Vaccination and Antiviral Treatment Reduce the Time to Negative SARS-CoV-2 Swab: A Real-Life Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Management
2.2. Endpoints
2.3. Data Collection
2.4. SARS-CoV-2 Detection in Respiratory Specimens
2.5. Ethics
2.6. Statistical Analysis
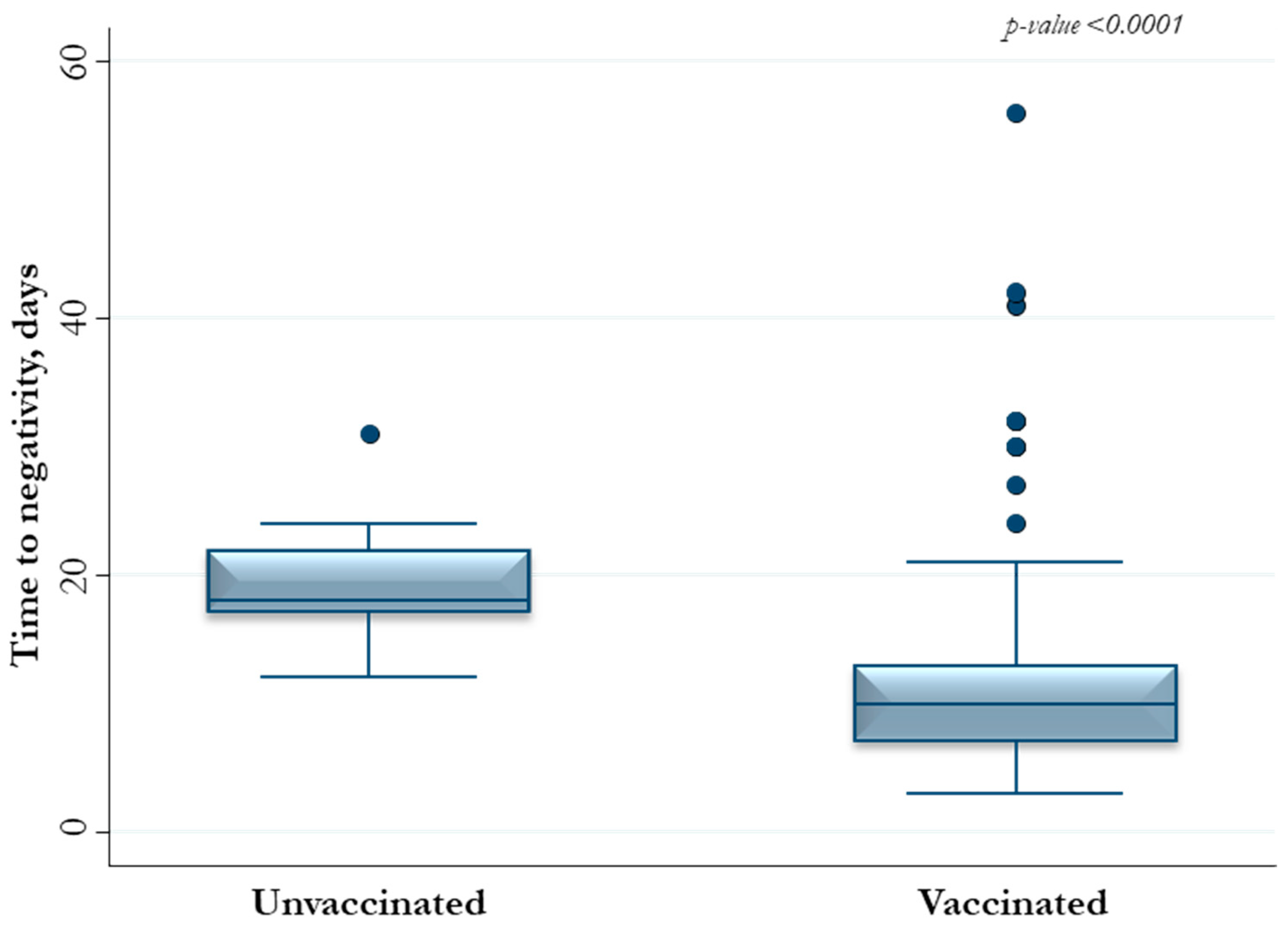
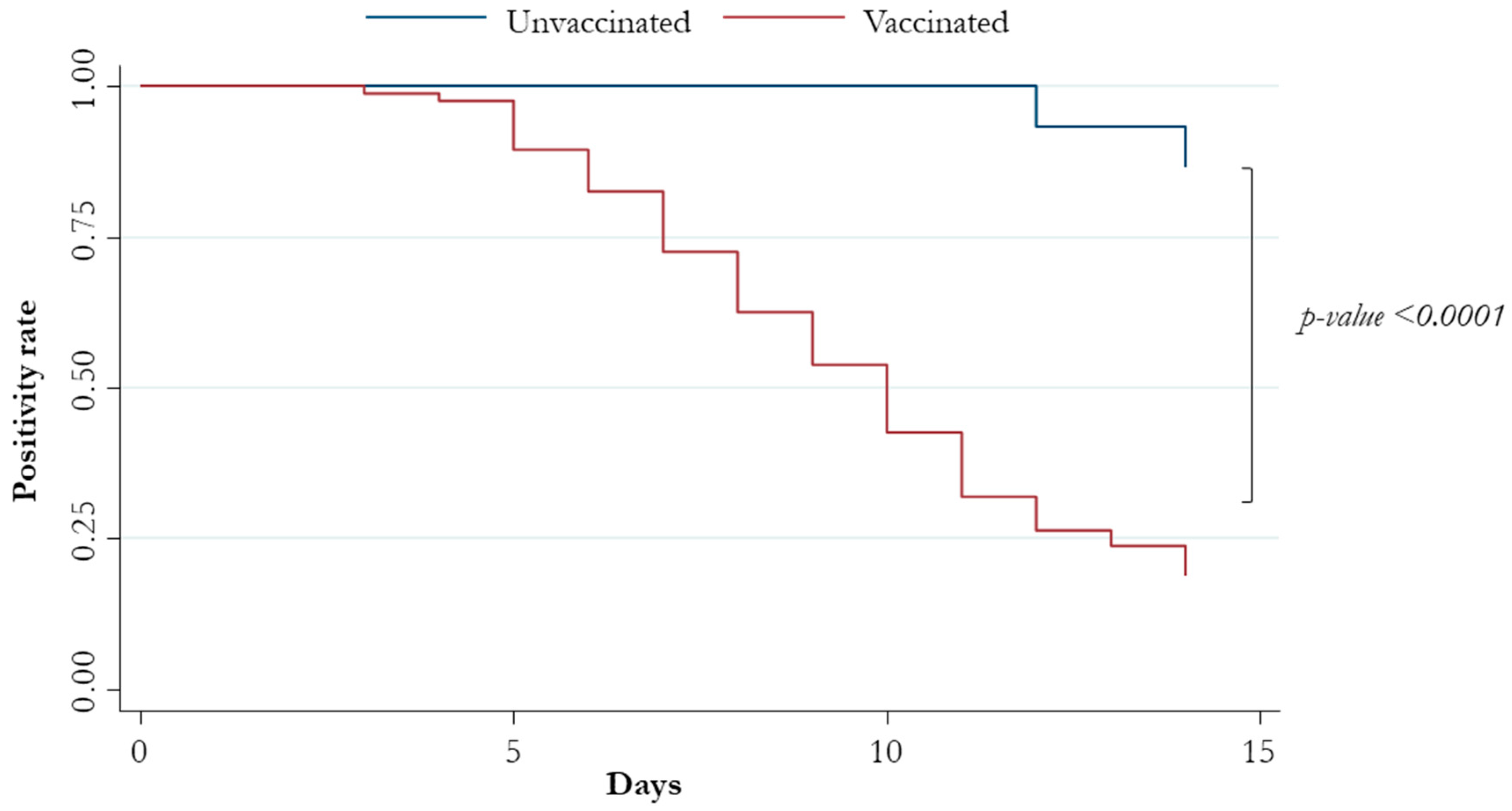
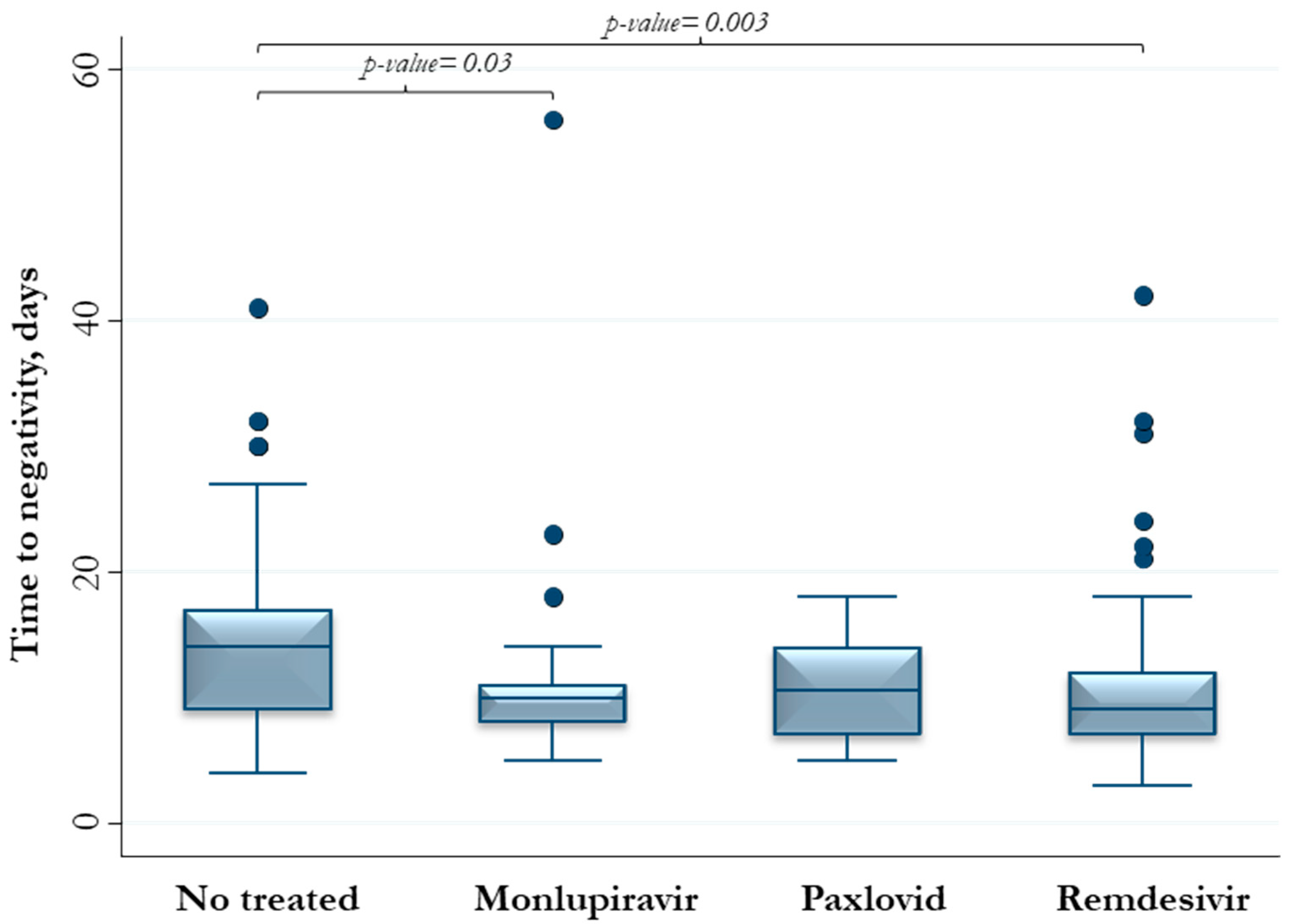
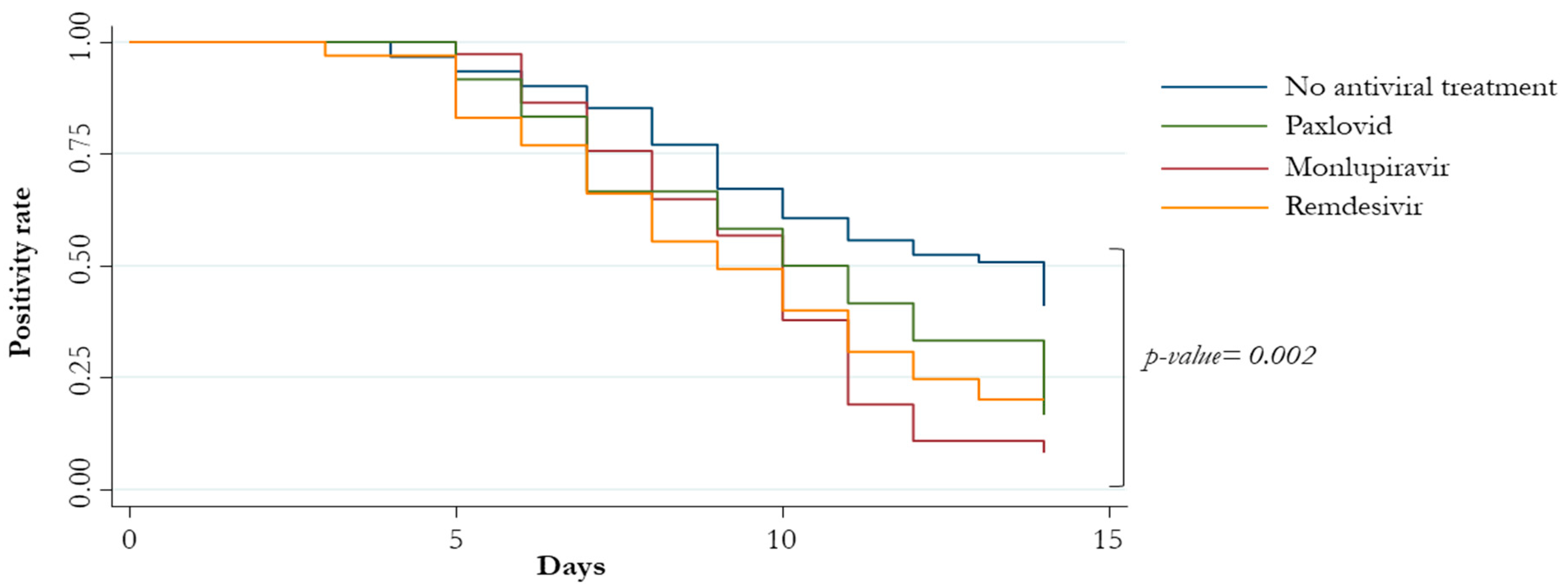
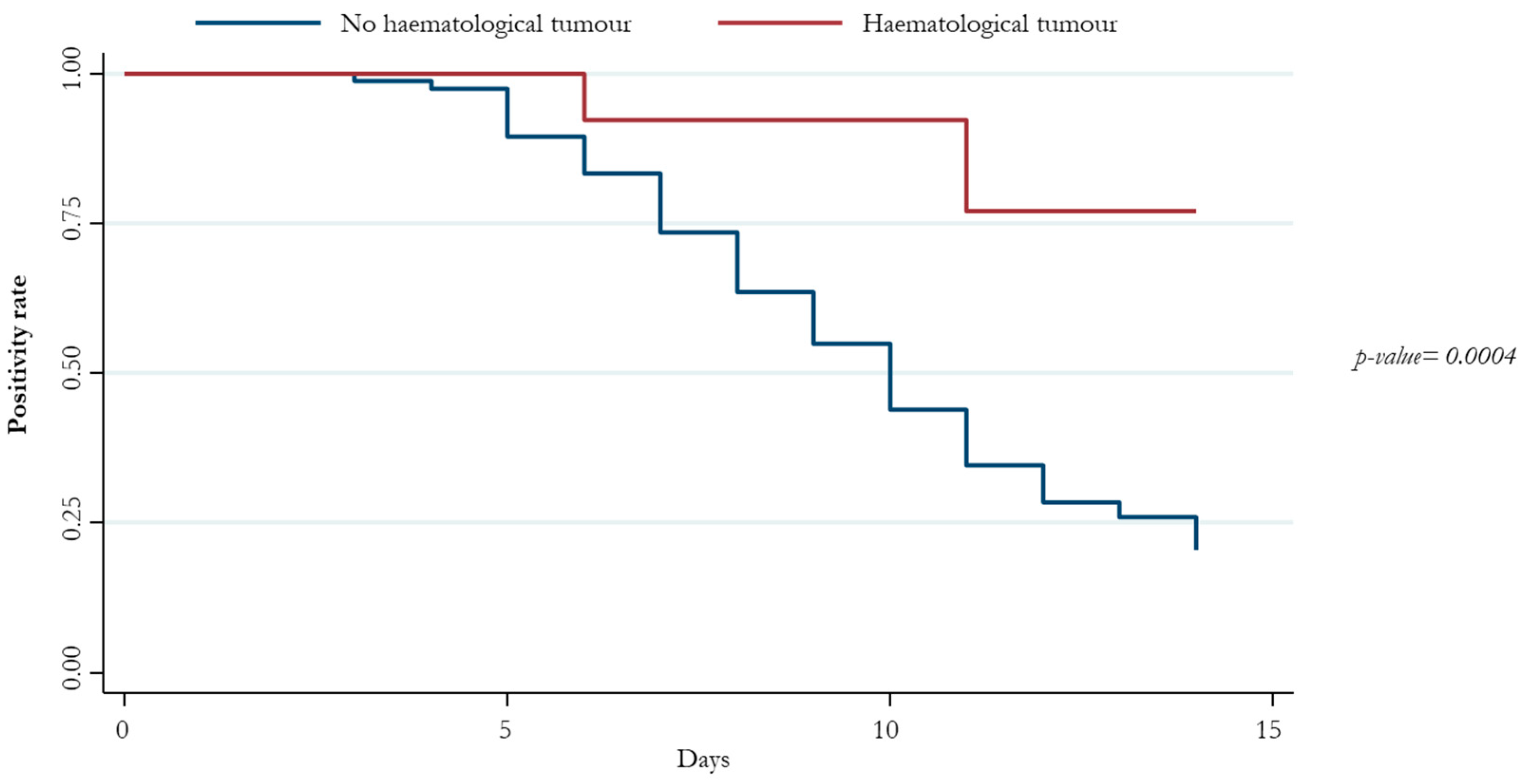
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ezzikouri, S.; Nourlil, J.; Benjelloun, S.; Kohara, M.; Tsukiyama-Kohara, K. Coronavirus Disease 2019—Historical Context, Virology, Pathogenesis, Immunotherapy, and Vaccine Development. Hum. Vaccines Immunother. 2020, 16, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Fiore, V.; Princic, E.; Geremia, N.; Panu Napodano, C.M.; Muredda, A.A.; Maida, I.; Madeddu, G.; Babudieri, S. Predictors of Infection, Symptoms Development, and Mortality in People with SARS-CoV-2 Living in Retirement Nursing Homes. PLoS ONE 2021, 16, e0248009. [Google Scholar] [CrossRef] [PubMed]
- Geremia, N.; De Vito, A.; Gunnella, S.; Fiore, V.; Princic, E.; Panu Napodano, C.; Madeddu, G.; Babudieri, S. A Case of Vasculitis-Like Skin Eruption Associated With COVID-19. Infect. Dis. Clin. Pract. 2020, 28, e30–e31. [Google Scholar] [CrossRef]
