Viral–Bacterial Interactions That Impact Viral Thermostability and Transmission
Abstract
:1. Introduction
2. Intestinal Bacteria Promote Viral Replication and Pathogenesis
3. Bacteria Can Bind Directly to Viruses
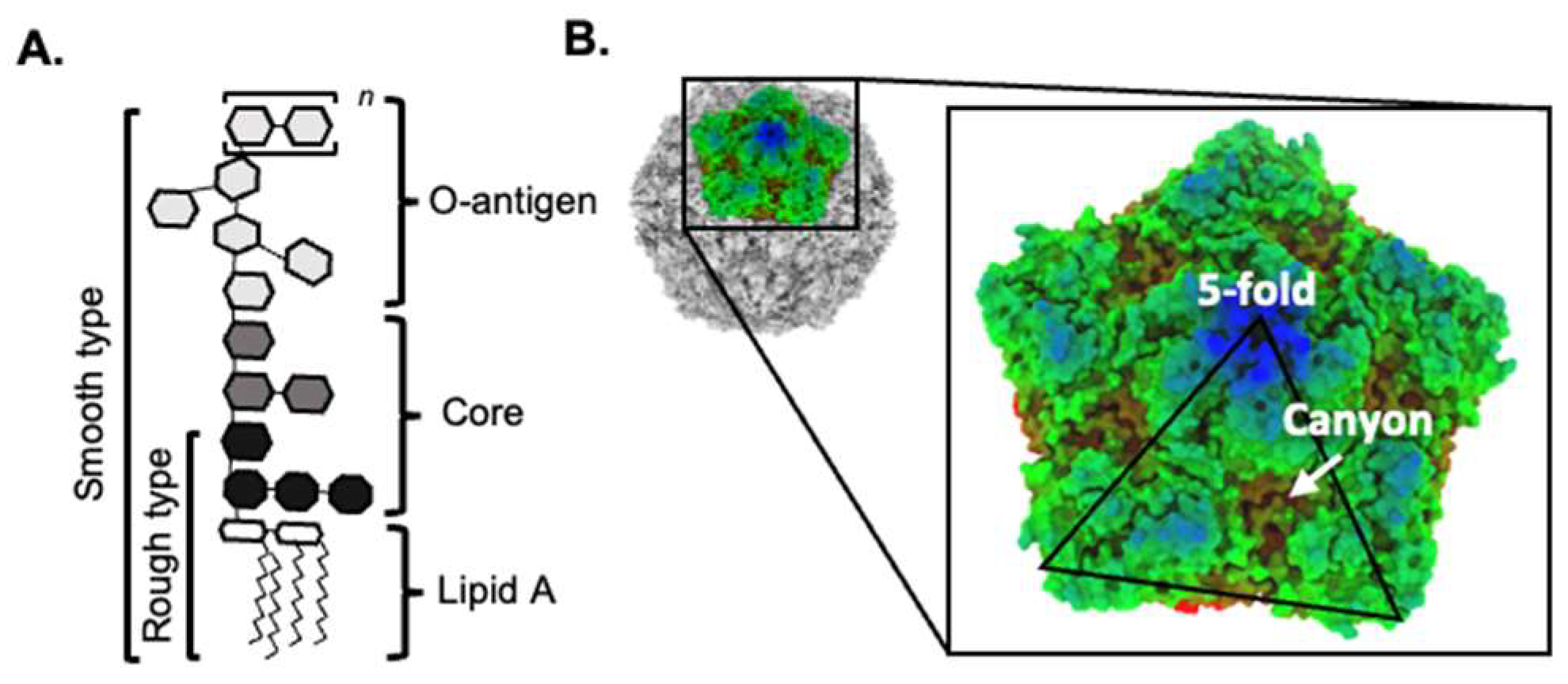
4. Bacteria Can Promote the Thermostability of Viruses
5. Wastewater and Transmission
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 22 October 2023).
