Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections
Abstract
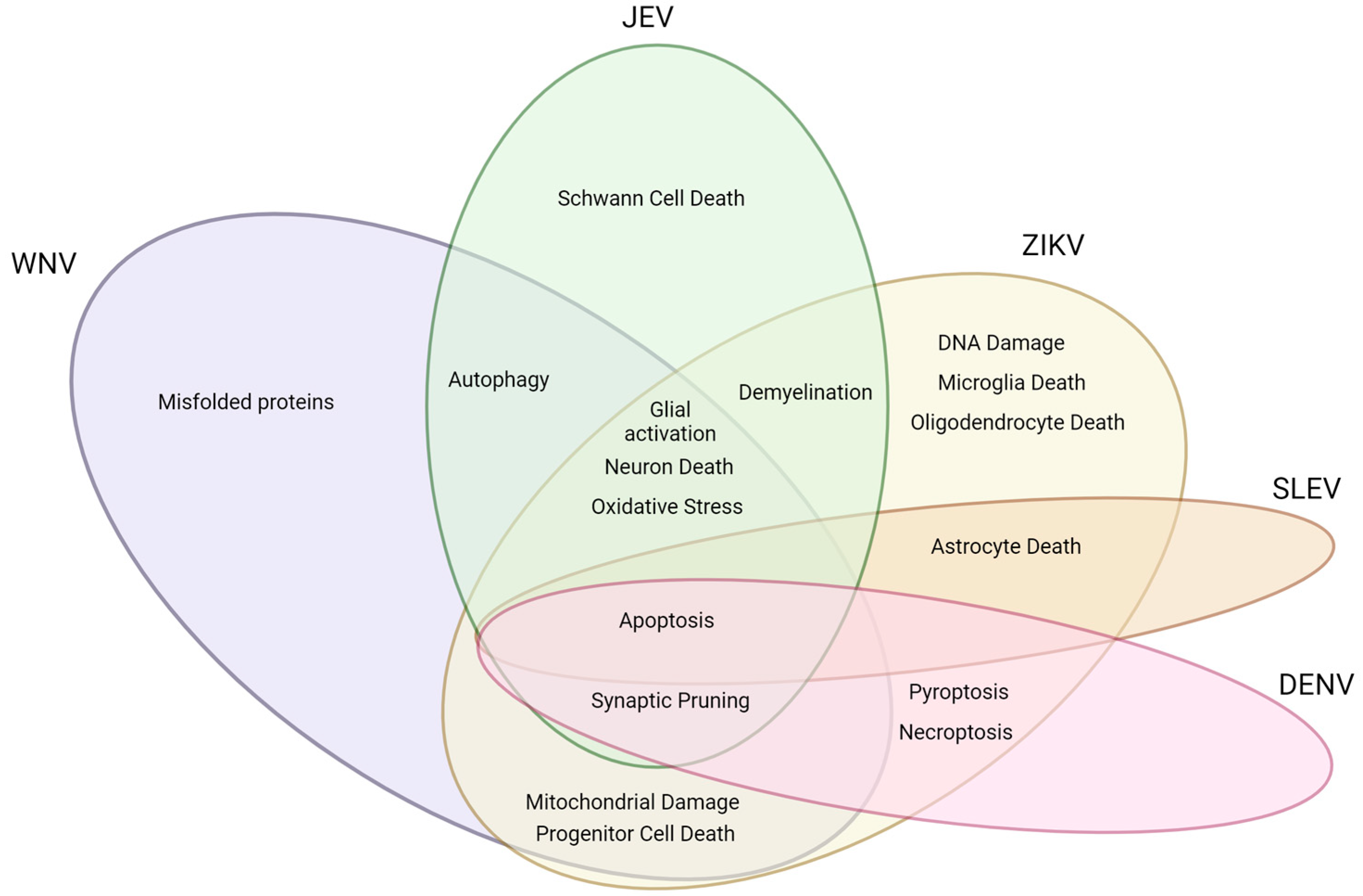
:1. Introduction
2. Flaviviruses and Associated Mechanisms of Neural Dysfunction
2.1. West Nile Virus
2.2. Japanese Encephalitis Virus
2.3. Zika Virus
2.4. Dengue Virus
2.5. St. Louis Encephalitis Virus
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus Genome Organization, Expression, and Replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Knipe, D.M.; Howley, P. Fields Virology (Knipe, Fields Virology)-2 Volume Set; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 2, ISBN 978-1-4511-0563-6. [Google Scholar]
- Gerold, G.; Bruening, J.; Weigel, B.; Pietschmann, T. Protein Interactions during the Flavivirus and Hepacivirus Life Cycle. Mol. Cell. Proteomics. 2017, 16, S75–S91. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Barrows, N.J.; Campos, R.K.; Liao, K.-C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.-C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Bernardo-Menezes, L.C.; Agrelli, A.; de Oliveira, A.S.L.E.; de Moura, R.R.; Crovella, S.; Brandão, L.A.C. An Overview of Zika Virus Genotypes and Their Infectivity. Rev. Soc. Bras. Med. Trop. 2022, 55, e0263-2022. [Google Scholar] [CrossRef]
- Fiacre, L.; Pagès, N.; Albina, E.; Richardson, J.; Lecollinet, S.; Gonzalez, G. Molecular Determinants of West Nile Virus Virulence and Pathogenesis in Vertebrate and Invertebrate Hosts. Int. J. Mol. Sci. 2020, 21, 9117. [Google Scholar] [CrossRef]
- Diamond, M.S.; Edgil, D.; Roberts, T.G.; Lu, B.; Harris, E. Infection of Human Cells by Dengue Virus Is Modulated by Different Cell Types and Viral Strains. J. Virol. 2000, 74, 7814–7823. [Google Scholar] [CrossRef]
- Lotz, S.K.; Blackhurst, B.M.; Reagin, K.L.; Funk, K.E. Microbial Infections Are a Risk Factor for Neurodegenerative Diseases. Front. Cell Neurosci. 2021, 15, 691136. [Google Scholar] [CrossRef]
- Levine, K.S.; Leonard, H.L.; Blauwendraat, C.; Iwaki, H.; Johnson, N.; Bandres-Ciga, S.; Ferrucci, L.; Faghri, F.; Singleton, A.B.; Nalls, M.A. Virus Exposure and Neurodegenerative Disease Risk across National Biobanks. Neuron 2023. [Google Scholar] [CrossRef]
- Weatherhead, J.E.; Miller, V.E.; Garcia, M.N.; Hasbun, R.; Salazar, L.; Dimachkie, M.M.; Murray, K.O. Long-Term Neurological Outcomes in West Nile Virus–Infected Patients: An Observational Study. Am. J. Trop. Med. Hyg. 2015, 92, 1006–1012. [Google Scholar] [CrossRef]
- Sejvar, J.J. West Nile Virus: An Historical Overview. Ochsner. J. 2003, 5, 6–10. [Google Scholar] [PubMed]
- Madden, K. West Nile Virus Infection and Its Neurological Manifestations. Clin. Med. Res. 2003, 1, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-H.; Wang, T. West Nile Virus Induced Cell Death in the Central Nervous System. Pathogens 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Klee, A.L.; Maidin, B.; Edwin, B.; Poshni, I.; Mostashari, F.; Fine, A.; Layton, M.; Nash, D. Long-Term Prognosis for Clinical West Nile Virus Infection. Emerg. Infect. Dis. 2004, 10, 1405–1411. [Google Scholar] [CrossRef]
- Montgomery, R.R. Age-related Alterations in Immune Responses to West Nile Virus Infection. Clin. Exp. Immunol. 2017, 187, 26–34. [Google Scholar] [CrossRef]
- Omalu, B.I.; Shakir, A.A.; Wang, G.; Lipkin, W.I.; Wiley, C.A. Fatal Fulminant Pan-Meningo-Polioencephalitis Due to West Nile Virus. Brain Pathol. 2003, 13, 465–472. [Google Scholar] [CrossRef]
- Guarner, J.; Shieh, W.-J.; Hunter, S.; Paddock, C.D.; Morken, T.; Campbell, G.L.; Marfin, A.A.; Zaki, S.R. Clinicopathologic Study and Laboratory Diagnosis of 23 Cases with West Nile Virus Encephalomyelitis. Hum. Pathol. 2004, 35, 983–990. [Google Scholar] [CrossRef]
- Armah, H.B.; Wang, G.; Omalu, B.I.; Tesh, R.B.; Gyure, K.A.; Chute, D.J.; Smith, R.D.; Dulai, P.; Vinters, H.V.; Kleinschmidt-DeMasters, B.K.; et al. Systemic Distribution of West Nile Virus Infection: Postmortem Immunohistochemical Study of Six Cases. Brain Pathol. 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Grubaugh, N.D.; Massey, A.; Shives, K.D.; Stenglein, M.D.; Ebel, G.D.; Beckham, J.D. West Nile Virus Population Structure, Injury, and Interferon-Stimulated Gene Expression in the Brain From a Fatal Case of Encephalitis. Open Forum. Infect. Dis. 2015, 3, ofv182. [Google Scholar] [CrossRef]
- Cheeran, M.C.-J.; Hu, S.; Sheng, W.S.; Rashid, A.; Peterson, P.K.; Lokensgard, J.R. Differential Responses of Human Brain Cells to West Nile Virus Infection. J. NeuroVirology 2005, 11, 512–524. [Google Scholar] [CrossRef]
