Antiviral Potential of Azelastine against Major Respiratory Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Viruses, Primers and Cells
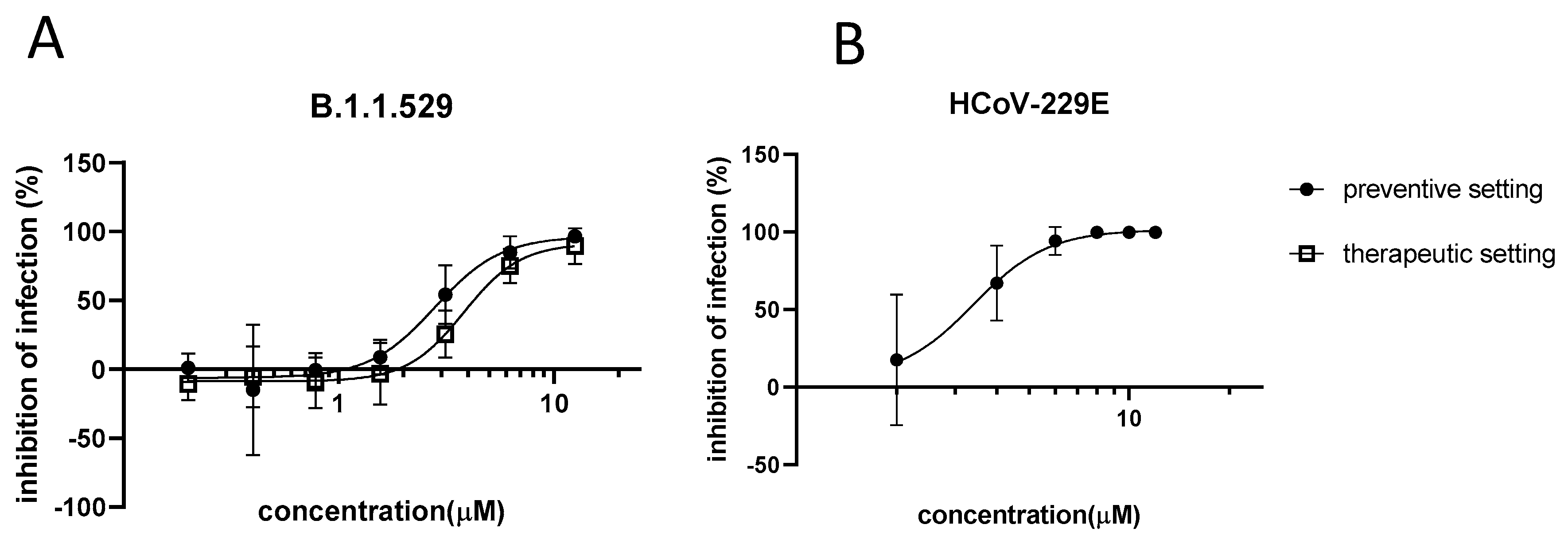
2.3. Infection of the Vero-TMPRSS2/ACE2 Cell Line by SARS-CoV-2 Omicron
2.4. Infection Model with 229E
2.5. In Vitro Testing against RSV
2.6. Influenza Induced Deregulation Model Using the Human Nasal 3D Tissue MucilAirTM
2.7. Efficacy Read-Out Monitoring in Influenza Model
2.8. Cytotoxicity Assays
2.9. Data Analysis and Statistical Analysis
3. Results
3.1. In Vitro Activity of Azelastine against Coronaviruses
3.2. Prophylactic and Therapeutic Efficacy of Azelastine on RSV Infection of Hep-2 Cells
3.3. Protective Effect of Azelastine in a Human Nasal 3D Tissue Infection Model of Influenza A
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, Y.; Mei, X.; Bushe, E.; Campbell, H.; Nair, H. Global Hospital Admissions and In-Hospital Mortality Associated with All-cause and virus-Specific acute Lower Respiratory Infections in Children and Adolescents Aged 5–19 Years between 1995 and 2019: A Systematic Review and Modelling Study. BMJ Glob. Health 2021, 6, 6014. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Lower Respiratory Infections in 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [CrossRef]
- Taubenberger, J.K.; Morens, D.M. Influenza: The Once and Future Pandemic. Public Health Rep. 2010, 125, 15–26. [Google Scholar] [CrossRef]
- Gray, G.C.; Robie, E.R.; Studstill, C.J.; Nunn, C.L. Mitigating Future Respiratory Virus Pandemics: New Threats and Approaches to Consider. Viruses 2021, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Baviskar, K.P.; Vaghela, D.A.; Raut, S.S.; Bedse, A.P. Nasal Sprays for Treating COVID-19: A Scientific Note The Post-COVID Era-Advances and Challenges In. Pharmacol. Rep. 2023, 75, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, Y.; Yang, H.; Yan, J.; Li, X.; Li, Z.; Zhao, Y.; Liang, H.; Wang, H. Advances in Next-Generation Coronavirus Vaccines in Response to Future Virus Evolution. Vaccines 2022, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Bedi, R.; Bayless, N.L.; Glanville, J. Challenges and Progress in Designing Broad-Spectrum Vaccines Against Rapidly Mutating Viruses. Annu. Rev. Biomed. Data Sci. 2023, 6, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Lo, C.W.; Einav, S. Preparing for the Next Viral Threat with Broad-Spectrum Antivirals. J. Clin. Investig. 2023, 133, e170236. [Google Scholar] [CrossRef]
- Ianevski, A.; Simonsen, R.M.; Myhre, V.; Tenson, T.; Oksenych, V.; Bjørås, M.; Bjørås, B.; Kainov, D.E. DrugVirus.Info 2.0: An Integrative Data Portal for Broad-Spectrum Antivirals (BSA) and BSA-Containing Drug Combinations (BCCs). Nucleic Acids Res. 2022, 50, W272–W275. [Google Scholar] [CrossRef]
- Andersen, P.I.; Ianevski, A.; Lysvand, H.; Vitkauskiene, A.; Oksenych, V.; Bjørås, M.; Telling, K.; Lutsar, I.; Dumpis, U.; Irie, Y.; et al. Discovery and Development of Safe-in-Man Broad-Spectrum Antiviral Agents. Int. J. Infect. Dis. 2020, 93, 268–276. [Google Scholar] [CrossRef]
