Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines
Abstract
:1. Introduction
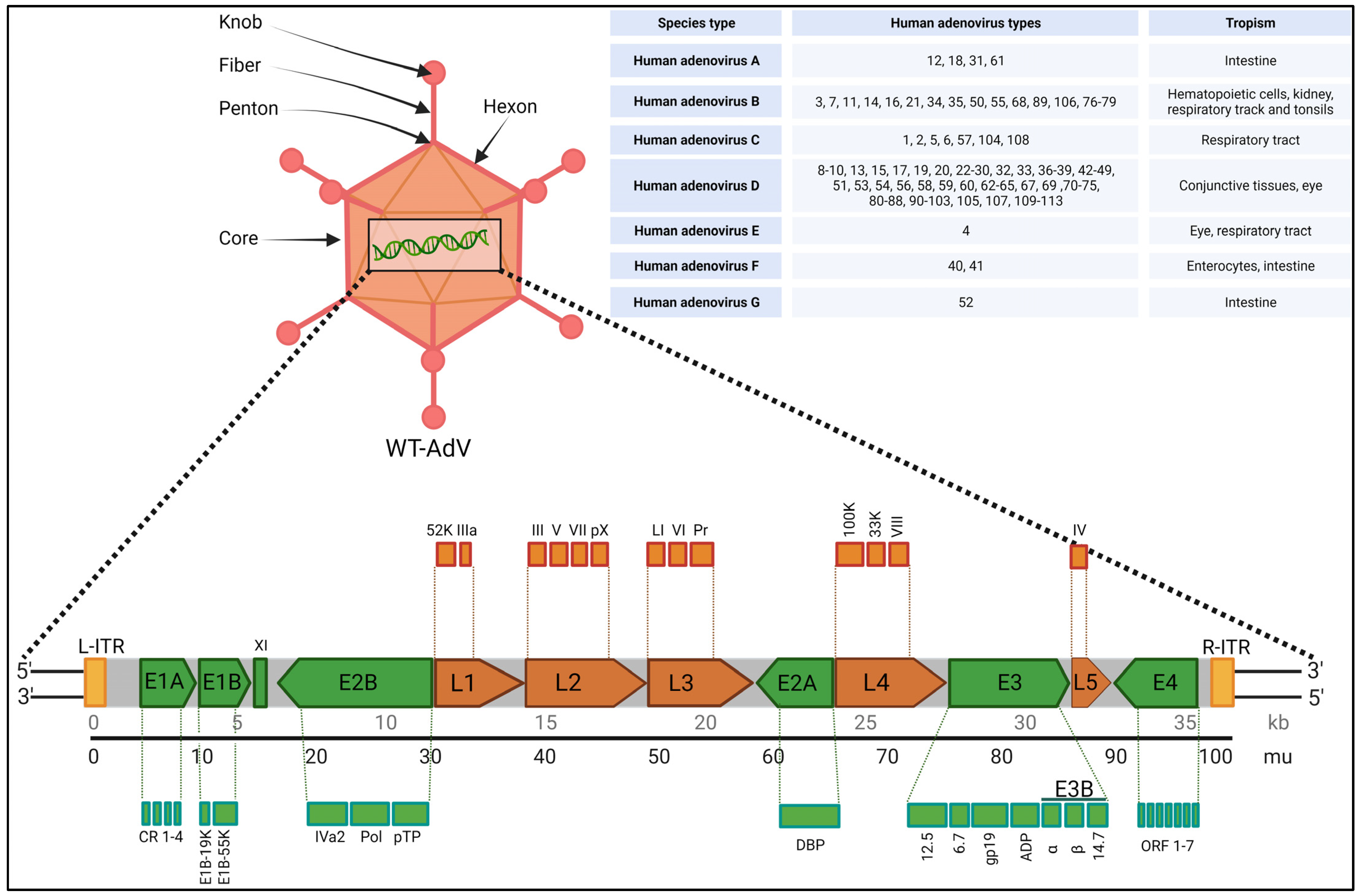
2. Biology, Species Types, Infection and Toxicity
3. Recombinant Adenoviruses: Safety, Modifications, Advantages, and Disadvantages
- (i)
- First-generation adenovirus (FGAdV) vectors: These vectors remove the E1 and E3 regions, allowing for increased cargo capacity by eliminating non-essential genetic information. These vectors cannot replicate on their own and rely on packaging cells, such as human embryonic kidney 293 cells expressing E1 protein, for production. The vector is capable of carrying up to 8.2 kb of foreign DNA cargo with higher immunogenicity [44]. These were used in certain earlier gene therapies. Vaccines often use FGAdV to trigger an apt immune response Modifications of early genes mostly depends on specific application.
- (ii)
- Conditionally replicating adenovirus (C-RAdV) vectors: These modified viral vectors involve the removal of E1 and/or E2 units that make it replication-deficient [45]. The vector is capable of replicating selectively in tumor cells, but not in normal cells, due to their abnormal retinoblastoma protein [46]. The engineering of tumor-specific gene promoters into the AdV genome is used to control the induction of viral replication to construct C-RAvD, which is regularly used in oncolytic virotherapy.
- (iii)
- Second-generation adenovirus (SGAdV) vectors: This generation of adenovirus was created to hold more genetic material (up to 12 Kb). The FGAdVs, despite E1 region deletion, induce strong host immune responses due to E1A-like factors in human cells. The SGAdV with E2 and E4 deletions was created to address this, but it still triggers host immune responses, resulting in reduced transgene expression in target cells [47]. More gene deletion was created by removing genes such as E2 and/or E4, in combination with E1 and E4 gene deletions. These are used in certain gene therapies for genetic disorders, vaccines, and certain types of cancers.
- (iv)
- Third-generation adenovirus (TGAdV) vectors: FGAdV and SGAdV vectors, despite E1–E4 region deletion, exhibit substantial immunogenicity and cytotoxicity. To accommodate larger therapeutic genes, third-generation adenovirus vectors were developed by removing the entire native adenovirus genome except for essential elements, thus raising the cargo limit to 36 kb. These ‘gutless adenovirus vectors’ enable high-level gene expression with minimal immune response. Production requires a co-introduced ‘helper adenovirus,’ but contamination concerns have led to the development of contamination-free methods using plasmids as helpers [48]. Incorporating stuffer DNA for efficient encapsidation in third-generation vectors has variable effects on transduction efficiency, with conflicting reports in the literature. These vectors do not have many regulatory genes and also do not have genetic elements, such as packing signals [48,49]. These gutless and safer vectors are regularly used in certain gene therapies for genetic disorders, vaccines, and certain types of cancers.
4. Recombinant Adenovirus Vector Production Methods
- (i)
- The traditional method/homologous recombination: The classical method for obtaining E1-deleted rAdVs involves homologous recombination of two DNA vectors. One vector contains a sequence mapping to the gene of interest at the left end of the adenovirus genome, while the other carries a sequence overlapping the 3′ viral segment and extending to the adenovirus genome’s right ITRs. This method is mostly used for generating FGAdV. This recombination process takes place in E-1-expressing cells such as HEK-293 cells. However, this laborious method is very inefficient in terms of recombination events and time consumption [50].
- (ii)
- Cre-LoxP-mediated recombination: To overcome limitations of the classical method, a Cre-lox site-specific recombination approach was developed. It involves three components: (a) a recombinant adenovirus with two loxP sites; (b) a shuttle vector containing ITR, an expression cassette, a packaging signal, and a loxP site; and (c) a 293-Cre cell line expressing Cre-recombinase. Transfection of the shuttle vector, containing the gene of interest and viral DNA, into a 293-Cre cell creates an adenovirus genome capable of replication, but it cannot be packaged. Recombination occurs between the loxP sites of the generated adenovirus genome and the shuttle vector, yielding the desired recombinant adenovirus. One drawback is the presence of parental adenovirus in the preparation, which persists even after multiple passages in 293-Cre cells, requiring careful recombinant virus verification [47,51].
- (iii)
- The AdEasy approach: This method employs HEK 293 cells to minimize homologous recombination issues, leveraging recombination in microorganisms such as yeast and bacterial cells. For instance, the AdEasy system aids in recovering the recombinant E. coli clone, primarily by introducing expression cassettes into the E1 region. After purifying recombinant plasmid DNA, it releases the viral chromosome and is subsequently transfected into the cell line. This system predominantly relies on E. coli rather than mammalian cells, benefiting from the bacterial machinery’s homologous capabilities [2,47].
- (iv)
- Use of Helper AdV for making TGAdV: The genome size of a virion should be within the range of 27.7–37 kb for proper packaging [52]. As discussed earlier third generational AdV/gutless AdV are helper-dependent adenovirus vector. TGAdV genomes include a noncoding eukaryotic ‘stuffer’, adenovirus ITRs, and a packaging signal [53]. In contrast, the helper virus (HV) lacks E1 and contains a packaging signal flanked by loxP sites. Infecting 293-Cre cells allows for the removal of the packaging signal from the helper virus genome, making it unpackageable but still capable of DNA replication, complementing TGAdV genome replication and encapsidation. An alternative TGAdV production system based on FLP/FRT site-specific recombination has also been developed, with similar results [54]. During packaging, helper virus contamination can be reduced via the Cre/LoxP or FLP/FRT systems if necessary [2,47]. Guo et al. recently conducted a study on restriction assembly for making a novel adenovirus vector. This is an easy-to-use method without the need for sophisticated instruments [55].