- Luo, H.; Winkelmann, E.R.; Zhu, S.; Ru, W.; Mays, E.; Silvas, J.A.; Vollmer, L.L.; Gao, J.; Peng, B.-H.; Bopp, N.E.; et al. Peli1 Facilitates Virus Replication and Promotes Neuroinflammation during West Nile Virus Infection. J. Clin. Investig. 2018, 128, 4980–4991. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.D.; Siddharthan, V.; Wang, H.; Hall, J.O.; Skirpstunas, R.T.; Olsen, A.L.; Nordstrom, J.L.; Koenig, S.; Johnson, S.; Diamond, M.S. West Nile Virus—Induced Acute Flaccid Paralysis Is Prevented by Monoclonal Antibody Treatment When Administered after Infection of Spinal Cord Neurons. J. NeuroVirology 2008, 14, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, R.C.; Jordan Steel, J.; Moon, S.L.; Soltani, E.; Geiss, B.J. Oxidative Stress Influences Positive Strand RNA Virus Genome Synthesis and Capping. Virology 2015, 475, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Suen, W.W.; Prow, N.A.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus Challenge Alters the Transcription Profiles of Innate Immune Genes in Rabbit Peripheral Blood Mononuclear Cells. Front. Vet. Sci. 2015, 2, 76. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Ke, Y.; Luo, C.; Gozal, D.; Liu, R. Depletion of Reduced Glutathione Enhances Motor Neuron Degeneration in Vitro and in Vivo. Neuroscience 2007, 144, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Nikam, S.; Nikam, P.; Ahaley, S.K.; Sontakke, A.V. Oxidative Stress in Parkinson’s Disease. Indian J. Clin. Biochem. 2009, 24, 98–101. [Google Scholar] [CrossRef]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA Damage in Mild Cognitive Impairment and Late-Stage Alzheimer’s Disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef]
- del Carmen Parquet, M.; Kumatori, A.; Hasebe, F.; Morita, K.; Igarashi, A. West Nile Virus-Induced Bax-Dependent Apoptosis. FEBS Lett. 2001, 500, 17–24. [Google Scholar] [CrossRef]
- Dejean, L.M.; Martinez-Caballero, S.; Manon, S.; Kinnally, K.W. Regulation of the Mitochondrial Apoptosis-Induced Channel, MAC, by BCL-2 Family Proteins. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 191–201. [Google Scholar] [CrossRef]
- Iyer, S.; Uren, R.T.; Dengler, M.A.; Shi, M.X.; Uno, E.; Adams, J.M.; Dewson, G.; Kluck, R.M. Robust Autoactivation for Apoptosis by BAK but Not BAX Highlights BAK as an Important Therapeutic Target. Cell Death Dis. 2020, 11, 268. [Google Scholar] [CrossRef]
- Roby, J.A.; Esser-Nobis, K.; Dewey-Verstelle, E.C.; Fairgrieve, M.R.; Schwerk, J.; Lu, A.Y.; Soveg, F.W.; Hemann, E.A.; Hatfield, L.D.; Keller, B.C.; et al. Flavivirus Nonstructural Protein NS5 Dysregulates HSP90 to Broadly Inhibit JAK/STAT Signaling. Cells 2020, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, M.C.; Michaelis, M.; Ogbomo, H.; Doerr, H.-W.; Cinatl, J. Inhibition of Apoptosis Prevents West Nile Virus Induced Cell Death. BMC Microbiol. 2007, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Shu, H.-B.; Pan, M.-G.; Goeddel, D.V. TRADD–TRAF2 and TRADD–FADD Interactions Define Two Distinct TNF Receptor 1 Signal Transduction Pathways. Cell 1996, 84, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Soung, A.L.; Davé, V.A.; Garber, C.; Tycksen, E.D.; Vollmer, L.L.; Klein, R.S. IL-1 Reprogramming of Adult Neural Stem Cells Limits Neurocognitive Recovery after Viral Encephalitis by Maintaining a Proinflammatory State. Brain Behav. Immun. 2022, 99, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Schafernak, K.T.; Bigio, E.H. West Nile Virus Encephalomyelitis with Polio-like Paralysis & Nigral Degeneration. Can. J. Neurol. Sci. 2006, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.; Song, J. Hsp70 Functions as a Negative Regulator of West Nile Virus Capsid Protein through Direct Interaction. Biochem. Biophys. Res. Commun. 2006, 347, 994–1000. [Google Scholar] [CrossRef]
- Albakova, Z.; Mangasarova, Y.; Albakov, A.; Gorenkova, L. HSP70 and HSP90 in Cancer: Cytosolic, Endoplasmic Reticulum and Mitochondrial Chaperones of Tumorigenesis. Front. Oncol. 2022, 12, 829520. [Google Scholar] [CrossRef]
- Rutledge, B.S.; Choy, W.-Y.; Duennwald, M.L. Folding or Holding?—Hsp70 and Hsp90 Chaperoning of Misfolded Proteins in Neurodegenerative Disease. J. Biol. Chem. 2022, 298. [Google Scholar] [CrossRef]
- Brinton, M.A. Replication Cycle and Molecular Biology of the West Nile Virus. Viruses 2013, 6, 13–53. [Google Scholar] [CrossRef]
- Kobayashi, S.; Orba, Y.; Yamaguchi, H.; Kimura, T.; Sawa, H. Accumulation of Ubiquitinated Proteins Is Related to West Nile Virus-Induced Neuronal Apoptosis. Neuropathology 2012, 32, 398–405. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yoshii, K.; Phongphaew, W.; Muto, M.; Hirano, M.; Orba, Y.; Sawa, H.; Kariwa, H. West Nile Virus Capsid Protein Inhibits Autophagy by AMP-Activated Protein Kinase Degradation in Neurological Disease Development. PLoS Pathog. 2020, 16, e1008238. [Google Scholar] [CrossRef] [PubMed]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Perez-Torres, C.; Frouin, A.; Wilton, D.K.; et al. A Complement-Microglial Axis Drives Synapse Loss during Virus-Induced Memory Impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Haddad, M.B.; Tierney, B.C.; Campbell, G.L.; Marfin, A.A.; Van Gerpen, J.A.; Fleischauer, A.; Leis, A.A.; Stokic, D.S.; Petersen, L.R. Neurologic Manifestations and Outcome of West Nile Virus Infection. JAMA 2003, 290, 511–515. [Google Scholar] [CrossRef]
- Oveisgharan, S.; Yu, L.; Poole, V.N.; Evia, A.M.; Barnes, L.L.; Schneider, J.A.; Arfanakis, K.; Bennett, D.A.; Buchman, A.S. Association of White Matter Hyperintensities With Pathology and Progression of Parkinsonism in Aging. JAMA Neurol. 2021, 78, 1494–1502. [Google Scholar] [CrossRef]
