EBV Impact in Peripheral Macrophages’ Polarization Cytokines in Pediatric Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Immunohistochemistry
2.3. Real-Time PCRA
2.4. Flow Cytometry
2.5. Statistical Analysis
3. Results
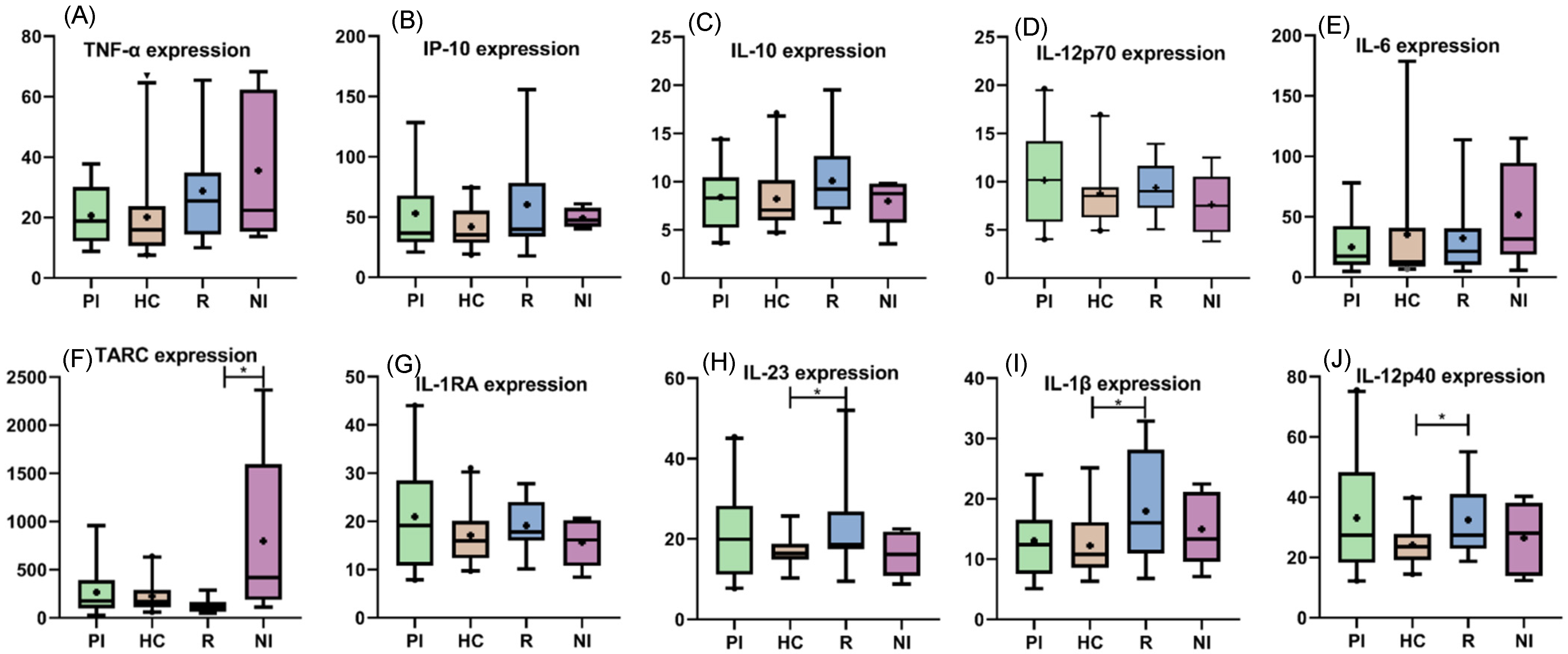
3.1. Peripheral Cytokines
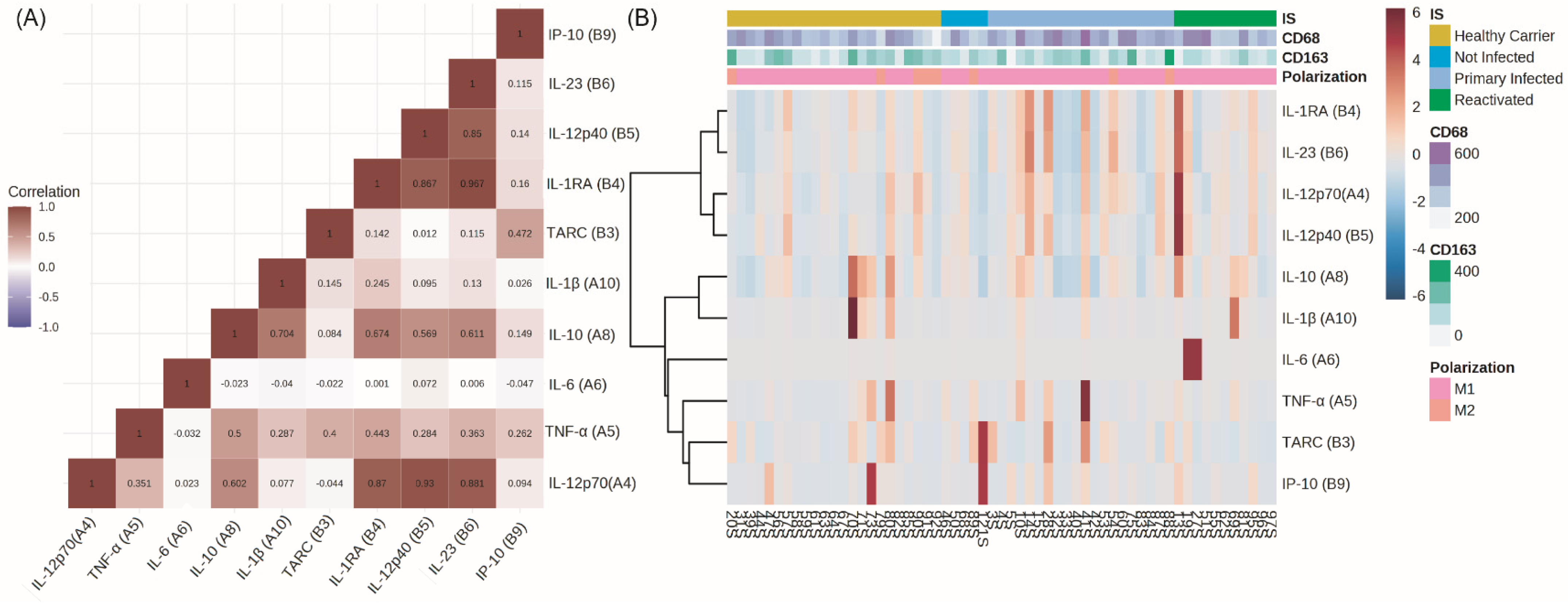
3.2. Peripheral and Tissue Cytokines
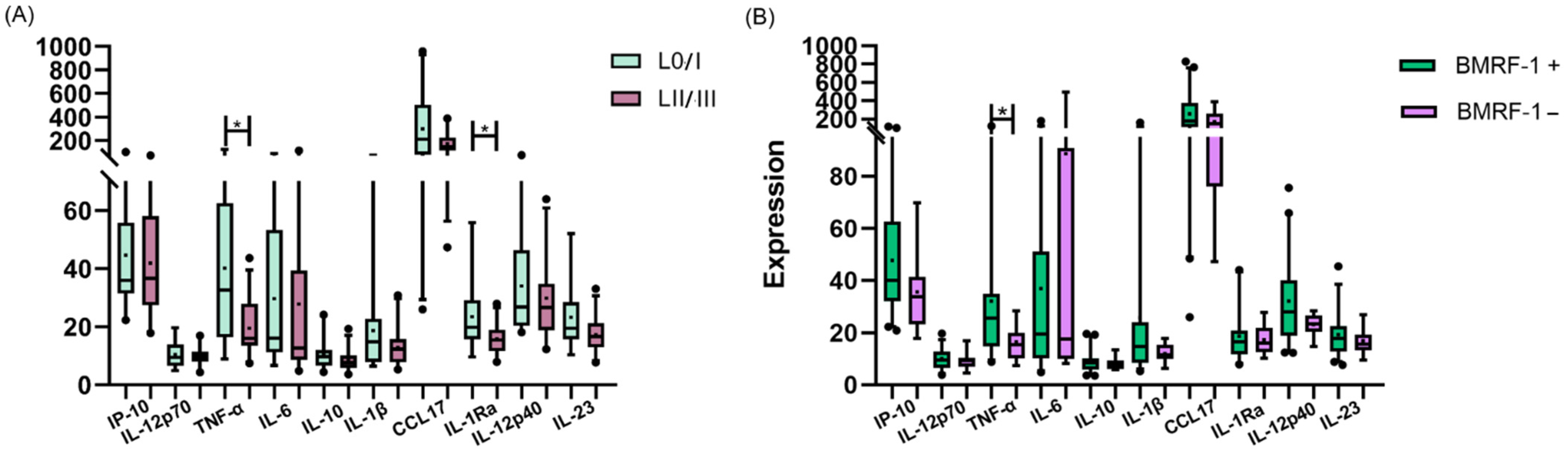
3.3. Peripheral Cytokines among EBV Infection Status
3.4. Peripheral Cytokines and Viral Protein Expression
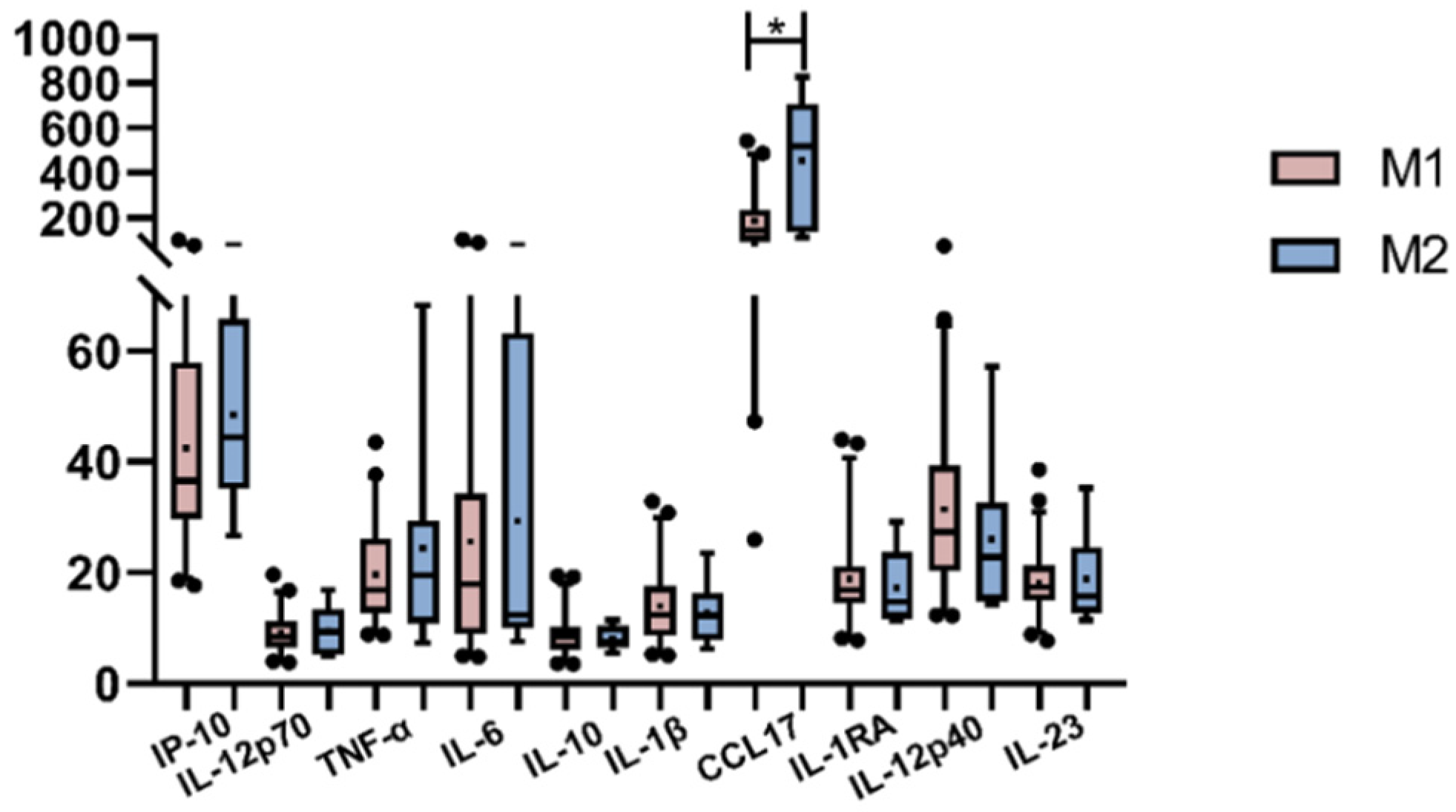
3.5. Peripheral Cytokines and Macrophages’ Polarization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The Interplay between Epstein-Barr Virus and B Lymphocytes: Implications for Infection, Immunity, and Disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, X.; Dai, R.; Li, Z.; Zhang, Y.; Xue, W.; Zhu, Y.; Feng, D.; Qin, L.; Wang, X.; et al. Phase Separation of Epstein-Barr Virus EBNA2 Protein Reorganizes Chromatin Topology for Epigenetic Regulation. Commun. Biol. 2021, 4, 967. [Google Scholar] [CrossRef] [PubMed]
- Raab-Traub, N. EBV-Induced Oncogenesis. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007; ISBN 9780521827140. [Google Scholar]
- Khan, G. Epstein-Barr Virus, Cytokines, and Inflammation: A Cocktail for the Pathogenesis of Hodgkin’s Lymphoma? Exp. Hematol. 2006, 34, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [PubMed]
- Chabay, P.A.; Preciado, M.V. EBV Primary Infection in Childhood and Its Relation to B-Cell Lymphoma Development: A Mini-Review from a Developing Region. Int. J. Cancer 2013, 133, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H., Jr. Primary Epstein-Barr Virus Infection. J. Clin. Virol. 2018, 102, 84–92. [Google Scholar] [CrossRef]
