 2021, 70, 877–889. [Google Scholar] [CrossRef] [PubMed]
- McGill, K.A.; Busse, W.W. Zileuton. Lancet 1996, 348, 519–524. [Google Scholar] [CrossRef]
- Hammock, B.D.; Wang, W.; Gilligan, M.M.; Panigrahy, D. Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? Am. J. Pathol. 2020, 190, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.D.S.P.; Campolina-Silva, G.H.; Queiroz-Junior, C.M.; de Oliveira, L.C.; Lacerda, L.D.S.B.; Gaggino, J.C.P.; de Souza, F.R.O.; de Meira Chaves, I.; Passos, I.B.; Teixeira, D.C. A Biosafety Level 2 Mouse Model for Studying Betacoronavirus-Induced Acute Lung Damage and Systemic Manifestations. J. Virol. 2021, 95, e0127621. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; de Moraes, A.P.; Rodrigues, D.M.; Gilioli, R.; de Oliveira-Filho, E.F.; Durães-Carvalho, R.; Arns, C.W. Coding-Complete Genome Sequence of Murine Hepatitis Virus Strain 3 from Brazil. Microbiol. Resour. Announc. 2021, 10, e00248-21. [Google Scholar] [CrossRef]
- Horvat, J.C.; Beagley, K.W.; Wade, M.A.; Preston, J.A.; Hansbro, N.G.; Hickey, D.K.; Kaiko, G.E.; Gibson, P.G.; Foster, P.S.; Hansbro, P.M. Neonatal chlamydial infection induces mixed T-cell responses that drive allergic airway disease. Am. J. Respir. Crit. Care Med. 2007, 176, 556–564. [Google Scholar] [CrossRef]
- Limjunyawong, N.; Fallica, J.; Horton, M.R.; Mitzner, W. Measurement of the Pressure-volume Curve in Mouse Lungs. J. Vis. Exp. 2015, 95, 52376. [Google Scholar]
- Robichaud, A.; Fereydoonzad, L.; Limjunyawong, N.; Rabold, R.; Allard, B.; Benedetti, A.; Martin, J.G.; Mitzner, W. Automated full-range pressure-volume curves in mice and rats. J. Appl. Physiol. 2017, 123, 746–756. [Google Scholar] [CrossRef]
- Soutiere, S.E.; Mitzner, W. On defining total lung capacity in the mouse. J. Appl. Physiol. 2004, 96, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Shintaku, T.; Ohba, T.; Niwa, H.; Kushikata, T.; Hirota, K.; Ono, K.; Matsuzaki, Y.; Imaizumi, T.; Kuwasako, K.; Sawamura, D.; et al. Effects of Propofol on Electrocardiogram Measures in Mice. J. Pharmacol. Sci. 2014, 126, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.; de Oliveira, M.S.; Soto-Blanco, B.; Melo, M.M. Computerized electrocardiography in healthy conscious guinea pigs (Cavia porcellus). Pesq. Vet. Bras. 2016, 36, 1203–1208. [Google Scholar] [CrossRef]
- Brant, F.; Miranda, A.S.; Esper, L.; Rodrigues, D.H.; Kangussu, L.M.; Bonaventura, D.; Soriani, F.M.; Pinho, V.; Souza, D.G.; Rachid, M.A.; et al. Role of the Aryl Hydrocarbon Receptor in the Immune Response Profile and Development of Pathology during Plasmodium berghei Anka Infection. Infect. Immun. 2014, 82, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Claser, C.; Nguee, S.Y.T.; Balachander, A.; Wu Howland, S.; Becht, E.; Gunasegaran, B.; Hartimath, S.V.; Lee, A.W.; Theng Theng Ho, J.; Bing Ong, C.; et al. Lung endothelial cell antigen cross-presentation to CD8+T cells drives malaria-associated lung injury. Nat. Commun. 2019, 10, 4241. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, F.H.G.; Ayala, T.S.; Martins, J.O. Lipid mediators are critical in resolving inflammation: A review of the emerging roles of eicosanoids in diabetes mellitus. BioMed. Res. Int. 2015, 2015, 568408. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.B.; Rothstein, J.D. Inhibition of cyclooxygenase-2 protects motor neurons in an organotypic model of amyotrophic lateral sclerosis. Ann. Neurol. 2000, 48, 792–795. [Google Scholar] [CrossRef]
- Brock, T.G.; Peters-Golden, M. Activation and regulation of cellular eicosanoid biosynthesis. Sci. World J. 2007, 7, 1273–1284. [Google Scholar] [CrossRef]
- Hoxha, M. What about COVID-19 and arachidonic acid pathway? Eur. J. Clin. Pharmacol. 2020, 76, 1501–1504. [Google Scholar] [CrossRef]