5. Applications of Recombinant Adenovirus Vector in Gene Therapy, Cancer, and Regenerative Medicine
5.1. Recombinant Adenovirus Vector Applied in Gene Therapy
5.2. Recombinant Adenovirus Vector Applied in Oncolytic Virotherapy
- Suicide genes: These genes make an enzyme that, when given a prodrug, triggers cell death. Suicide gene therapy approach is mostly used for solid tumors. After adenovirus is injected into the tumor (ITU), inactive prodrugs can be broken down into cytotoxic metabolites, leading to cell death. Adenovirus vectors have been designed to activate the p53 pathway, causing cell-cycle arrest and apoptosis in tumor cells, as many tumor types exhibit p53 dysfunction [62,63]. However, not all cancer cells lack p53. Various applications of adenoviruses in anticancer therapy have been explored beyond targeting p53 dysfunction. For example, herpes simplex virus thymidine kinase (HSV-TK) can convert prodrugs into cytotoxic compounds, such as converting fludarabine monophosphate (F-ara-AMP) into fluoroadenine or ganciclovir (GCV) into a cytotoxic nucleotide. It inhibits DNA polymerase and/or leads to incorporation into DNA and causes chain termination and tumoral cell death [64]. Additionally, the enzyme cytosine deaminase (CD) converts the prodrug 5-fluorocytosine (5-FC) into cytotoxic 5-fluorouridine (5-FU), causing DNA damage [65]. Other approaches include using varicella zoster virus-thymidine kinase (VZV-tk), purine nucleoside phosphorylase (PNP), and nitroreductase (NR). Modified adenovirus vectors delivering CD/HSV-TK chimeric enzymes, often in combination with other therapies such as radiotherapy, have shown effectiveness in clinical trials for various cancers, including prostate and pancreatic cancer [66,67]. Some vectors also carry additional genes, such as the human sodium iodide transporter (hNIS) for tumor imaging or pro-inflammatory cytokines such as IL-12 to trigger antitumor immune responses [68].
- Immunostimulatory genes: They introduce genes that regulate the immune system into the tumor cells, leading to a focused immune response. Adenovirus vectors can be equipped with immune-boosting genes to trigger the patient’s immune system against tumors. Delivering interferon (IFN)-β or IFN-α-2b using AdV vectors directly into the lungs has been shown to be a safe therapy for malignant pleural mesothelioma [69]. In Table 2, there are multiple active clinical trials ongoing using this strategy.
- Tumor suppressor drugs: These therapies reactivate the mutated tumor suppressor pathway. Tumor suppressor genes, such as p53, p16, RB, PTEN, and WWOX, are critical for controlling cell growth and differentiation under normal circumstances. Various strategies for tumor suppressor gene therapy exist. One such therapy, Gendicine, has been approved for clinical use. Combining p53 with treatments such as radiotherapy and chemotherapy has shown promise in treating cervical and liver cancers. Another approach involves inhibiting MDM2, a negative regulator of p53, with drugs such as RG7112, showing clinical activity in leukemia treatment [70]. Converting mutant p53 into a functional form using metallochaperones is another strategy, while vaccines targeting p53 mutant proteins have shown success in preclinical trials [71,72]. The adenovirus-mediated pRb94 has inhibited lung cancer cell growth, but wild-type Rb gene activation is challenging [73]. Additionally, introducing the PTEN gene can enhance the antitumor effect and reduce drug resistance in ovarian cancer cells. It has been evaluated with the rAdV approach as well [74].
5.3. Recombinant Adenovirus Vector Applied in Regenerative Medicine and Stem Cell Related Research
6. Vaccine Related Recombinant Adenovirus Development
7. Addressing Key Challenges for Enhanced Adenovirus Vector Performance
7.1. Pre-Existing Humoral and Cellular Immunity
7.2. Manufacturing Bottlenecks
7.3. Viral Vector Characterization Dilemma
7.4. Seroepidemiological Data
8. Recent Progress in the Field
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA. Approved Cellular and Gene Therapy Products; FDA: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 20 October 2023).
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, L.; Zhao, Y. Oncolytic adenovirus: A tool for reversing the tumor microenvironment and promoting cancer treatment (Review). Oncol. Rep. 2021, 45, 49. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Arenas, S.S.; Sirima, S.B.; Dzomo, G.R.T.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef]
- MacNeil, K.M.; Dodge, M.J.; Evans, A.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Adenoviruses in medicine: Innocuous pathogen, predator, or partner. Trends Mol. Med. 2023, 29, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy on trial. Science 2000, 288, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene Therapy Death Prompts Review of Adenovirus Vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, J.-L.; Feng, D.-Y.; Liu, X.-W.; Li, Y.; Wang, L.-F.; Yao, L.-B.; Zhang, H.; Zhang, J. The effect of adenovirus-conjugated NDRG2 on p53-mediated apoptosis of hepatocarcinoma cells through attenuation of nucleotide excision repair capacity. Biomaterials 2014, 35, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Syyam, A.; Nawaz, A.; Ijaz, A.; Sajjad, U.; Fazil, A.; Irfan, S.; Muzaffar, A.; Shahid, M.; Idrees, M.; Malik, K.; et al. Adenovirus vector system: Construction, history and therapeutic applications. BioTechniques 2022, 73, 297–305. [Google Scholar] [CrossRef]
- Berkner, K. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques 1998, 6, 616–629. [Google Scholar]
- Gallardo, J.; Pérez-Illana, M.; Martín-González, N.; San Martín, C. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021, 22, 5240. [Google Scholar] [CrossRef] [PubMed]
- Arrand, J.R.; Roberts, R.J. The nucleotide sequences at the termini of adenovirus-2 DNA. J. Mol. Biol. 1979, 128, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C. Looking Inside Adenovirus. Science 2010, 329, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Ahi, Y.S.; Mittal, S.K. Components of adenovirus genome packaging. Front. Microbiol. 2016, 7, 1503. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Human Adenovirus Gene Expression and Replication Is Regulated through Dynamic Changes in Nucleoprotein Structure throughout Infection. Viruses 2023, 15, 161. [Google Scholar] [CrossRef]
- Russell, W. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef]
- Baker, A.T.; Aguirre-Hernández, C.; Halldén, G.; Parker, A.L. Designer oncolytic adenovirus: Coming of age. Cancers 2018, 10, 201. [Google Scholar] [CrossRef]
- Goradel, N.H.; Baker, A.T.; Arashkia, A.; Ebrahimi, N.; Ghorghanlu, S.; Negahdari, B. Oncolytic virotherapy: Challenges and solutions. Curr. Probl. Cancer 2021, 45, 100639. [Google Scholar] [CrossRef]
- Sallard, E.; Zhang, W.; Aydin, M.; Schröer, K.; Ehrhardt, A. The Adenovirus Vector Platform: Novel Insights into Rational Vector Design and Lessons Learned from the COVID-19 Vaccine. Viruses 2023, 15, 204. [Google Scholar] [CrossRef]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T.; et al. Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep. 2019, 9, 1039. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccines Immunother. 2016, 12, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Volpers, C.; Kochanek, S. Adenoviral vectors for gene transfer and therapy. J. Gene Med. 2004, 6 (Suppl. S1), S164–S171. [Google Scholar] [CrossRef]
- Greber, U.F.; Flatt, J.W. Adenovirus entry: From infection to immunity. Annu. Rev. Virol. 2019, 6, 177–197. [Google Scholar] [CrossRef]
- Fessler, S.P.; Young, C.S. Control of adenovirus early gene expression during the late phase of infection. J. Virol. 1998, 72, 4049–4056. [Google Scholar] [CrossRef]
- Farley, D.C.; Brown, J.L.; Leppard, K.N. Activation of the early-late switch in adenovirus type 5 major late transcription unit expression by L4 gene products. J. Virol. 2004, 78, 1782–1791. [Google Scholar] [CrossRef]
- Abudoureyimu, M.; Lai, Y.; Tian, C.; Wang, T.; Wang, R.; Chu, X. Oncolytic Adenovirus—A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC. Front. Oncol. 2019, 9, 1182. [Google Scholar] [CrossRef]
- Tarakanova, V.L.; Wold, W.S. Adenovirus E1A and E1B-19K proteins protect human hepatoma cells from transforming growth factor beta1-induced apoptosis. Virus Res. 2010, 147, 67–76. [Google Scholar] [CrossRef]
- Yang, Z.R.; Wang, H.F.; Zhao, J.; Peng, Y.Y.; Wang, J.; Guinn, B.-A.; Huang, L.Q. Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther. 2007, 14, 599–615. [Google Scholar] [CrossRef]
- Watanabe, M.; Nishikawaji, Y.; Kawakami, H.; Kosai, K.-I. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses 2021, 13, 2502. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Gaden, F.; Franqueville, L.; Magnusson, M.K.; Hong, S.S.; Merten, M.D.; Lindholm, L.; Boulanger, P. Gene transduction and cell entry pathway of fiber-modified adenovirus type 5 vectors carrying novel endocytic peptide ligands selected on human tracheal glandular cells. J. Virol. 2004, 78, 7227–7247. [Google Scholar] [CrossRef]
- Ono, R.; Takayama, K.; Onishi, R.; Tokuoka, S.; Sakurai, F.; Mizuguchi, H. Treatment of Human Pancreatic Cancers Following Local and Systemic Administration of Oncolytic Adenovirus Serotype 35. Anticancer Res. 2023, 43, 537–546. [Google Scholar] [CrossRef]
- Dai, H.; Xi, H.; Huang, L.; Yuan, Z.; Liao, Y.; Tang, Y.; Liao, J.; Min, L.; Yu, Z. Molecular Epidemiology and Clinical Features Analysis of Respiratory Adenovirus Infections Reveals Correlations between Genotype, Inflammatory Biomarkers, and Disease Severity. Biomed. Res. Int. 2020, 2020, 4357910. [Google Scholar] [CrossRef] [PubMed]