- Klussmann, J.P.; Grosheva, M.; Meiser, P.; Lehmann, C.; Nagy, E.; Szijártó, V.; Nagy, G.; Konrat, R.; Flegel, M.; Holzer, F.; et al. Early Intervention with Azelastine Nasal Spray May Reduce Viral Load in SARS-CoV-2 Infected Patients. Sci. Rep. 2023, 13, 6839. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Blanco, M.A.; Ooi, E.E.; Sessions, O.M. RNA Viruses, Pandemics and Anticipatory Preparedness. Viruses 2022, 14, 2176. [Google Scholar] [CrossRef] [PubMed]
- Ternette, N.; Tippler, B.; Überla, K.; Grunwald, T. Immunogenicity and Efficacy of Codon Optimized DNA Vaccines Encoding the F-Protein of Respiratory Syncytial Virus. Vaccine 2007, 25, 7271–7279. [Google Scholar] [CrossRef] [PubMed]
- Essaidi-Laziosi, M.; Brito, F.; Benaoudia, S.; Royston, E.; Cagno, V.; Elanie Fernandes-Rocha, M.; Piuz, I.; Zdobnov, E.; Huang, S.; Constant, S.; et al. Propagation of Respiratory Viruses in Human Airway Epithelia Reveals Persistent Virus-Specific Signatures. J. Allergy Clin. Immunol. 2018, 141, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Riepler, L.; Rössler, A.; Falch, A.; Volland, A.; Borena, W.; Kimpel, J.; von Laer, D. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2021, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wiszniewski, L.; Constant, S. The Use of In Vitro 3D Cell Models in Drug Development for Respiratory Diseases. In Drug Discovery and Development; Kapetanovic, I.M., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Schreiber, S.S.; Kamahora, T.; Lai, M.M. Sequence Analysis of the Nucleocapsid Protein Gene of Human Coronavirus 229E. Virology 1989, 169, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Konrat, R.; Papp, H.; Kimpel, J.; Rössler, A.; Szijártó, V.; Nagy, G.; Madai, M.; Zeghbib, S.; Kuczmog, A.; Lanszki, Z.; et al. The Anti-Histamine Azelastine, Identified by Computational Drug Repurposing, Inhibits Infection by Major Variants of SARS-CoV-2 in Cell Cultures and Reconstituted Human Nasal Tissue. Front. Pharmacol. 2022, 13, 861295. [Google Scholar] [CrossRef]
- Fu Tseng, H.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of MRNA-1273 against SARS-CoV-2 Omicron and Delta Variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; Emmanuel San, J.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron Extensively but Incompletely Escapes Pfizer BNT162b2 Neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; Maclean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S. SARS-CoV-2 Omicron Is an Immune Escape Variant with an Altered Cell Entry Pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Akerman, A.; Milogiannakis, V.; Jean, T.; Esneau, C.; Silva, M.R.; Ison, T.; Fichter, C.; Lopez, J.A.; Chandra, D.; Naing, Z.; et al. Emergence and Antibody Evasion of BQ, BA.2.75 and SARS-CoV-2 Recombinant Sub-Lineages in the Face of Maturing Antibody Breadth at the Population Level. EBioMedicine 2023, 90, 104545. [Google Scholar] [CrossRef] [PubMed]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent Endemic Coronavirus Infection Is Associated with Less-Severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef] [PubMed]