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The Prevalence of Symptoms in 24,410 Adults Infected by the Novel Coronavirus (SARS-CoV-2; COVID-19): A Systematic Review and Meta-Analysis of 148 Studies from 9 Countries. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; De Vito, A.; Deiana, G.; Pes, C.; Giovanditto, F.; Fiore, V.; Lechien, J.R.; Saussez, S.; Policicchio, D.; Boccaletti, R.; et al. Systemic Inflammatory Markers and Psychophysical Olfactory Scores in Coronavirus Disease 2019 Patients: Is There Any Correlation? J. Laryngol. Otol. 2021, 135, 723–728. [Google Scholar] [CrossRef]
- Vaira, L.A.; De Vito, A.; Deiana, G.; Pes, C.; Giovanditto, F.; Fiore, V.; Lechien, J.R.; Le Bon, S.D.; Saussez, S.; Madeddu, G.; et al. Correlations between IL-6 Serum Level and Olfactory Dysfunction Severity in COVID-19 Patients: A Preliminary Study. Eur. Arch Otorhinolaryngol. 2022, 279, 811–816. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Casirivimab and Imdevimab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2022, 399, 665–676. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Colpani, A.; Saderi, L.; Puci, M.; Zauli, B.; Fiore, V.; Fois, M.; Meloni, M.C.; Bitti, A.; Di Castri, C.; et al. Impact of Early SARS-CoV-2 Antiviral Therapy on Disease Progression. Viruses 2022, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Colpani, A.; Bitti, A.; Zauli, B.; Meloni, M.C.; Fois, M.; Denti, L.; Bacciu, S.; Marcia, C.; Maida, I.; et al. Safety and Efficacy of Molnupiravir in SARS-CoV-2 Infected Patients: A Real-Life Experience. J. Med. Virol. 