- Bartsch, S.M.; Lopman, B.A.; Ozawa, S.; Hall, A.J.; Lee, B.Y. Global Economic Burden of Norovirus Gastroenteritis. PLoS ONE 2016, 11, e0151219. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.L.; Lee, Y.K. Microflora of the gastrointestinal tract: A review. Methods Mol. Biol. 2004, 268, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kuss, S.K.; Best, G.T.; Etheredge, C.A.; Pruijssers, A.J.; Frierson, J.M.; Hooper, L.V.; Dermody, T.S.; Pfeiffer, J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011, 334, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.; Case, L.K.; Kopaskie, K.; Kozlova, A.; MacDearmid, C.; Chervonsky, A.V.; Golovkina, T.V. Successful transmission of a retrovirus depends on the commensal microbiota. Science 2011, 334, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinje, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef]
- Baldridge, M.T.; Nice, T.J.; McCune, B.T.; Yokoyama, C.C.; Kambal, A.; Wheadon, M.; Diamond, M.S.; Ivanova, Y.; Artyomov, M.; Virgin, H.W. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 2015, 347, 266–269. [Google Scholar] [CrossRef]
- Robinson, C.M.; Jesudhasan, P.R.; Pfeiffer, J.K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 2014, 15, 36–46. [Google Scholar] [CrossRef]
- Robinson, C.M.; Woods Acevedo, M.A.; McCune, B.T.; Pfeiffer, J.K. Related Enteric Viruses Have Different Requirements for Host Microbiota in Mice. J. Virol. 2019, 93, e01339-19. [Google Scholar] [CrossRef]
- Dhalech, A.H.; Fuller, T.D.; Robinson, C.M. Specific bacterial cell wall components influence the stability of Coxsackievirus B3. J. Virol. 2021, 95, 22. [Google Scholar] [CrossRef]
- Berger, A.K.; Yi, H.; Kearns, D.B.; Mainou, B.A. Bacteria and bacterial envelope components enhance mammalian reovirus thermostability. PLoS Pathog. 2017, 13, e1006768. [Google Scholar] [CrossRef]
- Aguilera, E.R.; Nguyen, Y.; Sasaki, J.; Pfeiffer, J.K. Bacterial Stabilization of a Panel of Picornaviruses. mSphere 2019, 4, e00183-19. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.M.; Livingston, B.; Margolis, E.; Davis, A.; Meliopoulos, V.A.; Echlin, H.; Schultz-Cherry, S.; Rosch, J.W. Respiratory Bacteria Stabilize and Promote Airborne Transmission of Influenza A Virus. mSystems 2020, 5, e00762-20. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.K.; Jesudhasan, P.R.; Mayer, M.J.; Narbad, A.; Winter, S.E.; Pfeiffer, J.K. Bacteria Facilitate Enteric Virus Co-infection of Mammalian Cells and Promote Genetic Recombination. Cell Host Microbe 2018, 23, 77–88.e75. [Google Scholar] [CrossRef] [PubMed]
- Wilks, J.; Lien, E.; Jacobson, A.N.; Fischbach, M.A.; Qureshi, N.; Chervonsky, A.V.; Golovkina, T.V. Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission. Cell Host Microbe 2015, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Heggelund, J.E.; Varrot, A.; Imberty, A.; Krengel, U. Histo-blood group antigens as mediators of infections. Curr. Opin. Struct. Biol. 2017, 44, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Jiang, X. Histo-blood group antigens: A common niche for norovirus and rotavirus. Expert. Rev. Mol. Med. 2014, 16, e5. [Google Scholar] [CrossRef]
- Miura, T.; Sano, D.; Suenaga, A.; Yoshimura, T.; Fuzawa, M.; Nakagomi, T.; Nakagomi, O.; Okabe, S. Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J. Virol. 2013, 87, 9441–9451. [Google Scholar] [CrossRef]