- Waterlow, N.R.; Van Leeuwen, E.; Davies, N.G.; CMMID COVID-19 Working Group; Flasche, S.; Eggo, R.M. How Immunity from and Interaction with Seasonal Coronaviruses Can Shape SARS-CoV-2 Epidemiology. Proc. Natl. Acad. Sci. USA 2021, 118, e2108395118. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. Nature Reviews Immunology The Impact of Pre-Existing Cross-Reactive Immunity on SARS-CoV-2 Infection and Vaccine Responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, P.; Huttunen, M.; Iakubovskaia, A.; Maljanen, S.; Tauriainen, S.; Yatkin, E.; Pasternack, A.; Naves, R.; Toivonen, L.; Tähtinen, P.A.; et al. Coronavirus Spike Protein-Specific Antibodies Indicate Frequent Infections and Reinfections in Infancy and among BNT162b2-Vaccinated Healthcare Workers. Sci. Rep. 2023, 13, 8416. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Solanki, K.; Atre, R.; Lavrijsen, M.; Pan, Q.; Baig, M.S.; Li, P. Nirmatrelvir Exerts Distinct Antiviral Potency against Different Human Coronaviruses. Antivir. Res. 2023, 211, 105555. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanpour, M.M.; Tirado-Rives, J.; Deshmukh, M.; Ippolito, J.A.; Zhang, C.H.; Cabeza De Vaca, I.; Liosi, M.E.; Anderson, K.S.; Jorgensen, W.L. Identification of 14 Known Drugs as Inhibitors of the Main Protease of SARS-CoV-2. ACS Med. Chem. Lett. 2020, 11, 2526–2533. [Google Scholar] [CrossRef]
- Jain, R.; Mujwar, S. Repurposing Metocurine as Main Protease Inhibitor to Develop Novel Antiviral Therapy for COVID-19. Struct. Chem. 2020, 31, 2487–2499. [Google Scholar] [CrossRef]
- Odhar, H.A.; Ahjel, S.W.; Albeer, A.A.M.; Hashim, A.F.; Rayshan, A.M.; Humadi, S.S. Molecular Docking and Dynamics Simulation of FDA Approved Drugs with the Main Protease from 2019 Novel Coronavirus. Bioinformation 2020, 16, 236–244. [Google Scholar] [CrossRef]
- Bamia, A.; Sinane, M.; Naït-Saïdi, R.; Dhiab, J.; Keruzoré, M.; Nguyen, P.H.; Bertho, A.; Soubigou, F.; Halliez, S.; Blondel, M.; et al. Anti-Prion Drugs Targeting the Protein Folding Activity of the Ribosome Reduce PABPN1 Aggregation. Neurotherapeutics 2021, 18, 1137–1150. [Google Scholar] [CrossRef]
- Lane, T.R.; Ekins, S. Defending Antiviral Cationic Amphiphilic Drugs (CADs) That May Cause Drug-Induced Phospholipidosis Graphical Abstract HHS Public Access. J. Chem. Inf. Model. 2021, 61, 4125–4130. [Google Scholar] [CrossRef]
- Roingeard, P.; Eymieux, S.; Burlaud-Gaillard, J.; Hourioux, C.; Patient, R.; Blanchard, E. The Double-Membrane Vesicle (DMV): A Virus-Induced Organelle Dedicated to the Replication of SARS-CoV-2 and Other Positive-Sense Single-Stranded RNA Viruses. Cell. Mol. Life Sci. 2022, 79, 425. [Google Scholar] [CrossRef] [PubMed]