- Jagirdhar, G.S.K.; Pulakurthi, Y.S.; Chigurupati, H.D.; Surani, S. Gastrointestinal tract and viral pathogens. World J. Virol. 2023, 12, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Imaizumi, S.; Kamoi, K. Viral Conjunctivitis. Viruses 2023, 15, 676. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.J. Human adenovirus infections in pediatric population—An update on clinico-pathologic correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Richardson, S.E.; MacGregor, D.; Mahant, S.; Raghuram, K.; Bitnun, A. Adenovirus-Associated Central Nervous System Disease in Children. J. Pediatr. 2019, 205, 130–137. [Google Scholar] [CrossRef]
- de Pablo, P.J.; San Martín, C. Seeing and touching adenovirus: Complementary approaches for understanding assembly and disassembly of a complex virion. Curr. Opin. Virol. 2022, 52, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Avgousti, D.C.; Herrmann, C.; Kulej, K.; Pancholi, N.J.; Sekulic, N.; Petrescu, J.; Molden, R.C.; Blumenthal, D.; Paris, A.J.; Reyes, E.D.; et al. A core viral protein binds host nucleosomes to sequester immune danger signals. Nature 2016, 535, 173–177. [Google Scholar] [CrossRef]
- Schwartz, U.; Komatsu, T.; Huber, C.; Lagadec, F.; Baumgartl, C.; Silberhorn, E.; Nuetzel, M.; Rayne, F.; Basyuk, E.; Bertrand, E.; et al. Changes in adenoviral chromatin organization precede early gene activation upon infection. EMBO J. 2023, 42, e114162. [Google Scholar] [CrossRef]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef]
- Hoeben, R.C.; Uil, T.G. Adenovirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a013003. [Google Scholar] [CrossRef] [PubMed]
- Gorziglia, M.I.; Lapcevich, C.; Roy, S.; Kang, Q.; Kadan, M.; Wu, V.; Pechan, P.; Kaleko, M. Generation of an adenovirus vector lacking E1, E2a, E3, and all of E4 except open reading frame 3. J. Virol. 1999, 73, 6048–6055. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.; Radzniak, L.; Santoro, M.; Tsao, Y.-S.; Condon, R.G.G.; Lio, P.; Voloch, M.; Liu, Z. Development of a Serum-free Suspension Process for the Production of a Conditionally Replicating Adenovirus using A549 Cells. Cytotechnology 2005, 49, 161–171. [Google Scholar] [CrossRef]
- Johnson, L.; Shen, A.; Boyle, L.; Kunich, J.; Pandey, K.; Lemmon, M.; Hermiston, T.; Giedlin, M.; McCormick, F.; Fattaey, A. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell 2002, 1, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef]
- Liu, J.; Seol, D.-W. Helper virus-free gutless adenovirus (HF-GLAd): A new platform for gene therapy. BMB Rep. 2020, 53, 565. [Google Scholar] [CrossRef]
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-capacity adenoviral vectors: Expanding the scope of gene therapy. Int. J. Mol. Sci. 2020, 21, 3643. [Google Scholar] [CrossRef]
- Danthinne, X.; Imperiale, M.J. Production of first generation adenovirus vectors: A review. Gene Ther. 2000, 7, 1707–1714. [Google Scholar] [CrossRef]
- Duan, D.; Yue, Y.; Yan, Z.; Engelhardt, J.F. DNA Virus Vectors II. Mol. Ther. 2000, 1, S169–S184. [Google Scholar]
- Zhang, P.; Miao, D.; Zhang, Y.; Wang, M.; Hu, Z.; Lü, P.; Yao, Q. Cloning and rescue of the genome of Bombyx mori bidensovirus, and characterization of a recombinant virus. Virol. J. 2016, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Rosewell, A.; Vetrini, F.; Ng, P. Helper-Dependent Adenoviral Vectors. J. Genet. Syndr. Gene Ther. 2011. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.V.; Contzen, J.; Seemann, P.; Gossen, M. Site-specific chromosomal gene insertion: Flp recombinase versus Cas9 nuclease. Sci. Rep. 2017, 7, 17771. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, Y.; Chen, J.; Zou, X.; Hou, W.; Tan, W.; Hung, T.; Lu, Z. Restriction-Assembly: A Solution to Construct Novel Adenovirus Vector. Viruses 2022, 14, 546. [Google Scholar] [CrossRef]
- Zhao, Z.; Anselmo, A.C.; Mitragotri, S. Viral vector-based gene therapies in the clinic. Bioeng. Transl. Med. 2022, 7, e10258. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.A.; Siegfried, W.; Yoshimura, K.; Yoneyama, K.; Fukayama, M.; Stier, L.E.; Pääkkö, P.K.; Gilardi, P.; Stratford-Perricaudet, L.D.; Perricaudet, M.; et al. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science 1991, 252, 431–434. [Google Scholar] [CrossRef]
- Dormond, E.; Perrier, M.; Kamen, A. From the first to the third generation adenoviral vector: What parameters are governing the production yield? Biotechnol. Adv. 2009, 27, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rininger, J.; Fennell, A.; Schoukroun-Barnes, L.; Peterson, C.; Speidel, J. Capacity analysis for viral vector manufacturing: Is there enough? In Bioprocess International; Latham Biopharm Group: Cambridge, MA, USA, 2019. [Google Scholar]
- Sheldon, Y.; Yoo, B.C.; Kmiec, E.; Petrelli, N.; Tiesi, G.; Warner, S. Review of Safety Data for Adenovirus 5 as a Delivery Vector for Intratumoral Cancer Gene Therapy. Hum. Gene Ther. 2023, 34, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Bressy, C.; Hastie, E.; Grdzelishvili, V.Z. Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Mol. Ther.-Oncolytics 2017, 5, 20–40. [Google Scholar] [CrossRef]
- Wold, S.M.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Colombo, F.; Barzon, L.; Franchin, E.; Pacenti, M.; Pinna, V.; Danieli, D.; Zanusso, M.; Palù, G. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: Biological and clinical results. Cancer Gene Ther. 2005, 12, 835–848. [Google Scholar] [CrossRef]
- Freytag, S.O.; Stricker, H.; Lu, M.; Elshaikh, M.; Aref, I.; Pradhan, D.; Levin, K.; Kim, J.H.; Peabody, J.; Siddiqui, F.; et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 268–276. [Google Scholar] [CrossRef]
- Ayala, G.; Satoh, T.; Li, R.; Shalev, M.; Gdor, Y.; Aguilar-Cordova, E.; Frolov, A.; Wheeler, T.M.; Miles, B.J.; Rauen, K.; et al. Biological response determinants in HSV-tk+ ganciclovir gene therapy for prostate cancer. Mol. Ther. 2006, 13, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, T.; Terao, S.; Hinata, N.; Tanaka, K.; Takenaka, A.; Hara, I.; Sugimura, K.; Matsuo, M.; Hamada, K.; Fuji, K.; et al. Long-term outcome of phase I/II clinical trial of Ad-OC-TK/VAL gene therapy for hormone-refractory metastatic prostate cancer. Hum. Gene Ther. 2007, 18, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.; Barton, K.; Zhang, Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013, 20, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shi, Q.; Zhang, H.; Yang, K.; Ke, Y.; Wang, Y.; Qiao, L. Advances in the techniques and methodologies of cancer gene therapy. Discov. Med. 2019, 27, 45–55. [Google Scholar] [PubMed]
- Andreeff, M.; Kelly, K.R.; Yee, K.; Assouline, S.; Strair, R.; Popplewell, L.; Bowen, D.; Martinelli, G.; Drummond, M.W.; Vyas, P.; et al. Results of the Phase I Trial of RG7112, a Small-Molecule MDM2 Antagonist in Leukemia. Clin. Cancer Res. 2016, 22, 868–876. [Google Scholar] [CrossRef]
- Blanden, A.R.; Yu, X.; Loh, S.N.; Levine, A.J.; Carpizo, D.R. Reactivating mutant p53 using small molecules as zinc metallochaperones: Awakening a sleeping giant in cancer. Drug Discov. Today 2015, 20, 1391–1397. [Google Scholar] [CrossRef]
- Ellebaek, E.; Engell-Noerregaard, L.; Iversen, T.Z.; Froesig, T.M.; Munir, S.; Hadrup, S.R.; Andersen, M.H.; Svane, I.M. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: Results from a phase II trial. Cancer Immunol. Immunother 2012, 61, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Q.; Zhang, P.; Chen, F.; Cheng, Y. Role of adenovirus-mediated retinoblastoma 94 in the treatment of human non-small cell lung cancer. Mol. Med. Rep. 2015, 11, 3349–3353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, X.; Xu, C.; Yang, J.; Xiang, J.; Tao, M.; Xie, Y. Synergistic tumor suppression by adenovirus-mediated ING4/PTEN double gene therapy for gastric cancer. Cancer Gene Ther. 2016, 23, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; Mccormick, F.; Von Hoff, D.D.; Kirn, D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997, 3, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.D.; Berk, A.J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 1987, 156, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Ganly, I.; Khuri, F.; Arseneau, J.; Kuhn, J.; Mccarty, T.; Landers, S.; Maples, P.; Romel, L.; Randlev, B.; et al. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: A phase II trial. Cancer Res. 2000, 60, 6359–6366. [Google Scholar]
- Garber, K. China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006, 98, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Babiker, H.M.; Riaz, I.B.; Husnain, M.; Borad, M.J. Oncolytic virotherapy including Rigvir and standard therapies in malignant melanoma. Oncolytic Virother. 2017, 6, 11–18. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Li, L.; Wu, J.; Zhang, H.; Zhang, H.; Lei, T.; Xu, B. Oncolytic adenovirus: Prospects for cancer immunotherapy. Front. Microbiol. 2021, 12, 707290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, C.; Miao, J.; Wang, Z.; Wang, Z.; Cheng, Z.; Wang, P.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, Y. A Tumor-Targeted Replicating Oncolytic Adenovirus Ad-TD-nsIL12 as a Promising Therapeutic Agent for Human Esophageal Squamous Cell Carcinoma. Cells 2020, 9, 2438. [Google Scholar] [CrossRef] [PubMed]