2022, 94, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Mengato, D.; Sasset, L.; Ferrari, A.; Gardin, S.; Scaglione, V.; Bonadiman, N.; Calandrino, L.; Cavinato, S.; Trivellato, S.; et al. Molnupiravir and Nirmatrelvir/Ritonavir: Tolerability, Safety, and Adherence in a Retrospective Cohort Study. Viruses 2023, 15, 384. [Google Scholar] [CrossRef]
- De Vito, A.; Colpani, A.; Poliseno, M.; Diella, L.; Ieva, F.R.P.; Belati, A.; Papale, R.; Babudieri, S.; De Santis, L.; Saracino, A.; et al. What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study. Viruses 2023, 15, 1757. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin. Infect. Dis. 2022, 76, e342–e349. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and Safety of COVID-19 Vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Capone, S.; Fusco, F.M.; Milleri, S.; Borrè, S.; Carbonara, S.; Lo Caputo, S.; Leone, S.; Gori, G.; Maggi, P.; Cascio, A.; et al. GRAd-COV2 Vaccine Provides Potent and Durable Humoral and Cellular Immunity to SARS-CoV-2 in Randomized Placebo-Controlled Phase 2 Trial. Cell Rep. Med. 2023, 4, 101084. [Google Scholar] [CrossRef]
- Ghaffari Darab, M.; Keshavarz, K.; Sadeghi, E.; Shahmohamadi, J.; Kavosi, Z. The Economic Burden of Coronavirus Disease 2019 (COVID-19): Evidence from Iran. BMC Health Serv. Res. 2021, 21, 132. [Google Scholar] [CrossRef]
- Rice, D.P.; Hodgson, T.A.; Kopstein, A.N. The Economic Costs of Illness: A Replication and Update. Health Care Financ. Rev. 1985, 7, 61. [Google Scholar] [PubMed]