- Juttukonda, M.R.; Franco, G.; Englot, D.J.; Lin, Y.-C.; Petersen, K.J.; Trujillo, P.; Hedera, P.; Landman, B.A.; Kang, H.; Donahue, M.J.; et al. White Matter Differences between Essential Tremor and Parkinson Disease. Neurology 2019, 92, e30–e39. [Google Scholar] [CrossRef]
- Dadar, M.; Gee, M.; Shuaib, A.; Duchesne, S.; Camicioli, R. Cognitive and Motor Correlates of Grey and White Matter Pathology in Parkinson’s Disease. NeuroImage Clin. 2020, 27, 102353. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.R.; Guttmann, C.R.G.; Wei, X.; Warfield, S.K.; Hall, C.; Schmidt, J.A.; Kikinis, R.; Wolfson, L.I. Older People with Impaired Mobility Have Specific Loci of Periventricular Abnormality on MRI. Neurology 2002, 58, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Soumaré, A.; Elbaz, A.; Zhu, Y.; Maillard, P.; Crivello, F.; Tavernier, B.; Dufouil, C.; Mazoyer, B.; Tzourio, C. White Matter Lesions Volume and Motor Performances in the Elderly. Ann. Neurol. 2009, 65, 706–715. [Google Scholar] [CrossRef]
- Baezner, H.; Blahak, C.; Poggesi, A.; Pantoni, L.; Inzitari, D.; Chabriat, H.; Erkinjuntti, T.; Fazekas, F.; Ferro, J.M.; Langhorne, P.; et al. Association of Gait and Balance Disorders with Age-Related White Matter Changes: The LADIS Study. Neurology 2008, 70, 935–942. [Google Scholar] [CrossRef]
- Bui, B.; Byun, J.; Jacobs, J.; Liu, A.K. Multiple Sclerosis in a Patient With Prior West Nile Encephalitis. Cureus 2022, 14, e28935. [Google Scholar] [CrossRef]
- Steele, K.E.; Linn, M.J.; Schoepp, R.J.; Komar, N.; Geisbert, T.W.; Manduca, R.M.; Calle, P.P.; Raphael, B.L.; Clippinger, T.L.; Larsen, T.; et al. Pathology of Fatal West Nile Virus Infections in Native and Exotic Birds during the 1999 Outbreak in New York City, New York. Vet. Pathol. 2000, 37, 208–224. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Iglesias, J.; Eriksson, J.; Grize, F.; Tomassini, M.; Villa, A.E.P. Dynamics of Pruning in Simulated Large-Scale Spiking Neural Networks. Biosystems 2005, 79, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.; Soung, A.; Vollmer, L.L.; Kanmogne, M.; Last, A.; Brown, J.; Klein, R.S. T Cells Promote Microglia-Mediated Synaptic Elimination and Cognitive Dysfunction during Recovery from Neuropathogenic Flaviviruses. Nat. Neurosci. 2019, 22, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Jani, C.; Walker, A.; Al Omari, O.; Patel, D.; Heffess, A.; Wolpow, E.; Page, S.; Bourque, D. Acute Transverse Myelitis in West Nile Virus, a Rare Neurological Presentation. IDCases 2021, 24, e01104. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, A.; Liu, A. A Case of West Nile Encephalitis That Develops into a Disease of Deep White Matter on MRI over a Two-Week Span. Case Rep. Neurol. Med. 2016, 2016, e4389691. [Google Scholar] [CrossRef]
- Reagin, K.L.; Funk, K.E. The Role of Antiviral CD8+ T Cells in Cognitive Impairment. Curr. Opin. Neurobiol. 2022, 76, 102603. [Google Scholar] [CrossRef]
- Funk, K.E.; Klein, R.S. CSF1R Antagonism Limits Local Restimulation of Antiviral CD8+ T Cells during Viral Encephalitis. J. Neuroinflamm. 2019, 16, 22. [Google Scholar] [CrossRef]
- Shrestha, B.; Diamond, M.S. Role of CD8+ T Cells in Control of West Nile Virus Infection. J. Virol. 2004, 78, 8312–8321. [Google Scholar] [CrossRef]
- Shrestha, B.; Pinto, A.K.; Green, S.; Bosch, I.; Diamond, M.S. CD8+ T Cells Use TRAIL to Restrict West Nile Virus Pathogenesis by Controlling Infection in Neurons. J. Virol. 2012, 86, 8937–8948. [Google Scholar] [CrossRef]
- Shrestha, B.; Diamond, M.S. Fas Ligand Interactions Contribute to CD8+ T-Cell-Mediated Control of West Nile Virus Infection in the Central Nervous System. J. Virol. 2007, 81, 11749–11757. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Samuel, M.A.; Diamond, M.S. CD8+ T Cells Require Perforin to Clear West Nile Virus from Infected Neurons. J. Virol. 2006, 80, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Zhang, G.; Takeuchi, H.; Kawanokuchi, J.; Wang, J.; Sonobe, Y.; Jin, S.; Takada, N.; Komatsu, Y.; Suzumura, A. Interferon-γ Directly Induces Neurotoxicity through a Neuron Specific, Calcium-Permeable Complex of IFN-γ Receptor and AMPA GluRl Receptor. FASEB J. 2008, 22, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.F.; Soung, A.L.; Yang, W.; Ai, S.; Kanmogne, M.; Davé, V.A.; Artyomov, M.; Magee, J.A.; Klein, R.S. Single-Cell RNA Transcriptome Analysis of CNS Immune Cells Reveals CXCL16/CXCR6 as Maintenance Factors for Tissue-Resident T Cells That Drive Synapse Elimination. Genome Med. 2022, 14, 108. [Google Scholar] [CrossRef]
- Su, W.; Saravia, J.; Risch, I.; Rankin, S.; Guy, C.; Chapman, N.M.; Shi, H.; Sun, Y.; Kc, A.; Li, W.; et al. CXCR6 Orchestrates Brain CD8+ T Cell Residency and Limits Mouse Alzheimer’s Disease Pathology. Nat. Immunol. 2023, 24, 1735–1747. [Google Scholar] [CrossRef]
- Reagin, K.L.; Funk, K.E. CD8+ T Cells Pump the Brakes on Alzheimer’s Disease. Nat. Immunol. 2023, 24, 1597–1598. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Soung, A.; Klein, R.S. Viral Encephalitis and Neurologic Diseases: Focus on Astrocytes. Trends Mol. Med. 2018, 24, 950–962. [Google Scholar] [CrossRef]
- Daniels, B.P.; Jujjavarapu, H.; Durrant, D.M.; Williams, J.L.; Green, R.R.; White, J.P.; Lazear, H.M.; Gale, M.; Diamond, M.S.; Klein, R.S. Regional Astrocyte IFN Signaling Restricts Pathogenesis during Neurotropic Viral Infection. J. Clin. Investig. 2017, 127, 843–856. [Google Scholar] [CrossRef]