- Kesari, S.; Bessudo, A.; Gastman, B.R.; Conley, A.P.; Villaflor, V.M.; Nabell, L.M.; Madere, D.; Chacon, E.; Spencer, C.; Li, L.; et al. BETA PRIME: Phase I study of AdAPT-001 as monotherapy and combined with a checkpoint inhibitor in superficially accessible, treatment-refractory solid tumors. Future Oncol. 2022, 18, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.R.; Daluiski, A.; Stevenson, S.; Jolla, L.; Wu, L.; McALLISTER, P.; Lee, Y.P.; Kabo, J.M.; Finerman, G.A.; Berk, A.J.; et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. JBJS 1999, 81, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ries, J.; Gelse, K.; Kloss, F.; von der Mark, K.; Wiltfang, J.; Neukam, F.W.; Schneider, H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: A comparison of adenoviral vectors and liposomes. Gene Ther. 2003, 10, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Haydon, R.C.; Cheng, H.; Ishikawa, A.; Nenadovich, N.; Jiang, W.; Zhou, L.; Breyer, B.; Feng, T.; Gupta, P.; et al. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine 2003, 28, 755. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Kang, Q.; Luo, J.; He, T.-C.; Haydon, R.C.; Mass, D.P. Characterization of adenovirus-mediated gene transfer in rabbit flexor tendons. J. Hand Surg. 2005, 30, 136–141. [Google Scholar] [CrossRef]
- Bolt, P.; Clerk, A.N.; Luu, H.H.; Kang, Q.; Kummer, J.L.; Deng, Z.L.; Olson, K.; Primus, F.; Montag, A.G.; He, T.C.; et al. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. JBJS 2007, 89, 1315–1320. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, M.M.; Ene, C.; Lang, F.F.; Kan, P. Perfusion-guided endovascular super-selective intra-arterial infusion for treatment of malignant brain tumors. J. Neurointerv. Surg. 2022, 14, 533–538. [Google Scholar] [CrossRef]
- Wang, H.; Germond, A.; Li, C.; Gil, S.; Kim, J.; Kiem, H.P.; Lieber, A. In vivo HSC transduction in rhesus macaques with an HDAd5/3+ vector targeting desmoglein 2 and transiently overexpressing cxcr4. Blood Adv. 2022, 6, 4360–4372. [Google Scholar] [CrossRef] [PubMed]
- Haruta, K.; Takeuchi, S.; Yamaguchi, M.; Horiba, K.; Suzuki, T.; Torii, Y.; Narita, A.; Muramatsu, H.; Takahashi, Y.; Ito, Y.; et al. Droplet Digital PCR Development for Adenovirus Load Monitoring in Children after Hematopoietic Stem Cell Transplantation. J. Mol. Diagn. 2023, 25, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, E.T.; Gaydos, J.C.; Allen, R.G.; Top, F.H., Jr. Simultaneous administration of live, enteric-coated adenovirus types 4, 7, and 21 vaccines: Safety and immunogenicity. J. Infect. Dis. 1979, 140, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Hou, L.-H.; Li, J.-X.; Wu, S.-P.; Liu, P.; Zhang, G.-R.; Hu, Y.-M.; Meng, F.-Y.; Xu, J.-J.; Tang, R.; et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: Preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015, 385, 2272–2279. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; Vega, M.A.D.L.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Launay, O.; Lelievre, J.-D.; Lacabaratz, C.; Grande, S.; Goldstein, N.; Robinson, C.; Gaddah, A.; Bockstal, V.; Wiedemann, A.; et al. Safety and immunogenicity of a two-dose heterologous Ad26. ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): A randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2021, 21, 493–506. [Google Scholar] [CrossRef]
- Afolabi, M.O.; Ishola, D.; Manno, D.; Keshinro, B.; Bockstal, V.; Rogers, B.; Owusu-Kyei, K.; Serry-Bangura, A.; Swaray, I.; Lowe, B.; et al. Safety and immunogenicity of the two-dose heterologous Ad26. ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: A randomised, double-blind, controlled trial. Lancet Infect. Dis. 2022, 22, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Ishola, D.; Manno, D.; Afolabi, M.O.; Keshinro, B.; Bockstal, V.; Rogers, B.; Owusu-Kyei, K.; Serry-Bangura, A.; Swaray, I.; Lowe, B.; et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26. ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: A combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect. Dis. 2022, 22, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Anywaine, Z.; Barry, H.; Anzala, O.; Mutua, G.; Sirima, S.B.; Eholie, S.; Kibuuka, H.; Bétard, C.; Richert, L.; Lacabaratz, C.; et al. Safety and immunogenicity of 2-dose heterologous Ad26. ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: A randomised, placebo-controlled, multicentre Phase II clinical trial. PLoS Med. 2022, 19, e1003865. [Google Scholar] [CrossRef]
- Watson-Jones, D.; Kavunga-Membo, H.; Grais, R.F.; Ahuka, S.; Roberts, N.; Edmunds, W.J.; Choi, E.M.; Roberts, C.H.; Edwards, T.; Camacho, A.; et al. Protocol for a phase 3 trial to evaluate the effectiveness and safety of a heterologous, two-dose vaccine for Ebola virus disease in the Democratic Republic of the Congo. BMJ Open 2022, 12, e055596. [Google Scholar] [CrossRef]
- Wong, G.; Mendoza, E.J.; Plummer, F.A.; Gao, G.F.; Kobinger, G.P.; Qiu, X. From bench to almost bedside: The long road to a licensed Ebola virus vaccine. Expert Opin. Biol. Ther. 2018, 18, 159–173. [Google Scholar] [CrossRef]
- Lévy, Y.; Lane, C.; Piot, P.; Beavogui, A.H.; Kieh, M.; Leigh, B.; Doumbia, S.; D’Ortenzio, E.; Lévy-Marchal, C.; Pierson, J.; et al. Prevention of Ebola virus disease through vaccination: Where we are in 2018. Lancet 2018, 392, 787–790. [Google Scholar] [CrossRef]
- Mwesigwa, B.; Houser, K.V.; Hofstetter, A.R.; Ortega-Villa, A.M.; Naluyima, P.; Kiweewa, F.; Nakabuye, I.; Yamshchikov, G.V.; Andrews, C.; O’Callahan, M.; et al. Safety, tolerability, and immunogenicity of the Ebola Sudan chimpanzee adenovirus vector vaccine (cAd3-EBO S) in healthy Ugandan adults: A phase 1, open-label, dose-escalation clinical trial. Lancet Infect. Dis. 2023, 23, 1408–1417. [Google Scholar] [CrossRef]
- Barry, H.; Mutua, G.; Kibuuka, H.; Anywaine, Z.; Sirima, S.B.; Meda, N.; Anzala, O.; Eholie, S.; Bétard, C.; Richert, L.; et al. Safety and immunogenicity of 2-dose heterologous Ad26. ZEBOV, MVA-BN-Filo Ebola vaccination in healthy and HIV-infected adults: A randomised, placebo-controlled Phase II clinical trial in Africa. PLoS Med. 2021, 18, e1003813. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Yang, X.; Sun, J.; Ding, X.; Chen, Z.; Su, W.; Cai, L.; Hou, A.; Sun, B.; Gao, F.; et al. An Adenovirus-Based Recombinant Herpes Simplex Virus 2 (HSV-2) Therapeutic Vaccine Is Highly Protective against Acute and Recurrent HSV-2 Disease in a Guinea Pig Model. Viruses 2023, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Wiehe, K.; Borrrow, P.; Saunders, K.O.; Korber, B.; Wagh, K.; McMichael, A.J.; Kelsoe, G.; Hahn, B.H.; Alt, F.; et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 2023, 23, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Sekaly, R.-P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef]
- Churchyard, G.J.; Morgan, C.; Adams, E.; Hural, J.; Graham, B.S.; Moodie, Z.; Grove, D.; Gray, G.; Bekker, L.-G.; McElrath, M.J.; et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS ONE 2011, 6, e21225. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Liu, J.; Li, H.; Johnson, J.A.; Walsh, S.R.; Kleinjan, J.A.; Engelson, B.A.; Peter, L.; Abbink, P.; Milner, D.A.; et al. Induction of HIV-1–specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J. Infect. Dis. 2015, 211, 518–528. [Google Scholar] [CrossRef]
- Fuchs, J.D.; Alex, P.; Bart, R.; Frahm, N.; Morgan, C.; Gilbert, P.B.; Kochar, N.; DeRosa, S.C.; Tomaras, G.D.; Wagner, T.M.; et al. Safety and immunogenicity of a recombinant adenovirus serotype 35-vectored HIV-1 vaccine in adenovirus serotype 5 seronegative and seropositive individuals. J. AIDS Clin. Res. 2015, 6, 1000461. [Google Scholar] [CrossRef]
- Check Hayden, E. Mystery of HIV vaccine failure deepens. Nature 2009. [Google Scholar] [CrossRef]
- Baden, L.R.; Walsh, S.R.; Seaman, M.S.; Tucker, R.P.; Krause, K.H.; Patel, A.; Johnson, J.A.; Kleinjan, J.; Yanosick, K.E.; Perry, J.; et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J. Infect. Dis. 2013, 207, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Cuburu, N.; Khan, S.; Thompson, C.D.; Kim, R.; Vellinga, J.; Zahn, R.; Lowy, D.R.; Scheper, G.; Schiller, J.T. Adenovirus vector-based prime-boost vaccination via heterologous routes induces cervicovaginal CD8(+) T cell responses against HPV16 oncoproteins. Int. J. Cancer 2018, 142, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Chen, H.; Apkarian, M.; Affrime, M.; Bock, B.; Kim, K.; Mukherjee, N.; Nolan, G.P.; McNeal, M.M. Performance of BioFire array or QuickVue influenza A + B test versus a validation qPCR assay for detection of influenza A during a volunteer A/California/2009/H1N1 challenge study. Virol. J. 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Liebowitz, D.; Gottlieb, K.; Kolhatkar, N.S.; Garg, S.J.; Asher, J.M.; Nazareno, J.; Kim, K.; McIlwain, D.R.; Tucker, S.N. Efficacy, immunogenicity, and safety of an oral influenza vaccine: A placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 2020, 20, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Rejinold, N.S.; Piao, H.; Ryu, Y.B.; Kwon, H.J.; Lee, I.C.; Seo, J.I.; Yoo, H.H.; Jin, G.W.; Choy, J.H. The Next Generation COVID-19 Antiviral; Niclosamide-Based Inorganic Nanohybrid System Kills SARS-CoV-2. Small 2023, e2305148. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Bittaye, M.; Flaxman, A.; Lopez, F.R.; Bellamy, D.; Kupke, A.; Mair, C.; Makinson, R.; Sheridan, J.; Rohde, C.; et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: A dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 816–826. [Google Scholar] [CrossRef]
- Bosaeed, M.; Balkhy, H.H.; Almaziad, S.; Aljami, H.A.; Alhatmi, H.; Alanazi, H.; Alahmadi, M.; Jawhary, A.; Alenazi, M.W.; Almasoud, A.; et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): An open-label, non-randomised, dose-escalation, phase 1b trial. Lancet Microbe 2022, 3, e11–e20. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Infectious Diseases. Respiratory syncytial virus. In Red Book: 2006 Report of Committee on Infectious Diseases; Kimberlin, D.W., Ed.; American Academy of Pediatrics: Elk Grove Village, USA, 2006. [Google Scholar]
- WHO. Guidelines on the Quality, Safety and Efficacy of Respiratory Syncytial Virus Vaccines; WHO: Geneva, Switzerland, 2019.