- Cegolon, L.; Pol, R.; Simonetti, O.; Larese Filon, F.; Luzzati, R. Molnupiravir, Nirmatrelvir/Ritonavir, or Sotrovimab for High-Risk COVID-19 Patients Infected by the Omicron Variant: Hospitalization, Mortality, and Time until Negative Swab Test in Real Life. Pharmaceuticals 2023, 16, 721. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Ingenito, C.; D’Ambrosio, B.; Ranieri, C.; Iuliucci, M.R.; Iervolino, M.; Primiano, F.; Buonerba, L.; Busto, G.; Ferrara, C.; et al. The Effect of Vaccination against COVID-19 in Cancer Patients: Final Results of the COICA Trial. Oncology 2022, 100, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.; Yu, R.; Qian, X.; Huang, Y.; Chen, X.; Lin, H.; Zheng, H.; Zhang, Y.; Lin, J.; et al. The Effects of Vaccination on the Disease Severity and Factors for Viral Clearance and Hospitalization in Omicron-Infected Patients: A Retrospective Observational Cohort Study from Recent Regional Outbreaks in China. Front. Cell. Infect. Microbiol. 2022, 12, 1636. [Google Scholar] [CrossRef] [PubMed]
- Del Borgo, C.; Garattini, S.; Bortignon, C.; Carraro, A.; Di Trento, D.; Gasperin, A.; Grimaldi, A.; De Maria, S.G.; Corazza, S.; Tieghi, T.; et al. Effectiveness, Tolerability and Prescribing Choice of Antiviral Molecules Molnupiravir, Remdesivir and Nirmatrelvir/r: A Real-World Comparison in the First Ten Months of Use. Viruses 2023, 15, 1025. [Google Scholar] [CrossRef]
- de San Segundo Reyes, M.; Granizo Martinez, J.J.; Veiga Crespo, M.C.; Sanchiz Ruiz, A.; Camacho Munoz, I.; Sanchez-Uriz, M.A. Factors Associated with the Duration of SARS-CoV-2 Infection in Healthcare Professionals at a Second-Level Public Hospital in the Community of Madrid (Spain) during the Sixth Wave. Rev. Esp. Salud Publica 2023, 97, e202302012. [Google Scholar]