- Jayasooriya, S.; de Silva, T.I.; Njie-jobe, J.; Sanyang, C.; Leese, A.M.; Bell, A.I.; McAulay, K.A.; Yanchun, P.; Long, H.M.; Dong, T.; et al. Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children. PLoS Pathog. 2015, 11, e1004746. [Google Scholar]
- Hislop, A.D.; Taylor, G.S. T-Cell Responses to EBV. Curr. Top. Microbiol. Immunol. 2015, 391, 325–353. [Google Scholar]
- Azzi, T.; Lünemann, A.; Murer, A.; Ueda, S.; Béziat, V.; Malmberg, K.-J.; Staubli, G.; Gysin, C.; Berger, C.; Münz, C.; et al. Role for Early-Differentiated Natural Killer Cells in Infectious Mononucleosis. Blood 2014, 124, 2533–2543. [Google Scholar] [CrossRef]
- Chijioke, O.; Müller, A.; Feederle, R.; Barros, M.H.M.; Krieg, C.; Emmel, V.; Marcenaro, E.; Leung, C.S.; Antsiferova, O.; Landtwing, V.; et al. Human Natural Killer Cells Prevent Infectious Mononucleosis Features by Targeting Lytic Epstein-Barr Virus Infection. Cell Rep. 2013, 5, 1489–1498. [Google Scholar] [CrossRef]
- Lünemann, A.; Rowe, M.; Nadal, D. Innate Immune Recognition of EBV. Curr. Top. Microbiol. Immunol. 2015, 391, 265–287. [Google Scholar] [PubMed]
- Vistarop, A.G.; Cohen, M.; Huaman, F.; Irazu, L.; Rodriguez, M.; De Matteo, E.; Preciado, M.V.; Chabay, P.A. The Interplay between Local Immune Response and Epstein-Barr Virus-Infected Tonsillar Cells Could Lead to Viral Infection Control. Med. Microbiol. Immunol. 2018, 207, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Moyano, A.; Ferressini Gerpe, N.M.; De Matteo, E.; Preciado, M.V.; Chabay, P. M1 Macrophage Polarization Prevails in Epstein-Barr Virus-Infected Children in an Immunoregulatory Environment. J. Virol. 