- Hussmann, K.L.; Samuel, M.A.; Kim, K.S.; Diamond, M.S.; Fredericksen, B.L. Differential Replication of Pathogenic and Nonpathogenic Strains of West Nile Virus within Astrocytes. J. Virol. 2013, 87, 2814–2822. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.; Vasek, M.J.; Vollmer, L.L.; Sun, T.; Jiang, X.; Klein, R.S. Astrocytes Decrease Adult Neurogenesis during Virus-Induced Memory Dysfunction via IL-1. Nat. Immunol. 2018, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.S.; Jeswani, V.; Pal, N.; Bohra, B.; Vishwakarma, V.; Bapat, A.A.; Patnaik, Y.P.; Khanna, N.; Shukla, R. Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines. Vaccines 2023, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Basu, A. Japanese Encephalitis—A Pathological and Clinical Perspective. PLoS Neglected Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef]
- Johnson, R.T.; Burke, D.S.; Elwell, M.; Leake, C.J.; Nisalak, A.; Hoke, C.H.; Lorsomrudee, W. Japanese Encephalitis: Immunocytochemical Studies of Viral Antigen and Inflammatory Cells in Fatal Cases. Ann. Neurol. 1985, 18, 567–573. [Google Scholar] [CrossRef]
- Wong, K.T.; Ng, K.Y.; Ong, K.C.; Ng, W.F.; Shankar, S.K.; Mahadevan, A.; Radotra, B.; Su, I.J.; Lau, G.; Ling, A.E.; et al. Enterovirus 71 Encephalomyelitis and Japanese Encephalitis Can Be Distinguished by Topographic Distribution of Inflammation and Specific Intraneuronal Detection of Viral Antigen and RNA. Neuropathol. Appl. Neurobiol. 2012, 38, 443–453. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Zhao, J.-X.; Yamamoto, T.; Konno, H. Immunohistochemical Demonstration of Viral Antigens in Japanese Encephalitis. Acta Neuropathol. 1986, 70, 79–81. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Samutpong, A.; Lerdsamran, H.; Prasertsopon, J.; Yasawong, M.; Govitrapong, P.; Puthavathana, P.; Kitidee, K. Elevation of Cleaved P18 Bax Levels Associated with the Kinetics of Neuronal Cell Death during Japanese Encephalitis Virus Infection. Int. J. Mol. Sci. 2019, 20, 5016. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Z.; Guo, X.; Zhang, J.; Cheng, G.; Hu, X.; Zhang, W.; Gu, C. Japanese Encephalitis Virus Induces Apoptosis by Activating the RIG-1 Signaling Pathway. Arch. Virol. 2023, 168, 169. [Google Scholar] [CrossRef]
- Bhaskar, M.; Mukherjee, S.; Basu, A. Involvement of RIG-I Pathway in Neurotropic Virus-Induced Acute Flaccid Paralysis and Subsequent Spinal Motor Neuron Death. mBio 2021, 12, e0271221. [Google Scholar] [CrossRef]
- Guo, F.; Yu, X.; Xu, A.; Xu, J.; Wang, Q.; Guo, Y.; Wu, X.; Tang, Y.; Ding, Z.; Zhang, Y.; et al. Japanese Encephalitis Virus Induces Apoptosis by Inhibiting Foxo Signaling Pathway. Vet. Microbiol. 2018, 220, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Bhattacharyya, S.; Nain, M.; Kaur, M.; Sood, V.; Gupta, V.; Khasa, R.; Abdin, M.Z.; Vrati, S.; Kalia, M. Japanese Encephalitis Virus Replication Is Negatively Regulated by Autophagy and Occurs on LC3-I- and EDEM1-Containing Membranes. Autophagy 2014, 10, 1637. [Google Scholar] [CrossRef]
- Su, H.-L.; Liao, C.-L.; Lin, Y.-L. Japanese Encephalitis Virus Infection Initiates Endoplasmic Reticulum Stress and an Unfolded Protein Response. J. Virol. 2002, 76, 4162–4171. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Summers, P.L.; Ray, P.; Asafo-Adjei, E. Cytopathology of PC12 Cells Infected with Japanese Encephalitis Virus. Virchows. Archiv. B Cell Pathol. 1993, 63, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xin, X.; Wang, T.; Wan, J.; Ou, Y.; Yang, Z.; Yu, Q.; Zhu, L.; Guo, Y.; Wu, Y.; et al. Japanese Encephalitis Virus Induces Apoptosis and Encephalitis by Activating the PERK Pathway. J. Virol. 2019, 93, e00887-19. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-T.; Chang, B.-L.; Liang, J.-J.; Tsai, H.-J.; Lee, Y.-L.; Lin, R.-J.; Lin, Y.-L. Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation. PLoS Pathog. 2015, 11, e1004750. [Google Scholar] [CrossRef]
- de A Boleti, A.P.; de O Cardoso, P.H.; F Frihling, B.E.; E Silva, P.S.; de Moraes, L.F.R.N.; Migliolo, L. Adipose Tissue, Systematic Inflammation, and Neurodegenerative Diseases. Neural. Regen. Res. 2023, 18, 38–46. [Google Scholar] [CrossRef]
- Fan, F.; Liu, T.; Wang, X.; Ren, D.; Liu, H.; Zhang, P.; Wang, Z.; Liu, N.; Li, Q.; Tu, Y.; et al. ClC-3 Expression and Its Association with Hyperglycemia Induced HT22 Hippocampal Neuronal Cell Apoptosis. J. Diabetes Res. 2016, 2016, e2984380. [Google Scholar] [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; MacLaurin, J.G.; Lagace, D.C.; Park, D.S.; Slack, R.S. Mitochondrial Dysfunction Underlies Cognitive Defects as a Result of Neural Stem Cell Depletion and Impaired Neurogenesis. Hum. Mol. Genet. 2017, 26, 3327–3341. [Google Scholar] [CrossRef]
- Lannes, N.; Neuhaus, V.; Scolari, B.; Kharoubi-Hess, S.; Walch, M.; Summerfield, A.; Filgueira, L. Interactions of Human Microglia Cells with Japanese Encephalitis Virus. Virol. J. 2017, 14, 8. [Google Scholar] [CrossRef]