- Tummino, T.A.; Rezelj, V.V.; Fischer, B.; Fischer, A.; O’Meara, M.J.; Monel, B.; Vallet, T.; White, K.M.; Zhang, Z.; Alon, A.; et al. Drug-Induced Phospholipidosis Confounds Drug Repurposing for SARS-CoV-2. Science 2021, 373, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M.; Lavie, A. PH-Dependent Mechanisms of Influenza Infection Mediated by Hemagglutinin. Front. Mol. Biosci. 2021, 8, 777095. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory Syncytial Virus Entry and How to Block It. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Toprak, M.; Noory, M.A.; Robertson, G.P. Effect of Lysosomotropic Molecules on Cellular Homeostasis. Pharmacol. Res. 2017, 117, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kühnl, A.; Musiol, A.; Heitzig, N.; Johnson, D.E.; Ehrhardt, C.; Grewal, T.; Gerke, V.; Ludwig, S.; Rescher, U. Late Endosomal/Lysosomal Cholesterol Accumulation Is a Host Cell-Protective Mechanism Inhibiting Endosomal Escape of Influenza A Virus. mBio 2018, 9, e01345-18. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, L.; Xie, Z. Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus. Front. Cell Infect. Microbiol. 2022, 12, 858629. [Google Scholar] [CrossRef]
- Simon, M.W. The Efficacy of Azelastine in the Prophylaxis of Acute Upper Respiratory Tract Infections. Pediatr. Asthma Allergy Immunol. 2003, 16, 275–282. [Google Scholar] [CrossRef]
- ICTRP Search Portal. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00031059 (accessed on 24 October 2023).

| Primer | Sequence | Reference |
|---|---|---|
| N protein (229E) fw | 5′ TCTGCCAAGAGTCTTGCTCG 3′ | [17] |
| N protein (229E) rev | 5′ AGCATAGCAGCTGTTGACGG 3′ | [17] |
| E gene (SARS-CoV-2) fw | 5′ ACA GGT ACG TTA ATA GTT AAT AGC GT 3′ | [18] |
| E gene (SARS-CoV-2) rev | 5′ ATA TTG CAG CAG TAC GCA CAC A3′ | [18] |
| E gene (SARS-CoV-2) probe | FAM-ACA CTA GCC ATC CTT ACT GCG CTT CG-BHQ1 | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischhuber, K.; Bánki, Z.; Kimpel, J.; Kragl, N.; Rössler, A.; Bolze, A.; Muellauer, B.; Angerer, J.; Nagy, G.; Nagy, E.; et al. Antiviral Potential of Azelastine against Major Respiratory Viruses. Viruses 2023, 15, 2300. https://doi.org/10.3390/v15122300
Fischhuber K, Bánki Z, Kimpel J, Kragl N, Rössler A, Bolze A, Muellauer B, Angerer J, Nagy G, Nagy E, et al. Antiviral Potential of Azelastine against Major Respiratory Viruses. Viruses. 2023; 15(12):2300. https://doi.org/10.3390/v15122300
Chicago/Turabian StyleFischhuber, Katrin, Zoltán Bánki, Janine Kimpel, Natalie Kragl, Annika Rössler, Annika Bolze, Brigitte Muellauer, Joachim Angerer, Gábor Nagy, Eszter Nagy, and et al. 2023. "Antiviral Potential of Azelastine against Major Respiratory Viruses" Viruses 15, no. 12: 2300. https://doi.org/10.3390/v15122300
APA StyleFischhuber, K., Bánki, Z., Kimpel, J., Kragl, N., Rössler, A., Bolze, A., Muellauer, B., Angerer, J., Nagy, G., Nagy, E., & Szijarto, V. (2023). Antiviral Potential of Azelastine against Major Respiratory Viruses. Viruses, 15(12), 2300. https://doi.org/10.3390/v15122300