2022, 96, e0143421. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A.; Martinez Estrada, F. Macrophage Heterogeneity in Tissues: Phenotypic Diversity and Functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar]
- Cassetta, L.; Cassol, E.; Poli, G. Macrophage Polarization in Health and Disease. Sci. World J. 2011, 11, 2391–2402. [Google Scholar] [CrossRef]
- Fendl, B.; Berghoff, A.S.; Preusser, M.; Maier, B. Macrophage and Monocyte Subsets as New Therapeutic Targets in Cancer Immunotherapy. ESMO Open 2023, 8, 100776. [Google Scholar]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-Associated Macrophages Are a Distinct M2 Polarised Population Promoting Tumour Progression: Potential Targets of Anti-Cancer Therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef]
- Sarkar, T.; Dhar, S.; Chakraborty, D.; Pati, S.; Bose, S.; Panda, A.K.; Basak, U.; Chakraborty, S.; Mukherjee, S.; Guin, A.; et al. FOXP3/HAT1 Axis Controls Treg Infiltration in the Tumor Microenvironment by Inducing CCR4 Expression in Breast Cancer. Front. Immunol. 2022, 13, 740588. [Google Scholar] [CrossRef]
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological Analysis Reveals a PD-L1-Associated Microenvironmental Niche for Reed-Sternberg Cells in Hodgkin Lymphoma. Blood 2017, 130, 2420–2430. [Google Scholar] [CrossRef]