- Liao, S.-L.; Raung, S.-L.; Chen, C.-J. Japanese Encephalitis Virus Stimulates Superoxide Dismutase Activity in Rat Glial Cultures. Neurosci. Lett. 2002, 324, 133–136. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhao, Z.; Anees, A.; Li, Y.; Ashraf, U.; Chen, Z.; Song, Y.; Chen, H.; Cao, S.; Ye, J. P21-Activated Kinase 4 Signaling Promotes Japanese Encephalitis Virus-Mediated Inflammation in Astrocytes. Front. Cell. Infect. Microbiol. 2017, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.S.A.; Kipar, A.; Jarman, R.G.; Gibbons, R.V.; Perng, G.C.; Flanagan, B.; Mongkolsirichaikul, D.; Gessel, Y.V.; Solomon, T. Neuropathogenesis of Japanese Encephalitis in a Primate Model. PLoS Neglected Trop. Dis. 2014, 8, e2980. [Google Scholar] [CrossRef]
- Swarup, V.; Das, S.; Ghosh, S.; Basu, A. Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J. Neurochem. 2007, 103, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. Why Does Viral RNA Sometimes Persist after Recovery from Acute Infections? PLoS Biol. 2022, 20, e3001687. [Google Scholar] [CrossRef]
- Thongtan, T.; Cheepsunthorn, P.; Chaiworakul, V.; Rattanarungsan, C.; Wikan, N.; Smith, D.R. Highly Permissive Infection of Microglial Cells by Japanese Encephalitis Virus: A Possible Role as a Viral Reservoir. Microbes Infect. 2010, 12, 37–45. [Google Scholar] [CrossRef]
- Tseng, Y.-F.; Wang, C.-C.; Liao, S.-K.; Chuang, C.-K.; Chen, W.-J. Autoimmunity-Related Demyelination in Infection by Japanese Encephalitis Virus. J. Biomed. Sci. 2011, 18, 20. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Yang, X.; Guo, T.; Wang, L.; Zhao, Z.; Sun, H.; Hou, X.; Ding, X.; Dou, C.; et al. Guillain–Barré Syndrome Associated with JEV Infection. N. Engl. J. Med. 2020, 383, 1188–1190. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Wang, Z.; Wang, G.; Fu, S.; Li, F.; Yang, L.; Yuan, Y.; Shen, K.; Wang, H.; et al. Peripheral Nerve Injury Induced by Japanese Encephalitis Virus in C57BL/6 Mouse. J. Virol. 2023, 97, e01658-22. [Google Scholar] [CrossRef]
- Song, B.-H.; Yun, S.-I.; Woolley, M.; Lee, Y.-M. Zika Virus: History, Epidemiology, Transmission, and Clinical Presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef]
- Grant, R.; Flechelles, O.; Elenga, N.; Tressières, B.; Gaete, S.; Hebert, J.-C.; Schaub, B.; Djossou, F.; Mallard, A.; Delver, L.; et al. Consequences of In Utero Zika Virus Exposure and Adverse Pregnancy and Early Childhood Outcomes: A Prospective Cohort Study. Viruses 2022, 14, 2755. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Rodriguez, A.; Almiron, M.; Sanhueza, A.; Ramon, P.; de Oliveira, W.K.; Coelho, G.E.; Badaró, R.; Cortez, J.; Ospina, M.; et al. Zika Virus and the Guillain-Barré Syndrome–Case Series from Seven Countries. N. Engl. J. Med. 2016, 375, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Fontes, B.M. Zika Virus-Related Hypertensive Iridocyclitis. Arq. Bras. Oftalmol. 2016, 79, 63. [Google Scholar] [CrossRef] [PubMed]
- van den Pol, A.N.; Mao, G.; Yang, Y.; Ornaghi, S.; Davis, J.N. Zika Virus Targeting in the Developing Brain. J. Neurosci. 2017, 37, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Schultz, V.; Barrie, J.A.; Donald, C.L.; Crawford, C.L.; Mullin, M.; Anderson, T.J.; Solomon, T.; Barnett, S.C.; Linington, C.; Kohl, A.; et al. Oligodendrocytes Are Susceptible to Zika Virus Infection in a Mouse Model of Perinatal Exposure: Implications for CNS Complications. Glia 2021, 69, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, G.; Abraham, R.; Griffin, D.E. Human Schwann Cells Are Susceptible to Infection with Zika and Yellow Fever Viruses, but Not Dengue Virus. Sci. Rep. 2019, 9, 9951. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.-F.; Xu, Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 2016, 19, 672. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Q.; Jiang, Y.; Ye, Q.; Xu, D.; Gao, F.; Xu, J.W.; Wang, R.; Zhu, X.; Shi, L.; et al. Disruption of Glial Cell Development by Zika Virus Contributes to Severe Microcephalic Newborn Mice. Cell Discov. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Alejandro, B.; Hetman, M.; Hattab, E.M.; Joiner, J.; Schroten, H.; Ishikawa, H.; Chung, D.-H. Zika Virus Infects Pericytes in the Choroid Plexus and Enters the Central Nervous System through the Blood-Cerebrospinal Fluid Barrier. PLoS Pathog. 2020, 16, e1008204. [Google Scholar] [CrossRef]
- Wen, Z.; Song, H.; Ming, G. How Does Zika Virus Cause Microcephaly? Genes Dev. 2017, 31, 849–861. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Chin, T.-Y.; Chen, C.-P.; Chan, H.-L.; Wu, T.-Y. Zika Virus: An Emerging Challenge for Obstetrics and Gynecology. Taiwan J. Obstet. Gynecol. 2017, 56, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Beckman, D.; Seelke, A.M.; Bennett, J.; Dougherty, P.; Van Rompay, K.K.; Keesler, R.; Pesavento, P.A.; Coffey, L.L.; Morrison, J.H.; Bliss-Moreau, E. Neuroanatomical Abnormalities in a Nonhuman Primate Model of Congenital Zika Virus Infection. eLife 2022, 11, e64734. [Google Scholar] [CrossRef] [PubMed]