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.K.; et al. Efficacy and Safety of an Ad26.RSV.preF–RSV preF Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef]
- Stuart, A.S.; Virta, M.; Williams, K.; Seppa, I.; Hartvickson, R.; Greenland, M.; Omoruyi, E.; Bastian, A.R.; Haazen, W.; Salisch, N.; et al. Phase 1/2a Safety and Immunogenicity of an Adenovirus 26 Vector Respiratory Syncytial Virus (RSV) Vaccine Encoding Prefusion F in Adults 18–50 Years and RSV-Seropositive Children 12–24 Months. J. Infect. Dis. 2022, 227, 71–82. [Google Scholar] [CrossRef]
- Sadoff, J.; De Paepe, E.; Haazen, W.; Omoruyi, E.; Bastian, A.R.; Comeaux, C.; Heijnen, E.; Strout, C.; Schuitemaker, H.; Callendret, B. Safety and Immunogenicity of the Ad26.RSV.preF Investigational Vaccine Coadministered with an Influenza Vaccine in Older Adults. J. Infect. Dis. 2021, 223, 699–708. [Google Scholar] [CrossRef]
- GSK. US FDA Approves GSK’s Arexvy, the World’s First Respiratory Syncytial Virus (RSV) Vaccine for Older Adults; GSK: London, UK, 2023. [Google Scholar]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.; Pahud, B.A.; Llapur, C.; Baker, J.; Marc, G.P.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants; FDA: Silver Spring, MD, USA, 2023. [Google Scholar]
- Hao, M.; Bian, T.; Fu, G.; Chen, Y.; Fang, T.; Zhao, C.; Liu, S.; Yu, C.; Li, J.; Chen, W. An adenovirus-vectored RVF vaccine confers complete protection against lethal RVFV challenge in A129 mice. Front. Microbiol. 2023, 14, 1114226. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard; WHO: Geneva, Switzerland, 2023.
- Clemens, S.A.C.; Milan, E.P.; Sprinz, E.; Neto, J.C.; Pacciarini, F.; Li, P.; Chen, H.L.; Smolenov, I.; Pollard, A.; Clemens, R. Homologous and Heterologous Boosting of the Chadox1-S1-S COVID-19 Vaccine with the SCB-2019 Vaccine Candidate: A Randomized, Controlled, Phase 2 Study. Open Forum Infect. Dis. 2022, 9, ofac418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Li, Y.-H.; Guan, X.-H.; Hou, L.-H.; Wang, W.-J.; Li, J.-X.; Wu, S.-P.; Wang, B.-S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.M.; Self, W.H.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; Ghamande, S.A.; et al. Effectiveness of the Ad26.COV2.S (Johnson & Johnson) Coronavirus Disease 2019 (COVID-19) Vaccine for Preventing COVID-19 Hospitalizations and Progression to High Disease Severity in the United States. Clin. Infect. Dis. 2022, 75 Suppl. S2, S159–S166. [Google Scholar] [PubMed]
- Noushad, M.; Nassani, M.Z.; Samran, A.; Dimashkieh, M.R.; Al-Awar, M.S. COVID-19 and herpes zoster: A call to action. Front. Public Health 2023, 11, 1200353. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.; Merelie, S.; Sebastian, S.; Lambe, T. Preclinical immunogenicity of an adenovirus-vectored vaccine for herpes zoster. Hum. Vaccines Immunother. 2023, 19, 2175558. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Kulkarni, M.A.; Brownstein, J.S.; Mekaru, S.R.; Hay, S.I.; Groot, E.; et al. Anticipating the international spread of Zika virus from Brazil. Lancet 2016, 387, 335–336. [Google Scholar] [CrossRef]
- Xu, K.; Song, Y.; Dai, L.; Zhang, Y.; Lu, X.; Xie, Y.; Zhang, H.; Cheng, T.; Wang, Q.; Huang, Q.; et al. Recombinant chimpanzee adenovirus vaccine AdC7-M/E protects against Zika virus infection and testis damage. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Guo, Q.; Chan, J.F.-W.; Poon, V.K.-M.; Wu, S.; Chan, C.C.-S.; Hou, L.; Yip, C.C.-Y.; Ren, C.; Cai, J.-P.; Zhao, M.; et al. Immunization with a novel human type 5 adenovirus-vectored vaccine expressing the premembrane and envelope proteins of Zika virus provides consistent and sterilizing protection in multiple immunocompetent and immunocompromised animal models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef]
- Salisch, N.C.; Stephenson, K.E.; Williams, K.; Cox, F.; van der Fits, L.; Heerwegh, D.; Truyers, C.; Habets, M.N.; Kanjilal, D.G.; Larocca, R.A.; et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26. ZIKV. 001, an Ad26-vectored anti–Zika virus vaccine. Ann. Intern. Med. 2021, 174, 585–594. [Google Scholar] [CrossRef]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef] [PubMed]
- Bacon, A.; Teixeira, M.; Costa, V.; Bone, P.; Simmons, J.; Drew, J. Generation of a thermostable, oral Zika vaccine that protects against virus challenge in non-human primates. Vaccine 2023, 41, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Dold, C.; Marsay, L.; Wang, N.; Silva-Reyes, L.; Clutterbuck, E.; Paterson, G.K.; Sharkey, K.; Wyllie, D.; Beernink, P.T.; Hill, A.V.; et al. An adenoviral-vectored vaccine confers seroprotection against capsular group B meningococcal disease. Sci. Transl. Med. 2023, 15, eade3901. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.-H.; Lowrie, D.B.; Fan, X.-Y. Research Advances for Virus-vectored Tuberculosis Vaccines and Latest Findings on Tuberculosis Vaccine Development. Front. Immunol. 2022, 13, 895020. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Wieland, C.W.; Rodriguez, A.; Weverling, G.J.; Mintardjo, R.; Gillissen, G.; Vogels, R.; Skeiky, Y.A.W.; Hone, D.M.; Sadoff, J.C.; et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect. Immun. 2007, 75, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Bolton, D.L.; Lackner, A.A.; Kaushal, D.; Aye, P.P.; Mehra, S.; Blanchard, J.L.; Didier, P.J.; Roy, C.J.; Rao, S.S.; et al. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J. Immunol. 2014, 193, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Abel, B.; Tameris, M.; Mansoor, N.; Gelderbloem, S.; Hughes, J.; Abrahams, D.; Makhethe, L.; Erasmus, M.; Kock, M.D.; van der Merwe, L.; et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am. J. Respir. Crit. Care Med. 2010, 181, 1407–1417. [Google Scholar] [CrossRef]
- Stylianou, E.; Harrington-Kandt, R.; Beglov, J.; Bull, N.; Pinpathomrat, N.; Swarbrick, G.M.; Lewinsohn, D.A.; Lewinsohn, D.M.; McShane, H. Identification and evaluation of novel protective antigens for the development of a candidate tuberculosis subunit vaccine. Infect. Immun. 2018, 86, e00014-18. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Thanthrige-Don, N.; Afkhami, S.; Lai, R.; Damjanovic, D.; Zganiacz, A.; Feng, X.; Yao, X.-D.; Rosenthal, K.L.; Medina, M.F.; et al. Novel chimpanzee adenovirus-vectored respiratory mucosal tuberculosis vaccine: Overcoming local anti-human adenovirus immunity for potent TB protection. Mucosal Immunol. 2015, 8, 1373–1387. [Google Scholar] [CrossRef]
- Afkhami, S.; Lai, R.; D’agostino, M.R.; Vaseghi-Shanjani, M.; Zganiacz, A.; Yao, Y.; Jeyanathan, M.; Xing, Z. Single-dose mucosal immunotherapy with chimpanzee adenovirus-based vaccine accelerates tuberculosis disease control and limits its rebound after antibiotic cessation. J. Infect. Dis. 2019, 220, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, S.; Harris, S.A.; Satti, I.; Hokey, D.A.; Dheenadhayalan, V.; Stockdale, L.; Thomas, Z.-R.M.; Minhinnick, A.; Wilkie, M.; Vermaak, S.; et al. A phase I, open-label trial, evaluating the safety and immunogenicity of candidate tuberculosis vaccines AERAS-402 and MVA85A, administered by prime-boost regime in BCG-vaccinated healthy adults. PLoS ONE 2015, 10, e0141687. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.-R.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime–MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Buck, E.; Finnigan, N.A. Malaria. In StatPearls; StatPearls Publishing Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ophorst, O.J.; Radosevic, K.; Havenga, M.J.E.; Pau, M.G.; Holterman, L.; Berkhout, B.; Goudsmit, J.; Tsuji, M. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 2006, 74, 313–320. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Zavala, F.; Nussenzweig, R.S.; Wilson, J.M.; Tsuji, M. Efficient induction of protective anti-malaria immunity by recombinant adenovirus. Vaccine 1998, 16, 1812–1817. [Google Scholar] [CrossRef]
- Sedegah, M.; Peters, B.; Hollingdale, M.R.; Ganeshan, H.D.; Huang, J.; Farooq, F.; Belmonte, M.N.; Belmonte, A.D.; Limbach, K.J.; Diggs, C.; et al. Vaccine Strain-Specificity of Protective HLA-Restricted Class 1 P. falciparum Epitopes. PLoS ONE 2016, 11, e0163026. [Google Scholar]
- Sedegah, M.; Hollingdale, M.R.; Farooq, F.; Ganeshan, H.; Belmonte, M.; Huang, J.; Abot, E.; Limbach, K.; Chuang, I.; Tamminga, C.; et al. Controlled Human Malaria Infection (CHMI) differentially affects cell-mediated and antibody responses to CSP and AMA1 induced by adenovirus vaccines with and without DNA-priming. Hum. Vaccines Immunother. 2015, 11, 2705–2715. [Google Scholar] [CrossRef]
- Sedegah, M.; Hollingdale, M.R.; Farooq, F.; Ganeshan, H.; Belmonte, M.; Kim, Y.; Peters, B.; Sette, A.; Huang, J.; McGrath, S.; et al. Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+ T cells targeting AMA1 class I epitopes. PLoS ONE 2014, 9, e106241. [Google Scholar] [CrossRef]
- Tamminga, C.; Sedegah, M.; Maiolatesi, S.; Fedders, C.; Reyes, S.; Reyes, A.; Vasquez, C.; Alcorta, Y.; Chuang, I.; Spring, M.; et al. Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum. Vaccines Immunother. 2013, 9, 2165–2177. [Google Scholar] [CrossRef]
- Tamminga, C.; Sedegah, M.; Regis, D.; Chuang, I.; Epstein, J.E.; Spring, M.; Mendoza-Silveiras, J.; McGrath, S.; Maiolatesi, S.; Reyes, S.; et al. Adenovirus-5-vectored P. falciparum vaccine expressing CSP and AMA1. Part B: Safety, immunogenicity and protective efficacy of the CSP component. PLoS ONE 2011, 6, e25868. [Google Scholar] [CrossRef]
- Sedegah, M.; Tamminga, C.; McGrath, S.; House, B.; Ganeshan, H.; Lejano, J.; Abot, E.; Banania, G.J.; Sayo, R.; Farooq, F.; et al. Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: Safety and immunogenicity in seronegative adults. PLoS ONE 2011, 6, e24586. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Recommends R21/Matrix-M Vaccine for Malaria Prevention in Updated Advice on Immunization; WHO: Geneva, Switzerland, 2023.