- Almand, E.A.; Moore, M.D.; Outlaw, J.; Jaykus, L.A. Human norovirus binding to select bacteria representative of the human gut microbiota. PLoS ONE 2017, 12, e0173124. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Bhar, S.; Hackett, S.; Engelken, H.; Joseph, R.; Keyhani, N.O.; Jones, M.K. Attach Me If You Can: Murine Norovirus Binds to Commensal Bacteria and Fungi. Viruses 2020, 12, 759. [Google Scholar] [CrossRef]
- Budicini, M.R.; Pfeiffer, J.K. Stabilization of Murine Norovirus by Bacteria. mSphere 2022, 7, e0004622. [Google Scholar] [CrossRef] [PubMed]
- Waldman, P.; Meseguer, A.; Lucas, F.; Moulin, L.; Wurtzer, S. Interaction of Human Enteric Viruses with Microbial Compounds: Implication for Virus Persistence and Disinfection Treatments. Environ. Sci. Technol. 2017, 51, 13633–13640. [Google Scholar] [CrossRef]
- Li, D.; Breiman, A.; le Pendu, J.; Uyttendaele, M. Binding to histo-blood group antigen-expressing bacteria protects human norovirus from acute heat stress. Front. Microbiol. 2015, 6, 659. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Z.; Chen, J.; Zou, S.; Jin, Y.; Zhang, R.; Sheng, Y.; Liao, N.; Hu, B.; Cheng, D. Effect of Direct Viral-Bacterial Interactions on the Removal of Norovirus From Lettuce. Front. Microbiol. 2021, 12, 731379. [Google Scholar] [CrossRef] [PubMed]
- Raev, S.A.; Amimo, J.O.; Saif, L.J.; Vlasova, A.N. Intestinal mucin-type O-glycans: The major players in the host-bacteria-rotavirus interactions. Gut Microbes 2023, 15, 2197833. [Google Scholar] [CrossRef] [PubMed]
- Filman, D.J.; Syed, R.; Chow, M.; Macadam, A.J.; Minor, P.D.; Hogle, J.M. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 1989, 8, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Macadam, A.J.; Arnold, C.; Howlett, J.; John, A.; Marsden, S.; Taffs, F.; Reeve, P.; Hamada, N.; Wareham, K.; Almond, J.; et al. Reversion of the attenuated and temperature-sensitive phenotypes of the Sabin type 3 strain of poliovirus in vaccinees. Virology 1989, 172, 408–414. [Google Scholar] [CrossRef]
- Zocher, G.; Mistry, N.; Frank, M.; Hahnlein-Schick, I.; Ekstrom, J.O.; Arnberg, N.; Stehle, T. A sialic acid binding site in a human picornavirus. PLoS Pathog. 2014, 10, e1004401. [Google Scholar] [CrossRef]
- Sun, Y.; Roznowski, A.P.; Tokuda, J.M.; Klose, T.; Mauney, A.; Pollack, L.; Fane, B.A.; Rossmann, M.G. Structural changes of tailless bacteriophage PhiX174 during penetration of bacterial cell walls. Proc. Natl. Acad. Sci. USA 2017, 114, 13708–13713. [Google Scholar] [CrossRef]
- Muckelbauer, J.K.; Kremer, M.; Minor, I.; Diana, G.; Dutko, F.J.; Groarke, J.; Pevear, D.C.; Rossmann, M.G. The structure of coxsackievirus B3 at 3.5 A resolution. Structure 1995, 3, 653–667. [Google Scholar] [CrossRef]
- Greaves, J.; North, D.; Bibby, K. Particle association and size fraction of molecular viral fecal pollution indicators in wastewater. Environ. Sci. Water Res. Technol. 2022, 8, 1814–1821. [Google Scholar] [CrossRef]
- Lawler, D.F.; Wilkes, D.R. Flocculation model testing: Particle sizes in a softening plant. J. Am. Water Work. Assoc. 1984, 76, 90–97. [Google Scholar] [CrossRef]
- Schaub, S.A.; Sagik, B.P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl. Microbiol. 1975, 30, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G.; Bettarel, Y.; Cattaneo, R.; Luef, B.; Maier, C.; Motegi, C.; Peduzzi, P.; Mari, X. Viral ecology of organic and inorganic particles in aquatic systems: Avenues for further research. Aquat. Microb. Ecol. 2009, 57, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K. Norovirus removal and particle association in a waste stabilization pond. Environ. Sci. Technol. 2008, 42, 9151–9157. [Google Scholar] [CrossRef]