- Bruno, P.F.; Cappuccilli, M.; Spazzoli, A.; De Liberali, M.; Sejdiu, B.; Napoli, M.; Minerva, V.; Semprini, S.; Dirani, G.; Sambri, V.; et al. COVID-19 Infection: Viral Clearance and Antibody Response in Dialysis Patients and Renal Transplant Recipients. Nephron 2021, 145, 363–370. [Google Scholar] [CrossRef]
- Martin-Blondel, G.; Marcelin, A.G.; Soulié, C.; Kaisaridi, S.; Lusivika-Nzinga, C.; Zafilaza, K.; Dorival, C.; Nailler, L.; Boston, A.; Ronchetti, A.M.; et al. Time to Negative PCR Conversion amongst High-Risk Patients with Mild-to-Moderate Omicron BA.1 and BA.2 COVID-19 Treated with Sotrovimab or Nirmatrelvir. Clin. Microbiol. Infect. 2023, 29, 543.e5–543.e9. [Google Scholar] [CrossRef]
- Mikulska, M.; Sepulcri, C.; Dentone, C.; Magne, F.; Balletto, E.; Baldi, F.; Labate, L.; Russo, C.; Mirabella, M.; Magnasco, L.; et al. Triple Combination Therapy with 2 Antivirals and Monoclonal Antibodies for Persistent or Relapsed Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Patients. Clin. Infect. Dis. 2023, 77, 280–286. [Google Scholar] [CrossRef]
- Li, B.; Chen, L.T.; Shi, L. Determinants of Hospitalization Costs among Moderate Cases of COVID-19. Inquiry 2022, 59, 00469580211059483. [Google Scholar] [CrossRef]
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef] [PubMed]
| Variables | Cohort (n = 175) |
|---|---|
| Males, n (%) | 97 (55.4) |
| Median (IQR) age, years | 77 (68–83) |