- Hastings, A.K.; Hastings, K.; Uraki, R.; Hwang, J.; Gaitsch, H.; Dhaliwal, K.; Williamson, E.; Fikrig, E. Loss of the TAM Receptor Axl Ameliorates Severe Zika Virus Pathogenesis and Reduces Apoptosis in Microglia. iScience 2019, 13, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Turpin, J.; El Safadi, D.; Lebeau, G.; Krejbich, M.; Chatelain, C.; Desprès, P.; Viranaïcken, W.; Krejbich-Trotot, P. Apoptosis during ZIKA Virus Infection: Too Soon or Too Late? Int. J. Mol. Sci. 2022, 23, 1287. [Google Scholar] [CrossRef]
- Martinot, A.J.; Abbink, P.; Afacan, O.; Prohl, A.K.; Bronson, R.; Hecht, J.L.; Borducchi, E.N.; Larocca, R.A.; Peterson, R.L.; Rinaldi, W.; et al. Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 2018, 173, 1111–1122.e10. [Google Scholar] [CrossRef]
- Lossia, O.V.; Conway, M.J.; Tree, M.O.; Williams, R.J.; Goldthorpe, S.C.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. Zika Virus Induces Astrocyte Differentiation in Neural Stem Cells. J. Neurovirol. 2018, 24, 52–61. [Google Scholar] [CrossRef]
- Ferraris, P.; Cochet, M.; Hamel, R.; Gladwyn-Ng, I.; Alfano, C.; Diop, F.; Garcia, D.; Talignani, L.; Montero-Menei, C.N.; Nougairède, A.; et al. Zika Virus Differentially Infects Human Neural Progenitor Cells According to Their State of Differentiation and Dysregulates Neurogenesis through the Notch Pathway. Emerg. Microbes Infect. 2019, 8, 1003–1016. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Regla-Nava, J.A.; Chai, G.; Sheets, N.; Tang, W.; Terskikh, A.V.; Shresta, S.; Gleeson, J.G. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell 2016, 19, 593–598. [Google Scholar] [CrossRef]
- Angel, J.P.; Daniels, B.P. Paradoxical Roles for Programmed Cell Death Signaling during Viral Infection of the Central Nervous System. Curr. Opin. Neurobiol. 2022, 77, 102629. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus Necroptosis: Similarities, Differences, and Crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Brault, M.; Oberst, A. Controlled Detonation: Evolution of Necroptosis in Pathogen Defense. Immunol. Cell Biol. 2017, 95, 131–136. [Google Scholar] [CrossRef]
- He, Z.; An, S.; Chen, J.; Zhang, S.; Tan, C.; Yu, J.; Ye, H.; Wu, Y.; Yuan, J.; Wu, J.; et al. Neural Progenitor Cell Pyroptosis Contributes to Zika Virus-Induced Brain Atrophy and Represents a Therapeutic Target. Proc. Natl. Acad. Sci. USA 2020, 117, 23869–23878. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yu, Y.; Gao, C.; Qi, X.; Cardona, C.J.; Xing, Z. RIPK3-Dependent Necroptosis Is Induced and Restricts Viral Replication in Human Astrocytes Infected with Zika Virus. Front. Cell. Infect. Microbiol. 2021, 11, 637710. [Google Scholar] [CrossRef]
- Daniels, B.P.; Kofman, S.B.; Smith, J.R.; Norris, G.T.; Snyder, A.G.; Kolb, J.P.; Gao, X.; Locasale, J.W.; Martinez, J.; Gale, M.; et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity 2019, 50, 64–76.e4. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, A.; de Laval, F.; Belleoud, D.; Briolant, S.; Matheus, S. Duration of Zika Viremia in Serum. Clin. Infect. Dis. 2018, 67, 1143–1144. [Google Scholar] [CrossRef]
- Bhatnagar, J.; Rabeneck, D.B.; Martines, R.B.; Reagan-Steiner, S.; Ermias, Y.; Estetter, L.B.C.; Suzuki, T.; Ritter, J.; Keating, M.K.; Hale, G.; et al. Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg. Infect. Dis. 2017, 23, 405–414. [Google Scholar] [CrossRef]
- Ireland, D.D.C.; Manangeeswaran, M.; Lewkowicz, A.P.; Engel, K.; Clark, S.M.; Laniyan, A.; Sykes, J.; Lee, H.-N.; McWilliams, I.L.; Kelley-Baker, L.; et al. Long-Term Persistence of Infectious Zika Virus: Inflammation and Behavioral Sequela in Mice. PLoS Pathog. 2020, 16, e1008689. [Google Scholar] [CrossRef]
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. N. Engl. J. Med. 2018, 378, 1377–1385. [Google Scholar] [CrossRef]
- Li, X.; Shang, B.; Li, Y.; Shi, Y.; Shao, C. IFNγ and TNFα Synergistically Induce Apoptosis of Mesenchymal Stem/Stromal Cells via the Induction of Nitric Oxide. Stem Cell Res. Ther. 2019, 10, 18. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Barros-Aragão, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; de Sousa, V.L.; Souza, A.S.; et al. Zika Virus Replicates in Adult Human Brain Tissue and Impairs Synapses and Memory in Mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.R.F.; Frontera, J.A.; Bispo de Filippis, A.M.; do Nascimento, O.J.M.; RIO-GBS-ZIKV Research Group. Neurologic Complications Associated With the Zika Virus in Brazilian Adults. JAMA Neurol. 2017, 74, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Bido-Medina, R.; Wirsich, J.; Rodríguez, M.; Oviedo, J.; Miches, I.; Bido, P.; Tusen, L.; Stoeter, P.; Sadaghiani, S. Impact of Zika Virus on Adult Human Brain Structure and Functional Organization. Ann. Clin. Transl. Neurol. 2018, 5, 752–762. [Google Scholar] [CrossRef]
- Schultz, V.; Cumberworth, S.L.; Gu, Q.; Johnson, N.; Donald, C.L.; McCanney, G.A.; Barrie, J.A.; Da Silva Filipe, A.; Linington, C.; Willison, H.J.; et al. Zika Virus Infection Leads to Demyelination and Axonal Injury in Mature CNS Cultures. Viruses 2021, 13, 91. [Google Scholar] [CrossRef]