- Symonds, E.M.; Verbyla, M.E.; Lukasik, J.O.; Kafle, R.C.; Breitbart, M.; Mihelcic, J.R. A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res. 2014, 65, 257–270. [Google Scholar] [CrossRef]
- Templeton, M.R.; Andrews, R.C.; Hofmann, R. Inactivation of particle-associated viral surrogates by ultraviolet light. Water Res. 2005, 39, 3487–3500. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater: Significance and Implications for Treatment and Disinfection Processes. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef]
- Qiao, Z.; Wigginton, K.R. Direct and Indirect Photochemical Reactions in Viral RNA Measured with RT-qPCR and Mass Spectrometry. Environ. Sci. Technol. 2016, 50, 13371–13379. [Google Scholar] [CrossRef]
- Pinon, A.; Vialette, M. Survival of Viruses in Water. Intervirology 2018, 61, 214–222. [Google Scholar] [CrossRef]
- Rehmann, C.R.; Soupir, M.L. Importance of interactions between the water column and the sediment for microbial concentrations in streams. Water Res. 2009, 43, 4579–4589. [Google Scholar] [CrossRef] [PubMed]
- Pitol, A.K.; Bischel, H.N.; Kohn, T.; Julian, T.R. Virus Transfer at the Skin-Liquid Interface. Environ. Sci. Technol. 2017, 51, 14417–14425. [Google Scholar] [CrossRef] [PubMed]
- Pitol, A.K.; Bischel, H.N.; Boehm, A.B.; Kohn, T.; Julian, T.R. Transfer of Enteric Viruses Adenovirus and Coxsackievirus and Bacteriophage MS2 from Liquid to Human Skin. Appl. Environ. Microbiol. 2018, 84, e01809-18. [Google Scholar] [CrossRef] [PubMed]
- Pitol, A.K.; Kohn, T.; Julian, T.R. Retention of E. coli and water on the skin after liquid contact. PLoS ONE 2020, 15, e0238998. [Google Scholar] [CrossRef] [PubMed]
- Bischel, H.N.; Caduff, L.; Schindelholz, S.; Kohn, T.; Julian, T.R. Health Risks for Sanitation Service Workers along a Container-Based Urine Collection System and Resource Recovery Value Chain. Environ. Sci. Technol. 2019, 53, 7055–7067. [Google Scholar] [CrossRef] [PubMed]
- Sobsey, M.D.; Dean, C.H.; Knuckles, M.E.; Wagner, R.A. Interactions and Survival of Enteric Viruses in Soil Materials. Appl. Environ. Microbiol. 1980, 40, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ginn, O.; Rocha-Melogno, L.; Bivins, A.; Lowry, S.; Cardelino, M.; Nichols, D.; Tripathi, S.N.; Soria, F.; Andrade, M.; Bergin, M.; et al. Detection and Quantification of Enteric Pathogens in Aerosols Near Open Wastewater Canals in Cities with Poor Sanitation. Environ. Sci. Technol. 2021, 55, 14758–14771. [Google Scholar] [CrossRef]
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Re, V.E. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef]
- Rocha-Melogno, L.; Crank, K.C.; Ginn, O.; Bergin, M.H.; Brown, J.; Gray, G.C.; Hamilton, K.A.; Bibby, K.; Deshusses, M.A. Quantitative microbial risk assessment of outdoor aerosolized pathogens in cities with poor sanitation. Sci. Total Environ. 2022, 827, 154233. [Google Scholar] [CrossRef]
- Antwi-Agyei, P.; Biran, A.; Peasey, A.; Bruce, J.; Ensink, J. A faecal exposure assessment of farm workers in Accra, Ghana: A cross sectional study. Bmc Public. Health 2016, 16, 587. [Google Scholar] [CrossRef]
- Gagne, M.J.; Savard, T.; Brassard, J. Interactions Between Infectious Foodborne Viruses and Bacterial Biofilms Formed on Different Food Contact Surfaces. Food Environ. Virol. 2022, 14, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, R.; Chassaing, B.; Zhang, B.; Gewirtz, A.T. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J. Infect. Dis. 2014, 210, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Mrukowicz, J.Z. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: A systematic review of published randomized, double-blind, placebo-controlled trials. J. Pediatr. Gastroenterol. Nutr. 2001, 33 (Suppl. S2), S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Kaila, M.; Isolauri, E.; Saxelin, M.; Arvilommi, H.; Vesikari, T. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch. Dis. Child. 1995, 72, 51–53. [Google Scholar] [CrossRef]