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Younis, B.M.; Osman, M.; Khalil, E.A.; Santoro, F.; Furini, S.; Wiggins, R.; Keding, A.; Carraro, M.; Musa, A.E.; Abdarahaman, M.A.; et al. Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan. Mol. Ther. 2021, 29, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, G.A.; Duncan, C.J.A.; Ewer, K.J.; Collins, K.A.; Elias, S.C.; Halstead, F.D.; Goodman, A.L.; Edwards, N.J.; Reyes-Sandoval, A.; Bird, P.; et al. Clinical assessment of a recombinant simian adenovirus ChAd63: A potent new vaccine vector. J. Infect. Dis. 2012, 205, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Freier, A.; Boussoffara, T.; Das, S.; Oswald, D.; Losch, F.O.; Selka, M.; Sacerdoti-Sierra, N.; Schönian, G.; Wiesmüller, K.H.; et al. Modular multiantigen T cell epitope–enriched DNA vaccine against human leishmaniasis. Sci. Transl. Med. 2014, 6, 234ra56. [Google Scholar] [CrossRef] [PubMed]
- Stäger, S.; Alexander, J.; Kirby, A.C.; Botto, M.; Van Rooijen, N.; Smith, D.F.; Brombacher, F.; Kaye, P.M. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat. Med. 2003, 9, 1287–1292. [Google Scholar] [CrossRef]
- Moreno, J.; Nieto, J.; Masina, S.; Cañavate, C.; Cruz, I.; Chicharro, C.; Carrillo, E.; Napp, S.; Reymond, C.; Kaye, P.; et al. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine 2007, 25, 5290–5300. [Google Scholar] [CrossRef]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Neglected Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef]
- Lacey, C.; Musa, A.; Khalil, E.T.; Younis, B.; Osman, M.; Wiggins, R.; Keding, A.; Kaye, P. LEISH2b—A phase 2b study to assess the safety, efficacy, and immunogenicity of the Leishmania vaccine ChAd63-KH in post-kala azar dermal leishmaniasis. Wellcome Open Res. 2022, 7, 200. [Google Scholar] [CrossRef]
- Abbass, M.A.; Church, J. Contemporary surgical management of colorectal cancer in Lynch syndrome. J. Surg. Oncol. 2023, 127, 1259–1263. [Google Scholar] [CrossRef]
- Richards, J.R.; Le, J.K. Cocaine Toxicity. In StatPearls; StatPearls Publishing Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Stephenson, R.J.; Toth, I. Anti-cocaine Vaccine Development: Where Are We Now and Where Are We Going? J. Med. Chem. 2023, 66, 7086–7100. [Google Scholar] [CrossRef]
- Havlicek, D.F.; Rosenberg, J.B.; De, B.P.; Hicks, M.J.; Sondhi, D.; Kaminsky, S.M.; Crystal, R.G. Cocaine vaccine dAd5GNE protects against moderate daily and high-dose “binge” cocaine use. PLoS ONE 2020, 15, e0239780. [Google Scholar] [CrossRef] [PubMed]
- Zak, D.E.; Andersen-Nissen, E.; Peterson, E.R.; Sato, A.; Hamilton, M.K.; Borgerding, J.; Krishnamurty, A.T.; Chang, J.T.; Adams, D.J.; Hensley, T.R.; et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc. Natl. Acad. Sci. USA 2012, 109, E3503–E3512. [Google Scholar] [CrossRef]
- Freitag, P.C.; Brandl, F.; Brücher, D.; Weiss, F.; Dreier, B.; Plückthun, A. Modular Adapters Utilizing Binders of Different Molecular Types Expand Cell-Targeting Options for Adenovirus Gene Delivery. Bioconjug. Chem. 2022, 33, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiang, Z.Q.; Li, Y.; Kurupati, R.K.; Jia, B.; Bian, A.; Zhou, D.M.; Hutnick, N.; Yuan, S.; Gray, C.; et al. Adenovirus-based vaccines: Comparison of vectors from three species of adenoviridae. J. Virol. 2010, 84, 10522–10532. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.M.; Da Costa, A.; Yamamoto, A.; Berry, D.; Lindsay, R.W.B.; Darrah, P.A.; Wang, L.; Cheng, C.; Kong, W.-P.; Gall, J.G.D.; et al. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 2013, 190, 2720–2735. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.T.; Hoeben, R.C. Non-human primate-derived adenoviruses for future use as oncolytic agents? Int. J. Mol. Sci. 2020, 21, 4821. [Google Scholar] [CrossRef]
- Brunetti-Pierri, N.; Ng, T.; Iannitti, D.A.; Cioffi, W.G.; Stapleton, G.; Law, M.; Breinholt, J.; Grove, N.; Rice, K.; Bauer, C.; et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum. Gene Ther. 2013, 24, 761–765. [Google Scholar] [CrossRef]
- Gonzalez-Aparicio, M.; Bunuales, M.; de Landazuri, I.O.; Prieto, J.; Hernandez--Alcoceba, R. Application of a split-Cre system for high-capacity adenoviral vector amplification. Biotechnol. J. 2023, 18, 2200227. [Google Scholar] [CrossRef]
- D’Alise, A.M.; Nocchi, L.; Garzia, I.; Seclì, L.; Infante, L.; Troise, F.; Cotugno, G.; Allocca, S.; Romano, G.; Lahm, A.; et al. Adenovirus Encoded Adjuvant (AdEnA) anti-CTLA-4, a novel strategy to improve Adenovirus based vaccines against infectious diseases and cancer. Front. Immunol. 2023, 14, 1156714. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, C.; Cox, T.M.; Li, C.; Son, Y.M.; Cheon, I.S.; Wu, Y.; Behl, S.; Taylor, J.J.; Chakaraborty, R.; et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 2022, 7, eadd4853. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.V.; Mittal, S.K. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin. Biol. Ther. 2010, 10, 1469–1487. [Google Scholar] [CrossRef]
- Blanche, F.; Cameron, B.; Barbot, A.; Ferrero, L.; Guillemin, T.; Guyot, S.; Somarriba, S.; Bisch, D. An improved anion-exchange HPLC method for the detection and purification of adenoviral particles. Gene Ther. 2000, 7, 1055–1062. [Google Scholar] [CrossRef]
- Huyghe, B.G.; Sutjipto, S.; Sugarman, B.J.; Horn, M.T.; Shepard, H.M.; Scandella, C.J.; Shabram, P.W.; Green, A.P.; Huang, J.J.; Scott, M.O.; et al. Purification of a type 5 recombinant adenovirus encoding human p53 by column chromatography. Hum. Gene Ther. 1995, 6, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Green, A.P.; Huang, J.J.; Scott, M.O.; Kierstead, T.D.; Beaupré, I.; Gao, G.-P.; Wilson, J.M.; Qu, G.; Burnham, M.S.; Chirmule, N.; et al. A new scalable method for the purification of recombinant adenovirus vectors. Hum. Gene Ther. 2002, 13, 1921–1934. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, B. Development of a Reference Material for Characterizing Adenovirus Vectors BETH HUTCHINS. BioProcess J. 2002, 1, 25–29. [Google Scholar] [CrossRef]
- Chen, G.; Li, H.; Gao, Y.; Zhao, H.; Yang, J.; Dong, L. Establishment of Digital PCR Method and Reference Material for Adenoviruses 40 and 41. Foodborne Pathog. Dis. 2023, 20, 453–459. [Google Scholar] [CrossRef]
- Ugwu, C.; Bejo, M.; Omar, A.; Isa, N.; Ideris, A. TaqMan probe-based qPCR method for specific detection and quantification of fowl adenovirus 8b challenge from chickens inoculated with live attenuated or inactivated virus. Open Vet. J. 2023, 13, 171–178. [Google Scholar] [CrossRef]
- Abdullah, O.; Fall, A.; Forman, M.; Howard, C.; Klein, E.; Mostafa, H.H. Respiratory Adenovirus Quantification with a Droplet Digital Polymerase Chain Reaction (ddPCR) Assay. Microbiol. Spectr. 2023, 11, e0026923. [Google Scholar] [CrossRef]