| BMI > 30 kg/m2, n (%) | 50 (28.6) |
| Chronic renal failure, n (%) | 28 (16.0) |
| Immunodeficit, n (%) | 17 (9.7) |
| Decompensated diabetes, n (%) | 34 (19.4) |
| Diabetes, n (%) | 46 (26.3) |
| COPD, n (%) | 38 (21.7) |
| Neurodevelopmental/neurodegenerative diseases, n (%) | 51 (29.1) |
| Dementia, n (%) | 28 (16.0) |
| Cerebrovascular events, n (%) | 26 (14.9) |
| Oncological disease, n (%) | 43 (24.6) |
| Metastasis, n (%) | 20 (11.4) |
| Hematological tumors, n (%) | 13 (9.4) |
| Cardiovascular diseases, n (%) | 69 (39.4) |
| Heart failure, n (%) | 65 (37.1) |
| Previous acute myocardial infarction, n (%) | 25 (14.3) |
| Vaccination, n (%) | 160 (91.4) |
| Fever, n (%) | 73 (41.7) |
| Cough, n (%) | 50 (28.6) |
| Pharyngodynia, n (%) | 19 (10.9) |
| Headache, n (%) | 15 (8.6) |
| Gastrointestinal symptoms, n (%) | 11 (6.3) |
| Dyspnea, n (%) | 35 (20.0) |
| GGO, n (%) | 31 (17.7) |
| Consolidation, n (%) | 31 (17.7) |
| Pulmonary embolism, n (%) | 3 (1.7) |
| Median (IQR) WBC (×103) | 7.1 (5.4–9.4) |
| Median (IQR) neutrophils | 5.3 (3.4–7.7) |
| Median (IQR) lymphocytes | 1.1 (0.8–1.5) |
| Median (IQR) NLR | 4.8 (2.8–8.2) |
| Median (IQR) ferritin | 250 (140–512) |
| Median (IQR) procalcitonin | 0.08 (0.03–0.31) |
| Median (IQR) urea | 35 (25–51) |
| Median (IQR) AST | 21 (16–31) |
| Median (IQR) ALT | 18 (11–28) |
| Median (IQR) De Ritis | 1.1 (0.8–1.6) |
| Median (IQR) LDH | 215 (180–280) |
| Median (IQR) CRP | 4.8 (1.7–10.0) |
| Median (IQR) D-Dimer | 1.4 (0.7–2.5) |
| Antiviral, n (%) | 114 (65.1) |