- Cumberworth, S.L.; Barrie, J.A.; Cunningham, M.E.; de Figueiredo, D.P.G.; Schultz, V.; Wilder-Smith, A.J.; Brennan, B.; Pena, L.J.; Freitas de Oliveira França, R.; Linington, C.; et al. Zika Virus Tropism and Interactions in Myelinating Neural Cell Cultures: CNS Cells and Myelin Are Preferentially Affected. Acta Neuropathol. Commun. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Kang, M.; Baek, S.; Lee, C.H.; Park, D. Zika Virus Infection Differentially Affects Genome-Wide Transcription in Neuronal Cells and Myeloid Dendritic Cells. PLoS ONE 2020, 15, e0231049. [Google Scholar] [CrossRef]
- Ghouzzi, V.E.; Bianchi, F.T.; Molineris, I.; Mounce, B.C.; Berto, G.E.; Rak, M.; Lebon, S.; Aubry, L.; Tocco, C.; Gai, M.; et al. ZIKA Virus Elicits P53 Activation and Genotoxic Stress in Human Neural Progenitors Similar to Mutations Involved in Severe Forms of Genetic Microcephaly and P53. Cell Death Dis. 2016, 7, e2440. [Google Scholar] [CrossRef]
- Rychlowska, M.; Agyapong, A.; Weinfeld, M.; Schang, L.M. Zika Virus Induces Mitotic Catastrophe in Human Neural Progenitors by Triggering Unscheduled Mitotic Entry in the Presence of DNA Damage While Functionally Depleting Nuclear PNKP. J. Virol. 2022, 96, e0033322. [Google Scholar] [CrossRef]
- Hammack, C.; Ogden, S.C.; Madden, J.C.; Medina, A.; Xu, C.; Phillips, E.; Son, Y.; Cone, A.; Giovinazzi, S.; Didier, R.A.; et al. Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Ledur, P.F.; Karmirian, K.; Pedrosa, C.d.S.G.; Souza, L.R.Q.; Assis-de-Lemos, G.; Martins, T.M.; Ferreira, J.D.C.C.G.; de Azevedo Reis, G.F.; Silva, E.S.; Silva, D.; et al. Zika Virus Infection Leads to Mitochondrial Failure, Oxidative Stress and DNA Damage in Human iPSC-Derived Astrocytes. Sci. Rep. 2020, 10, 1218. [Google Scholar] [CrossRef]
- Jayaraman, D.; Bae, B.-I.; Walsh, C.A. The Genetics of Primary Microcephaly. Annu. Rev. Genom. Hum. Genet. 2018, 19, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Bäck, A.T.; Lundkvist, Å. Dengue Viruses–an Overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The Global Burden of Dengue: An Analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Ahsan, O.; Mumtaz, H.; Tahir Khan, M.; Sah, R.; Waheed, Y. Tracing down the Updates on Dengue Virus-Molecular Biology, Antivirals, and Vaccine Strategies. Vaccines 2023, 11, 1328. [Google Scholar] [CrossRef]
- Morrone, S.R.; Lok, S.-M. Structural Perspectives of Antibody-Dependent Enhancement of Infection of Dengue Virus. Curr. Opin. Virol. 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Bastos, M.d.S.; Martins, V.d.C.A.; da Silva, N.L.; Jezine, S.; Pinto, S.; Aprigio, V.; Monte, R.L.; Fragoso, S.; Puccioni-Sohler, M. Importance of Cerebrospinal Fluid Investigation during Dengue Infection in Brazilian Amazonia Region. Mem. Inst. Oswaldo Cruz 2018, 114, e180450. [Google Scholar] [CrossRef]
- Trivedi, S.; Chakravarty, A. Neurological Complications of Dengue Fever. Curr. Neurol. Neurosci. Rep. 2022, 22, 515–529. [Google Scholar] [CrossRef]
- Ellul, M.; Solomon, T. Acute Encephalitis–Diagnosis and Management. Clin. Med. 2018, 18, 155–159. [Google Scholar] [CrossRef]
- Borawake, K.; Prayag, P.; Wagh, A.; Dole, S. Dengue Encephalitis. Indian J. Crit. Care Med. 2011, 15, 190–193. [Google Scholar] [CrossRef]
- Ganguly, M.; Giri, P.P.; Mukherjee, M.; Jagwani, H.; Banerjee, A. Dengue Associated Demyelinating Disorders–A Report of 2 Cases. Neurol. India 2022, 70, 1244. [Google Scholar] [CrossRef]
- Puccioni-Sohler, M.; Ornelas, A.M.M.; de Souza, A.S.; Cabral-Castro, M.J.; Ramos, J.T.M.A.; Rosadas, C.; Salgado, M.C.F.; Castiglione, A.A.; Ferry, F.; Peralta, J.M.; et al. First Report of Persistent Dengue-1-Associated Autoimmune Neurological Disturbance: Neuromyelitis Optica Spectrum Disorder. J. Neurovirol. 2017, 23, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.T.; Estofolete, C.F.; Zini, N.; Terzian, A.C.; Gongora, D.V.; Maia, I.L.; Nogueira, M.L. Transverse Myelitis as an Unusual Complication of Dengue Fever. Am. J. Trop. Med. Hyg. 2017, 96, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Simon, O.; Billot, S.; Guyon, D.; Daures, M.; Descloux, E.; Gourinat, A.C.; Molko, N.; Dupont-Rouzeyrol, M. Early Guillain-Barré Syndrome Associated with Acute Dengue Fever. J. Clin. Virol. 2016, 77, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Mustafá, Y.M.; Meuren, L.M.; Coelho, S.V.A.; de Arruda, L.B. Pathways Exploited by Flaviviruses to Counteract the Blood-Brain Barrier and Invade the Central Nervous System. Front. Microbiol. 2019, 10, 525. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Calderón-Peláez, M.-A.; Castellanos, J.E. In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium. PLoS ONE 2016, 11, e0157786. [Google Scholar] [CrossRef] [PubMed]
- da Conceição, T.M.; Rust, N.M.; Berbel, A.C.E.R.; Martins, N.B.; do Nascimento Santos, C.A.; Da Poian, A.T.; de Arruda, L.B. Essential Role of RIG-I in the Activation of Endothelial Cells by Dengue Virus. Virology 2013, 435, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Velandia-Romero, M.L.; Acosta-Losada, O.; Castellanos, J.E. In Vivo Infection by a Neuroinvasive Neurovirulent Dengue Virus. J. Neurovirol. 2012, 18, 374–387. [Google Scholar] [CrossRef]
- Basu, A.; Chaturvedi, U.C. Vascular Endothelium: The Battlefield of Dengue Viruses. FEMS Immunol. Med. Microbiol. 2008, 53, 287–299. [Google Scholar] [CrossRef]