- Santander-Parra, S.H.; Caza, M.; Nuñez, L. Detection, Quantification and Molecular Characterization of Fowl Adenoviruses Circulating in Ecuadorian Chicken Flocks during 2019–2021. Vet. Sci. 2023, 10, 115. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Fan, H.; Chen, M.; Hu, W.; Zhang, Q.; Jin, M.; Liu, G.L.; Huang, L. Versatile nanorobot hand biosensor for specific capture and ultrasensitive quantification of viral nanoparticles. Mater. Today Bio 2022, 16, 100444. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Jonveaux, J.; Wang, P.; Haller, F.M.; Gu, B.; Koulov, A.V.; Jahn, M. Proteomic Analysis of Adenovirus 5 by UHPLC-MS/MS: Development of a Robust and Reproducible Sample Preparation Workflow. ACS Omega 2022, 7, 36825–36835. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A. Epidemiology of Human and Animal Viral Diseases. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 140–148. [Google Scholar]
- Wang, B.; Li, J.; Wu, S.; Wang, Y.; Chen, Y.; Zhai, Y.; Song, X.; Zhao, Z.; Zhang, Z.; Zhang, J.; et al. A seroepidemiological survey of adenovirus type 7 circulation among healthy adults in China and in Sierra Leone, West Africa. Front. Public Health 2023, 11, 1095343. [Google Scholar] [CrossRef] [PubMed]
- Kosoltanapiwat, N.; Tongshoob, J.; Ampawong, S.; Reamtong, O.; Prasittichai, L.; Yindee, M.; Tongthainan, D.; Tulayakul, P.; Boonnak, K. Simian adenoviruses: Molecular and serological survey in monkeys and humans in Thailand. One Health 2022, 15, 100434. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Wang, C.; Chen, H.; Li, Y.; Liang, Z.; Xu, T.; Guo, M.; Zhu, B. Seroprevalence of neutralizing antibodies to human mastadenovirus serotypes 3 and 7 in healthy children from guangdong province. Heliyon 2023, 9, e16986. [Google Scholar] [CrossRef] [PubMed]
- Klann, P.J.; Wang, X.; Elfert, A.; Zhang, W.; Köhler, C.; Güttsches, A.-K.; Jacobsen, F.; Weyen, U.; Roos, A.; Ehrke-Schulz, E.; et al. Seroprevalence of Binding and Neutralizing Antibodies against 39 Human Adenovirus Types in Patients with Neuromuscular Disorders. Viruses 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Sapuła, M.; Suchacz, M.; Kozłowska, J.; Cybula, A.; Siwak, E.; Krankowska, D.; Wiercińska-Drapało, A. Adenovirus 36 Infection in People Living with HIV-An Epidemiological Study of Seroprevalence and Associations with Cardiovascular Risk Factors. Viruses 2022, 14, 1639. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, Q.; Deng, J.; Li, H.; Zhang, Y.; Chen, Z.; Ji, T.; Liu, W.; Zheng, X.; Ma, Q.; et al. Seroprevalence of neutralizing antibodies against adenovirus type 26 and 35 in healthy populations from Guangdong and Shandong provinces, China. Virol. Sin. 2022, 37, 716–723. [Google Scholar] [CrossRef]
- Ewbank, A.C.; Duarte-Benvenuto, A.; Zamana-Ramblas, R.; Sacristán, I.; Costa-Silva, S.; Carvalho, V.L.; de Mello, D.M.D.; da Silva, V.M.F.; Catão-Dias, J.L.; Sacristán, C. Herpesvirus and adenovirus surveillance in threatened wild West Indian (Trichechus manatus) and Amazonian manatees (Trichechus inunguis), Brazil. Acta Trop. 2023, 237, 106740. [Google Scholar] [CrossRef]
- Zolotukhin, S.; Trivedi, P.D.; Corti, M.; Byrne, B.J. Scratching the surface of RGD-directed AAV capsid engineering. Mol. Ther. 2021, 29, 3099–3100. [Google Scholar] [CrossRef]
- Bauerschmitz, G.J.; Guse, K.; Kanerva, A.; Menzel, A.; Herrmann, I.; Desmond, R.A.; Yamamoto, M.; Nettelbeck, D.M.; Hakkarainen, T.; Dall, P.; et al. Triple-targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol. Ther. 2006, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Diaconu, I.; Cerullo, V.; Pesonen, S.K.; Kanerva, A.; Joensuu, T.; Kairemo, K.; Laasonen, L.; Partanen, K.; Kangasniemi, L.; et al. Ad3-hTERT-E1A, a fully serotype 3 oncolytic adenovirus, in patients with chemotherapy refractory cancer. Mol. Ther. 2012, 20, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.A.; Lovatt, C.; Plein, A.R.; Davies, J.A.; Siebzehnrubl, F.A.; Parker, A.L. Engineering Adenoviral Vectors with Improved GBM Selectivity. Viruses 2023, 15, 1086. [Google Scholar] [CrossRef] [PubMed]
- Dickson, A.; Geerling, E.; Stone, E.T.; Hassert, M.; Steffen, T.L.; Makkena, T.; Smither, M.; Schwetye, K.E.; Zhang, J.; Georges, B.; et al. The role of vaccination route with an adenovirus-vectored vaccine in protection, viral control, and transmission in the SARS-CoV-2/K18-hACE2 mouse infection model. Front. Immunol. 2023, 14, 1188392. [Google Scholar] [CrossRef]
- Trivedi, P.D.; Yu, C.; Chaudhuri, P.; Johnson, E.J.; Caton, T.; Adamson, L.; Byrne, B.J.; Paulk, N.K.; Clément, N. Comparison of highly pure rAAV9 vector stocks produced in suspension by PEI transfection or HSV infection reveals striking quantitative and qualitative differences. Mol. Ther. Methods Clin. Dev. 2021, 24, 154–170. [Google Scholar] [CrossRef]
- Yu, C.; Trivedi, P.D.; Chaudhuri, P.; Bhake, R.; Johnson, E.J.; Caton, T.; Potter, M.; Byrne, B.J.; Clément, N. NaCl and KCl mediate log increase in AAV vector particles and infectious titers in a specific/timely manner with the HSV platform. Mol. Ther. Methods Clin. Dev. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Milligan, I.D.; Gibani, M.M.; Sewell, R.; Clutterbuck, E.A.; Campbell, D.; Plested, E.; Nuthall, E.; Voysey, M.; Silva-Reyes, L.; McElrath, M.J.; et al. Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA 2016, 315, 1610–1623. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim results of a phase 1–2a trial of Ad26. COV2. S COVID-19 vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; Busby, J.E.; et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021, 22, 107–117. [Google Scholar] [CrossRef]
- Green, J.L.; Osterhout, R.E.; Klova, A.L.; Merkwirth, C.; McDonnell, S.R.; Zavareh, R.B.; Fuchs, B.C.; Kamal, A.; Jakobsen, J.S. Molecular characterization of type I IFN-induced cytotoxicity in bladder cancer cells reveals biomarkers of resistance. Mol. Ther.-Oncolytics 2021, 23, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chan, K.; Chang, S.; Friedlander, T.; et al. NCCN Guidelines® Insights: Bladder Cancer, Version 2.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Nadofaragene Firadenovec: First Approval. Drugs 2023, 83, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ferring, P. First Bladder Cancer Patient Dosed with Commercially Available Intravesical Gene Therapy ADSTILADRIN® (Nadofaragene Firadenovec-Vncg); BusinessWire: Parsippany, NJ, USA, 2023; Available online: https://ferringusa.com/?press=first-bladder-cancer-patient-dosed-with-commercially-available-intravesical-gene-therapy-adstiladrin-nadofaragene-firadenovec-vncg (accessed on 20 October 2023).