| Molnupiravir, n (%) | 37 (21.1) |
| Nirmatrelvir/ritonavir, n (%) | 12 (6.9) |
| Remdesivir (3-day course), n (%) | 65 (37.1) |
| Monoclonal antibodies, n (%) | 44 (25.1) |
| Casirivimab/Imdevimab, n (%) | 8 (4.6) |
| Sotrovimab, n (%) | 36 (21.2) |
| Severe disease *, n (%) | 31 (17.7) |
| Median (IQR) time to negativity, days | 10 (7–14) |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Males | 1.02 (0.72–1.43) | 0.93 | 1.05 (0.73–1.51) | 0.79 |
| Age, years | 1.00 (0.99–1.01) | 0.77 | 0.99 (0.98–1.00) | 0.11 |
| Immunodeficit | 0.48 (0.23–0.98) | 0.04 | 0.71 (0.30–1.70) | 0.44 |
| Hematological tumors | 0.18 (0.06–0.57) | 0.03 | 0.21 (0.06–0.74) | 0.02 |
| Vaccination | 11.4 (2.82–46.2) | 0.001 | 12.5 (3.05–50.9) | <0.0001 |
| Headache | 1.89 (1.08–3.29) | 0.03 | 1.55 (0.85–2.81) | 0.15 |
| Exposure to antiviral therapies | 1.97 (1.34–2.90) | 0.001 | 2.32 (1.56–3.44) | <0.0001 |
| Patient | Negativization Time (Days) | Age | Gender | Obesity | COPD | Hematologic Cancer | Vaccination | GGO | Antiviral | Monoclonal Antibodies |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30.00 | 78 | Female | No | Yes | No | Yes | Yes | No | No |
| 2 | 56.00 | 87 | Female | No | No | Yes | Yes | No | Molnupiravir | No |
| 3 | 30.00 | 79 | Female | Yes | No | No | Yes | Yes | No | No |
| 4 | 30.00 | 77 | Male | No | No | Yes | Yes | No | No | No |
| 5 | 41.00 | 87 | Female | No | Yes | Yes | Yes | Yes | No | No |
| 6 | 32.00 | 78 | Female | Yes | No | No | Yes | No | No | No |