- Chien, Y.-W.; Wang, Y.-P.; Chi, C.-Y.; Shih, H.-I. Reinvestigation of the Risk of Stroke after Dengue Virus Infection: A Population-Based Cohort Study. J. Infect. Public Health 2023, 16, 1427–1434. [Google Scholar] [CrossRef]
- Carr, J.M.; Ashander, L.M.; Calvert, J.K.; Ma, Y.; Aloia, A.; Bracho, G.G.; Chee, S.-P.; Appukuttan, B.; Smith, J.R. Molecular Responses of Human Retinal Cells to Infection with Dengue Virus. Mediat. Inflamm. 2017, 2017, 3164375. [Google Scholar] [CrossRef]
- Ashander, L.M.; Lumsden, A.L.; Dawson, A.C.; Ma, Y.; Ferreira, L.B.; Oliver, G.F.; Appukuttan, B.; Carr, J.M.; Smith, J.R. Infection of Human Retinal Pigment Epithelial Cells with Dengue Virus Strains Isolated during Outbreaks in Singapore. Microorganisms 2022, 10, 310. [Google Scholar] [CrossRef]
- Salomão, N.; Rabelo, K.; Basílio-de-Oliveira, C.; Basílio-de-Oliveira, R.; Geraldo, L.; Lima, F.; dos Santos, F.; Nuovo, G.; Oliveira, E.R.A.; Paes, M. Fatal Dengue Cases Reveal Brain Injury and Viral Replication in Brain-Resident Cells Associated with the Local Production of Pro-Inflammatory Mediators. Viruses 2020, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Suwanmanee, S.; Luplertlop, N. Immunopathogenesis of Dengue Virus-Induced Redundant Cell Death: Apoptosis and Pyroptosis. Viral Immunol. 2017, 30, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jhan, M.-K.; Chen, C.-L.; Shen, T.-J.; Tseng, P.-C.; Wang, Y.-T.; Satria, R.D.; Yu, C.-Y.; Lin, C.-F. Polarization of Type 1 Macrophages Is Associated with the Severity of Viral Encephalitis Caused by Japanese Encephalitis Virus and Dengue Virus. Cells 2021, 10, 3181. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-R.; Tsai, T.-T.; Chen, C.-L.; Jhan, M.-K.; Tsai, C.-C.; Lee, Y.-C.; Chen, C.-H.; Lin, C.-F. Blockade of Dengue Virus Infection and Viral Cytotoxicity in Neuronal Cells in Vitro and in Vivo by Targeting Endocytic Pathways. Sci. Rep. 2017, 7, 6910. [Google Scholar] [CrossRef]
- Desprès, P.; Frenkiel, M.-P.; Ceccaldi, P.-E.; Duarte Dos Santos, C.; Deubel, V. Apoptosis in the Mouse Central Nervous System in Response to Infection with Mouse-Neurovirulent Dengue Viruses. J. Virol. 1998, 72, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Chen, B.-H.; Ma, S.-H.; Liu, C.-I.; Tsai, H.-P.; Wu, H.-C.; Jiang, S.-Y.; Yang, K.-D.; Shaio, M.-F. Potential Dengue Virus-Triggered Apoptotic Pathway in Human Neuroblastoma Cells: Arachidonic Acid, Superoxide Anion, and NF-κB Are Sequentially Involved. J. Virol. 2000, 74, 8680–8691. [Google Scholar] [CrossRef]
- Amorim, J.F.S.; Azevedo, A.S.; Costa, S.M.; Trindade, G.F.; Basílio-de-Oliveira, C.A.; Gonçalves, A.J.S.; Salomão, N.G.; Rabelo, K.; Amaral, R.; Geraldo, L.H.M.; et al. Dengue Infection in Mice Inoculated by the Intracerebral Route: Neuropathological Effects and Identification of Target Cells for Virus Replication. Sci. Rep. 2019, 9, 17926. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, X.; Hong, W.; Qiu, S.; Wang, J.; Yu, L.; Zeng, Y.; Tan, X.; Zhang, F. Slow Resolution of Inflammation in Severe Adult Dengue Patients. BMC Infect. Dis. 2016, 16, 291. [Google Scholar] [CrossRef]
- Simon, L.V.; Kong, E.L.; Graham, C. St Louis Encephalitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Azar, G.J.; Chappell, G.L.; Lawton, A.H.; Bond, J.O. Follow-up Studies of St. Louis Encephalitis in Florida: Sensorimotor Findings. Am. J. Public Health Nations Health 1966, 56, 1074–1081. [Google Scholar] [CrossRef]
- Greve, K.W.; Houston, R.J.; Adams, D.; Stanford, M.S.; Bianchini, K.J.; Clancy, A.; Rabito, F.J. The Neurobehavioural Consequences of St. Louis Encephalitis Infection. Brain Inj. 2002, 16, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.J.; Finley, K.H. Sequelae of Encephalitis; Report of a Study after the California Epidemic. Calif. Med. 1956, 84, 98–100. [Google Scholar]
- Parquet, M.C.; Kumatori, A.; Hasebe, F.; Mathenge, E.G.M.; Morita, K. St. Louis Encephalitis Virus Induced Pathology in Cultured Cells. Arch. Virol. 2002, 147, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.E.; Del Sarto, J.L.; Rocha, R.P.F.; Gomes, G.F.; Cramer, A.; Rachid, M.A.; Souza, D.G.; Nogueira, M.L.; Teixeira, M.M. Development of a Model of Saint Louis Encephalitis Infection and Disease in Mice. J. Neuroinflamm. 2017, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Zuza, A.L.; Barros, H.L.S.; de Mattos Silva Oliveira, T.F.; Chávez-Pavoni, J.H.; Zanon, R.G. Astrocyte Response to St. Louis Encephalitis Virus. Virus Res. 2016, 217, 92–100. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackhurst, B.M.; Funk, K.E. Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections. Viruses 2023, 15, 2200. https://doi.org/10.3390/v15112200
Blackhurst BM, Funk KE. Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections. Viruses. 2023; 15(11):2200. https://doi.org/10.3390/v15112200
Chicago/Turabian StyleBlackhurst, Britanie M., and Kristen E. Funk. 2023. "Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections" Viruses 15, no. 11: 2200. https://doi.org/10.3390/v15112200
APA StyleBlackhurst, B. M., & Funk, K. E. (2023). Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections. Viruses, 15(11), 2200. https://doi.org/10.3390/v15112200