- Moyano, A.; Ferressini, N.; De Matteo, E.; Preciado, M.V.; Chabay, P. PD-L1 Is Upregulated in CD163+ Tonsillar Macrophages from Children Undergoing EBV Primary Infection. Front. Immunol. 2022, 13, 940910. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. Natural Killer Cell Responses to Human Oncogenic γ-Herpesvirus Infections. Semin. Immunol. 2022, 60, 101652. [Google Scholar] [CrossRef]
- Ferressini Gerpe, N.M.; Vistarop, A.G.; Moyano, A.; De Matteo, E.; Preciado, M.V.; Chabay, P.A. Distinctive EBV Infection Characteristics in Children from a Developing Country. Int. J. Infect. Dis. 2020, 93, 139–145. [Google Scholar] [CrossRef]
- Edholm, E.-S.; Rhoo, K.H.; Robert, J. Evolutionary Aspects of Macrophages Polarization. Results Probl. Cell Differ. 2017, 62, 3–22. [Google Scholar]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.H.M.; Hassan, R.; Niedobitek, G. Tumor-Associated Macrophages in Pediatric Classical Hodgkin Lymphoma: Association with Epstein-Barr Virus, Lymphocyte Subsets, and Prognostic Impact. Clin. Cancer Res. 2012, 18, 3762–3771. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, O.; Barros, M.H.; De Matteo, E.; Garcia Lombardi, M.; Preciado, M.V.; Niedobitek, G.; Chabay, P. M1-like Macrophage Polarization Prevails in Young Children with Classic Hodgkin Lymphoma from Argentina. Sci. Rep. 2019, 9, 12687. [Google Scholar] [CrossRef]
- Hsi, E.D.; Li, H.; Nixon, A.B.; Schöder, H.; Bartlett, N.L.; LeBlanc, M.; Smith, S.; Kahl, B.S.; Leonard, J.P.; Evens, A.M.; et al. Serum Levels of TARC, MDC, IL-10, and Soluble CD163 in Hodgkin Lymphoma: A SWOG S0816 Correlative Study. Blood 2019, 133, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Odumade, O.A.; Porter, J.L.; Reyes-Genere, J.; Schmeling, D.O.; Bilgic, H.; Fan, D.; Baechler, E.C.; Balfour, H.H., Jr.; Hogquist, K.A. Primary EBV Infection Induces an Expression Profile Distinct from Other Viruses but Similar to Hemophagocytic Syndromes. PLoS ONE 2014, 9, e85422. [Google Scholar] [CrossRef] [PubMed]