| 7 | 42.00 | 44 | Male | No | No | Yes | Yes | No | Remdesivir | No |
| 8 | 32.00 | 89 | Female | No | No | No | Yes | No | No | No |
| 9 | 31.00 | 78 | Male | No | No | Yes | No | No | Remdesivir | Sotrovimab |
| 10 | 32.00 | 66 | Male | No | No | Yes | Yes | No | Remdesivir | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vito, A.; Moi, G.; Saderi, L.; Puci, M.V.; Colpani, A.; Firino, L.; Puggioni, A.; Uzzau, S.; Babudieri, S.; Sotgiu, G.; et al. Vaccination and Antiviral Treatment Reduce the Time to Negative SARS-CoV-2 Swab: A Real-Life Study. Viruses 2023, 15, 2180. https://doi.org/10.3390/v15112180
De Vito A, Moi G, Saderi L, Puci MV, Colpani A, Firino L, Puggioni A, Uzzau S, Babudieri S, Sotgiu G, et al. Vaccination and Antiviral Treatment Reduce the Time to Negative SARS-CoV-2 Swab: A Real-Life Study. Viruses. 2023; 15(11):2180. https://doi.org/10.3390/v15112180
Chicago/Turabian StyleDe Vito, Andrea, Giulia Moi, Laura Saderi, Mariangela V. Puci, Agnese Colpani, Laura Firino, Anna Puggioni, Sergio Uzzau, Sergio Babudieri, Giovanni Sotgiu, and et al. 2023. "Vaccination and Antiviral Treatment Reduce the Time to Negative SARS-CoV-2 Swab: A Real-Life Study" Viruses 15, no. 11: 2180. https://doi.org/10.3390/v15112180
APA StyleDe Vito, A., Moi, G., Saderi, L., Puci, M. V., Colpani, A., Firino, L., Puggioni, A., Uzzau, S., Babudieri, S., Sotgiu, G., & Madeddu, G. (2023). Vaccination and Antiviral Treatment Reduce the Time to Negative SARS-CoV-2 Swab: A Real-Life Study. Viruses, 15(11), 2180. https://doi.org/10.3390/v15112180