- Luster, A.D.; Tager, A.M. T-cell trafficking in asthma: Lipid mediators grease the way. Nat. Rev. Immunol. 2004, 4, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, T.; Izumi, T.; Shimizu, T. Leukotriene B4: Metabolism and signal transduction. Arch. Biochem. Biophys. 2001, 385, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Chambers, R.C. The mercurial nature of neutrophils: Still an enigma in ARDS? Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L217–L230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef]
- Dhawan, M.; Rabaan, A.A.; Fawarah, M.M.A.; Almuthree, S.A.; Alsubki, R.A.; Alfaraj, A.H.; Mashraqi, M.M.; Alshamrani, S.A.; Abduljabbar, W.A.; Alwashmi, A.S.; et al. Updated Insights into the T Cell-Mediated Immune Response against SARS-CoV-2: A Step towards Efficient and Reliable Vaccines. Vaccines 2023, 11, 101. [Google Scholar] [CrossRef]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Chia, W.N.; Tham, C.Y.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef]
- Grau-Expósito, J.; Sánchez-Gaona, N.; Massana, N.; Suppi, M.; Astorga-Gamaza, A.; Perea, D.; Rosado, J.; Falcó, A.; Kirkegaard, C.; Torrella, A.; et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Mortola, J.P. How to breathe? Respiratory mechanics and breathing pattern. Respir. Physiol. Neurobiol. 2019, 261, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.A.; Russo, A.M.; Chung, M.K.; Deering, T.F.; Lakkireddy, D.; Gopinathannair, R. COVID-19 and Cardiac Arrhythmias: A Contemporary Review. Curr. Treat. Options Cardiovasc. Med. 2022, 24, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetité, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef] [PubMed]
- Panda, R.; Castanheira, F.V.; Schlechte, J.M.; Surewaard, B.G.; Shim, H.B.; Zucoloto, A.Z.; Slavikova, Z.; Yipp, B.G.; Kubes, P.; McDonald, B. A functionally distinct neutrophil landscape in severe COVID-19 reveals opportunities for adjunctive therapies. JCI Insight 2022, 7, e152291. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Wong, P.; Pamer, E.G. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 2003, 21, 29–70. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Ribeiro, L.R.; Lima, K.V.B.; Lima, L.N.G.C. Adaptive immunity to SARS-CoV-2 infection: A systematic review. Front. Immunol. 2022, 13, 1001198. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.d.D.; Rabelo, R.A.N.; Oliveira, N.F.d.M.; Porto, S.L.T.; Andrade, A.C.d.S.P.; Queiroz-Junior, C.M.; Barbosa, C.L.N.; de Souza-Costa, L.P.; Santos, F.R.d.S.; Oliveira, F.B.R.; et al. A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus. Viruses 2023, 15, 2049. https://doi.org/10.3390/v15102049
Pereira RdD, Rabelo RAN, Oliveira NFdM, Porto SLT, Andrade ACdSP, Queiroz-Junior CM, Barbosa CLN, de Souza-Costa LP, Santos FRdS, Oliveira FBR, et al. A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus. Viruses. 2023; 15(10):2049. https://doi.org/10.3390/v15102049
Chicago/Turabian StylePereira, Rafaela das Dores, Rayane Aparecida Nonato Rabelo, Natália Fernanda de Melo Oliveira, Samuel Luiz Teixeira Porto, Ana Claudia dos Santos Pereira Andrade, Celso M. Queiroz-Junior, César Luís Nascimento Barbosa, Luiz Pedro de Souza-Costa, Felipe Rocha da Silva Santos, Fernando Bento Rodrigues Oliveira, and et al. 2023. "A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus" Viruses 15, no. 10: 2049. https://doi.org/10.3390/v15102049
APA StylePereira, R. d. D., Rabelo, R. A. N., Oliveira, N. F. d. M., Porto, S. L. T., Andrade, A. C. d. S. P., Queiroz-Junior, C. M., Barbosa, C. L. N., de Souza-Costa, L. P., Santos, F. R. d. S., Oliveira, F. B. R., da Silva, B. L. V., Umezu, H. L., Ferreira, R., da Silva, G. S. F., Cruz, J. S., Teixeira, M. M., Costa, V. V., & Machado, F. S. (2023). A 5-Lipoxygenase Inhibitor, Zileuton, Modulates Host Immune Responses and Improves Lung Function in a Model of Severe Acute Respiratory Syndrome (SARS) Induced by Betacoronavirus. Viruses, 15(10), 2049. https://doi.org/10.3390/v15102049