- Budiningsih, I.; Dachlan, Y.P.; Hadi, U.; Middeldorp, J.M. Quantitative Cytokine Level of TNF-α, IFN-γ, IL-10, TGF-β and Circulating Epstein-Barr Virus DNA Load in Individuals with Acute Malaria due to P. falciparum or P. vivax or Double Infection in a Malaria Endemic Region in Indonesia. PLoS ONE 2021, 16, e0261923. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano, A.; Ferressini Gerpe, N.; Amarillo, M.E.; De Matteo, E.; Preciado, M.V.; Caldirola, M.S.; Chabay, P. EBV Impact in Peripheral Macrophages’ Polarization Cytokines in Pediatric Patients. Viruses 2023, 15, 2105. https://doi.org/10.3390/v15102105
Moyano A, Ferressini Gerpe N, Amarillo ME, De Matteo E, Preciado MV, Caldirola MS, Chabay P. EBV Impact in Peripheral Macrophages’ Polarization Cytokines in Pediatric Patients. Viruses. 2023; 15(10):2105. https://doi.org/10.3390/v15102105
Chicago/Turabian StyleMoyano, Agustina, Natalia Ferressini Gerpe, Maria Eugenia Amarillo, Elena De Matteo, Maria Victoria Preciado, Maria Soledad Caldirola, and Paola Chabay. 2023. "EBV Impact in Peripheral Macrophages’ Polarization Cytokines in Pediatric Patients" Viruses 15, no. 10: 2105. https://doi.org/10.3390/v15102105
APA StyleMoyano, A., Ferressini Gerpe, N., Amarillo, M. E., De Matteo, E., Preciado, M. V., Caldirola, M. S., & Chabay, P. (2023). EBV Impact in Peripheral Macrophages’ Polarization Cytokines in Pediatric Patients. Viruses, 15(10), 2105. https://doi.org/10.3390/v15102105