- Majamaa, H.; Isolauri, E.; Saxelin, M.; Vesikari, T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.I.; Strauman, M.C.; Mozdzanowska, K.; Whittle, J.R.; Williams, K.L.; Sharpe, A.H.; Weiser, J.N.; Caton, A.J.; Hensley, S.E.; Erikson, J. Coinfection with Streptococcus pneumoniae modulates the B cell response to influenza virus. J. Virol. 2014, 88, 11995–12005. [Google Scholar] [CrossRef] [PubMed]
- Goncheva, M.I.; Conceicao, C.; Tuffs, S.W.; Lee, H.M.; Quigg-Nicol, M.; Bennet, I.; Sargison, F.; Pickering, A.C.; Hussain, S.; Gill, A.C.; et al. Staphylococcus aureus Lipase 1 Enhances Influenza A Virus Replication. mBio 2020, 11, 4. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Kimura, K.S.; Shilts, M.H.; Strickland, B.A.; Freeman, M.H.; Wessinger, B.C.; Gupta, V.; Brown, H.M.; Rajagopala, S.V.; Turner, J.H.; et al. SARS-CoV-2 infection and viral load are associated with the upper respiratory tract microbiome. J. Allergy Clin. Immunol. 2021, 147, 1226–1233.e1222. [Google Scholar] [CrossRef]
- Xu, R.; Lu, R.; Zhang, T.; Wu, Q.; Cai, W.; Han, X.; Wan, Z.; Jin, X.; Zhang, Z.; Zhang, C. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun. Biol. 2021, 4, 240. [Google Scholar] [CrossRef]
- Hernandez-Teran, A.; Mejia-Nepomuceno, F.; Herrera, M.T.; Barreto, O.; Garcia, E.; Castillejos, M.; Boukadida, C.; Matias-Florentino, M.; Rincon-Rubio, A.; Avila-Rios, S.; et al. Dysbiosis and structural disruption of the respiratory microbiota in COVID-19 patients with severe and fatal outcomes. Sci. Rep. 2021, 11, 21297. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V.; Margolis, E.; Schultz-Cherry, S. Astrovirus and the microbiome. Curr. Opin. Virol. 2019, 37, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Edens, F.W.; Parkhurst, C.R.; Qureshi, M.A.; Casas, I.A.; Havenstein, G.B. Atypical Escherichia coli strains and their association with poult enteritis and mortality syndrome. Poult. Sci. 1997, 76, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Saif, Y.M.; Heggen-Peay, C.L.; Edens, F.W.; Havenstein, G.B. Induction of functional defects in macrophages by a poult enteritis and mortality syndrome-associated turkey astrovirus. Avian Dis. 2001, 45, 853–861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robledo Gonzalez, L.; Tat, R.P.; Greaves, J.C.; Robinson, C.M. Viral–Bacterial Interactions That Impact Viral Thermostability and Transmission. Viruses 2023, 15, 2415. https://doi.org/10.3390/v15122415
Robledo Gonzalez L, Tat RP, Greaves JC, Robinson CM. Viral–Bacterial Interactions That Impact Viral Thermostability and Transmission. Viruses. 2023; 15(12):2415. https://doi.org/10.3390/v15122415
Chicago/Turabian StyleRobledo Gonzalez, Lorimar, Rachel P. Tat, Justin C. Greaves, and Christopher M. Robinson. 2023. "Viral–Bacterial Interactions That Impact Viral Thermostability and Transmission" Viruses 15, no. 12: 2415. https://doi.org/10.3390/v15122415
APA StyleRobledo Gonzalez, L., Tat, R. P., Greaves, J. C., & Robinson, C. M. (2023). Viral–Bacterial Interactions That Impact Viral Thermostability and Transmission. Viruses, 15(12), 2415. https://doi.org/10.3390/v15122415