| Adenovirus Related Genes | Functions |
|---|---|
| Capsid proteins II, III, IIIa, IV, VI, VIII, and IX | Structural proteins for capsid |
| Core proteins V, VII, X | Structural proteins for capsid |
| CR1β | Membrane glycoprotein, helps modulate the host immune response |
| E1A | Most important early gene, activates transcription of a number of viral genes as well as genes of the host cells |
| E1B55K | Binds to and inactivates p53, to block p53-mediated functions in the cell |
| E2A and E2B | Participates in replication of viral DNA |
| E3 RIDα and E3 RIDβ | Membrane proteins, plays role to prevent apoptosis |
| E3 gp19K | Membrane glycoprotein, blocks class I MHC protein insertion in the host cell membrane to prevent T cell recognition of viral infection |
| E3 14.7K | Defends the virus from host antiviral responses |
| E4 transcription unit | Multifunctional viral proteins regulate viral and host-cell gene expressions |
| IVa2, 52K, L1, and 100K | Encapsidation proteins, helps in proper assembly of viral capsids |
| L3 protease | Cleave precursor polypeptides of pTP, pVI, pVII, pVIII, and IIIa to produce the mature viral proteins |
| Terminal protein TP | Covalently attached to 5′ ends of the DNA, plays critical role in replication and long-term infectivity |
| SN | NCT Identifier | Trial Stage | Conditions | Transgene/Strategy | Route | Enrol. |
|---|---|---|---|---|---|---|
| 1 | NCT05124002 | Phase IV | Intrahepatic Cholangiocarcinoma | rHAdV 5 (H101) + HAIC | ITU | 66 |
| 2 | NCT04452591 | Phase III | Non-Muscle Invasive Bladder Cancer (NMIBC) | DDM in engineered oncolytic rAdV (CG0070) + n-dodecyl-B-D-maltoside | IVE | 110 |
| 3 | NCT03780049 | Phase III | Hepatocellular carcinoma | Hepatic artery infusion chemotherapy ± H101 | IV | 304 |
| 4 | NCT02928094 | Phase III | Refractory Angina Due to Myocardial Ischemia | HAdV 5 + FGF-4 | ICOI | 160 |
| 5 | NCT05664139 | Phase II | Liver Metastases from Malignant Melanoma | HAdV 5 + PD-1 mAb + Nab-paclitaxel | ITU/IV | 30 |
| 6 | NCT04111172 | Phase II | Gastrointestinal Adenocarcinoma | HAdV 5 + F35-hGCC-PADRE | IM | 81 |
| 7 | NCT03947190 | Phase II | Malaria Vaccine | ChAdV 63/MVA ME-TRAP + R21/Matrix-M | ID/DVI | 64 |
| 8 | NCT05419011 | Phase II | Lynch Syndrome | CEA/MUC1/Brachyury (TRI-AdV 5)+ IL-15 Superagonist N-803 | ITU | 186 |
| 9 | NCT05872841 | Phase II | Primary Hepatocellular Carcinoma | H101 Combined + TACE | ITU | 38 |
| 10 | NCT05564897 | Phase II | Bladder Cancer | Oncolytic AdV + PD-1 inhibitor (Camrelizumab) | IVE | 25 |
| 11 | NCT05419011 | Phase II | Lynch Syndrome | Tri-AdV 5 + IL-15 superagonist nogapendekin alfa inbakicept (N-803) | SC | 186 |
| 12 | NCT05234905 | Phase II | Cervical Cancer | H101 + Camrelizumab | ITU | 55 |
| 13 | NCT01913106 | Phase II | Prostate Cancer | AdV/RSC-TK + Brachytherapy | ITU | 25 |
| 14 | NCT03039751 | Phase II | Refractory Angina Pectoris | AdV + VEGF-D | IMCD | 180 |
| 15 | NCT04095689 | Phase II | Triple Negative Breast Cancer | Docetaxel + Pembrolizumab + AdV/IL-12 | ITU | 30 |
| 16 | NCT04495153 | Phase II | Non-Small Cell Lung Cancer | Aglatimagene besadenovec (CAN-2409 + prodrug) | ITU | 86 |
| 17 | NCT04416516 | Phase II | Basal Cell Carcinomas/Basal Cell Nevus Syndrome | ASN-002 + Hh inhibitor vismodegib | ITU | 18 |
| 18 | NCT04739046 | Phase II | Pancreatic cancer | HAdV 5-yCD/mutTKSR39rep-ADP (Theragene) | ITU | 12 |
| 19 | NCT05441410 | Phase I/II | Malaria | ME-TRAP + ChAdV 63 | IM | 30 |
| 20 | NCT04097002 | Phase I/IIa | Prostate Cancer | Improved AdV 5 (ORCA-010) | ITU | 24 |
| 21 | NCT05078866 | Phase Ib/II | Lynch Syndrome | AdV neoantigen priming vaccine GAdV-209-FSP + MVA tumor-specific neoantigen-boosting vaccine MVA-209-FSP | IM | 45 |
| 22 | NCT04673942 | Phase I/II | Refractory Solid Tumors | Replicative AdV 5 + TGF-β receptor-immunoglobulin Fc fusion trap (AdAPT-001) | ITU | 79 |
| 23 | NCT03754933 | Phase I/II | Head/Neck Cancer | rAdV expressing E. coli Purine nucleoside phosphorylase + fludarabine phosphate (Ad/PNP) | ITU | 10 |
| 24 | NCT02749331 | Phase I/II | Neuroendocrine Tumors | rAdV AdVince (CgA-E1AmiR122) | HAI | 35 |
| 25 | NCT02705196 | Phase I/II | Pancreatic Cancer | rAdV + TMZ-CD40L + 4-1BBL (LOAd703) | ITU | 55 |
| 26 | NCT05617040 | Phase I/II | Prostate Cancer | ChAdOx1-PCAQ + MVA-PCAQ + PSA + PAP + STEAP1+ 5T4 | IM/IV | 137 |
| 27 | NCT05914935 | Phase I | Malignant Tumors; Glioblastoma | AdV + rL-IFN | ITU | 6 |
| 28 | NCT04695327 | Phase I | Solid Tumors | TNFα + IL-2 + rAdV (TILT-123) | ITU | 18 |
| 29 | NCT05180851 | Phase I | Head and Neck Cancer; Melanoma; Ovarian/Cervical Carcinoma; Lung Cancer | rAdV L-IFN (YSCH-01) | ITU | 19-28 |
| 30 | NCT04053283 | Phase I | Metastatic or Advanced Epithelial Tumors | Tumor-selective transgene expressing AdV (NG-641) | IV | 186 |
| 31 | NCT05271318 | Phase I | Ovarian Cancer | rAdV TILT-123 + pembrolizumab [AdV 5/3-E2F-d24-hTNFa-IRES-hIL2] | ITU | 29 |
| 32 | NCT05222932 | Phase I | Melanoma; Head and Neck Squamous Cell Carcinoma | rAdV (TILT-123) + avelumab + anti-PD1 (L) (AVENTIL) [AdV 5/3-E2F-d24-hTNFa-IRES-hIL2] | ITU | 15 |
| 33 | NCT05165433 | Phase I | Metastatic or Advanced Epithelial Tumors | NG-350A vector + pembrolizumab (tumor-selective anti-CD40) | IV | 198 |
| 34 | NCT05686798 | Phase I | Progressive Astrocytoma; GBM; Brain Tumor | AdV 5-yCD/ mutTKSR39rep-ADP | ITU | 18 |
| 35 | NCT05043714 | Phase I | Metastatic or Advanced Epithelial Tumors (NEBULA) | NG-641 vector + nivolumab (NG-641) | IV | 30 |
| 36 | NCT04217473 | Phase I | Advanced Melanoma | TNFalpha + IL 2 coding rAdV TILT-123 (TUNINTIL) | ITU | 15 |
| 37 | NCT03896568 | Phase I | High-Grade Glioma | MSC-DNX-2401 (AdV 5-DNX-2401) | IA | 36 |
| 38 | NCT03740256 | Phase I | Advanced HER2 Positive Solid Tumors | HER2-specific CAR-T + ChAdVEC | ITU | 45 |
| 39 | NCT03896568 | Phase I | Recurrent High-Grade Glioma | BM-hMSCs + DNX-2401 (MSC-DNX-2401) | IA | 36 |
| 40 | NCT04053283 | Phase I | Metastatic Cancer, Epithelial Tumor | rAdV + FAP-TAc antibody/ CXCL9/CXCL10/IFN-α (NG-641) | IV | 186 |
| 41 | NCT03284268 | Phase I | Refractory Retinoblastoma (RTB) | rAdV (VCN-01) | IVE | 13 |
| 42 | NCT02455479 | Phase I | Cocaine-Dependent Individuals | Disruptive dAd5GNE | IV | 15 |
| 43 | NCT05076760 | Phase I | Non-Small Cell Lung Cancer | rAdV (MEM-288) + IFNβ + rCD40-ligand | ITU | 18 |
| 44 | NCT03878121 | Phase I | HIV | ADV 4-HIV envelope vaccine vectors [AdV 4-Env145NFL + AdV 4-Env150KN + VRC-HIVRGP096-00-VP (Trimer 4571)] | IN/IM | 300 |
| 45 | NCT04839042 | Phase I | COVID-19 Vaccine | AdV 6 vector (SC-AdV 6-1) | IM/IN/ IH | 190 |
| 46 | NCT05526183 | Phase I | COVID-19 Vaccine | AdV 5 CoVacHGMix (with equal amounts of CoVacHGA1320, CoVacHGB420, CoVacHGC720 and CoVacHGD1480 | IM | 36 |
| 47 | NCT05717699 | Phase I | Intrinsic Pontine Glioma | AdV 5-TD-nsIL12 + human non-secretory interleukin-12 | ITU | 18 |
| 48 | NCT05717712 | Phase I | Intrinsic Pontine Glioma | AdV 5-TD-nsIL12 + human non-secretory interleukin-12 | ITU | 18 |
| 49 | NCT03546361 | Phase I | Non-Small Cell Lung Cancer | CCL21-Gene modified dendritic cell (rAdV -CCL21-DC) + pembrolizumab | ITU + IV | 24 |
| 50 | NCT05991427 | Phase I | Zoster Disease | ChAdOx1-VZV | IM | 65 |
| SN | Name | Sponsor | AdV Type | Payload/ Antigen | Application |
|---|---|---|---|---|---|
| 1 | Jcovden | Johnson & Johnson (Solothurn, Switzerland) | AdV 26 | CoV2-S spike protein | COVID-19 vaccine |
| 2 | Convidecia | CanSino Biologics Inc. (Tianjin, China) | AdV 5 | SARS-CoV-2 spike protein | COVID-19 vaccine |
| 3 | Vaxzevria / Covishield | AstraZeneca/ University of Oxford (Oxford, UK) | ChAdV | ChAdOx1-S spike protein antigen | COVID-19 vaccine |
| 4 | Sputnik V | Gamaleya Research Institute (Moscow, Russia) | AdV 26 (prime) + AdV 5 (boost) | Spike protein (S) antigen | COVID-19 vaccine |
| 5 | Zabdeno | Johnson and Johnson (Solothurn, Switzerland) | AdV 26 | Ad26.ZEBOV | Ebola Vaccine |
| 6 | Adstiladrin | Ferring Pharmaceuticals (Parsippany-Troy Hills, NJ, USA) | AdV 5 | Nadofaragene firadenovec-vncg with IFNα2b | Gene Therapy for Bladder Cancer |
| 7 | H101/Oncorine | Shanghai Sunway Biotech (Shanghai, China) | AdV 5 | Tumor-specific | Cancer Therapy for Nasopharyngeal cancer |
| 8 | Gendicine | Shenzhen SiBiono GeneTech (Shenzhen, China) | AdV 5 | Anti-p53 | Cancer Therapy for Head and neck cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, P.D.; Byrne, B.J.; Corti, M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses 2023, 15, 2378. https://doi.org/10.3390/v15122378
Trivedi PD, Byrne BJ, Corti M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses. 2023; 15(12):2378. https://doi.org/10.3390/v15122378
Chicago/Turabian StyleTrivedi, Prasad D., Barry J. Byrne, and Manuela Corti. 2023. "Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines" Viruses 15, no. 12: 2378. https://doi.org/10.3390/v15122378






