Galectin-1 Modulates the Fusogenic Activity of Placental Endogenous Retroviral Envelopes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Plasmids
2.3. Recombinant Galectin-1 and Galectin-3
2.4. Production of Pseudotyped NL4.3 Viruses and Titration
2.5. Infection with Pseudotyped Viruses
2.6. Computational Analysis of MFSD2a Transcript Expression in Human Cell Lines
2.7. MTT Assay
2.8. Cell Fusion Assay
2.9. Immunolocalization of MFSD2a
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Galectin-1 Increases the Fusion Capacity of the Endogenous Retroviral Envelope Proteins Syncytin-2 and EnvP(b)
3.2. Syncytin-1, Syncytin-2, and VSV-G-Pseudotyped Viruses Differentially Infect Human and Simian Cell Lines
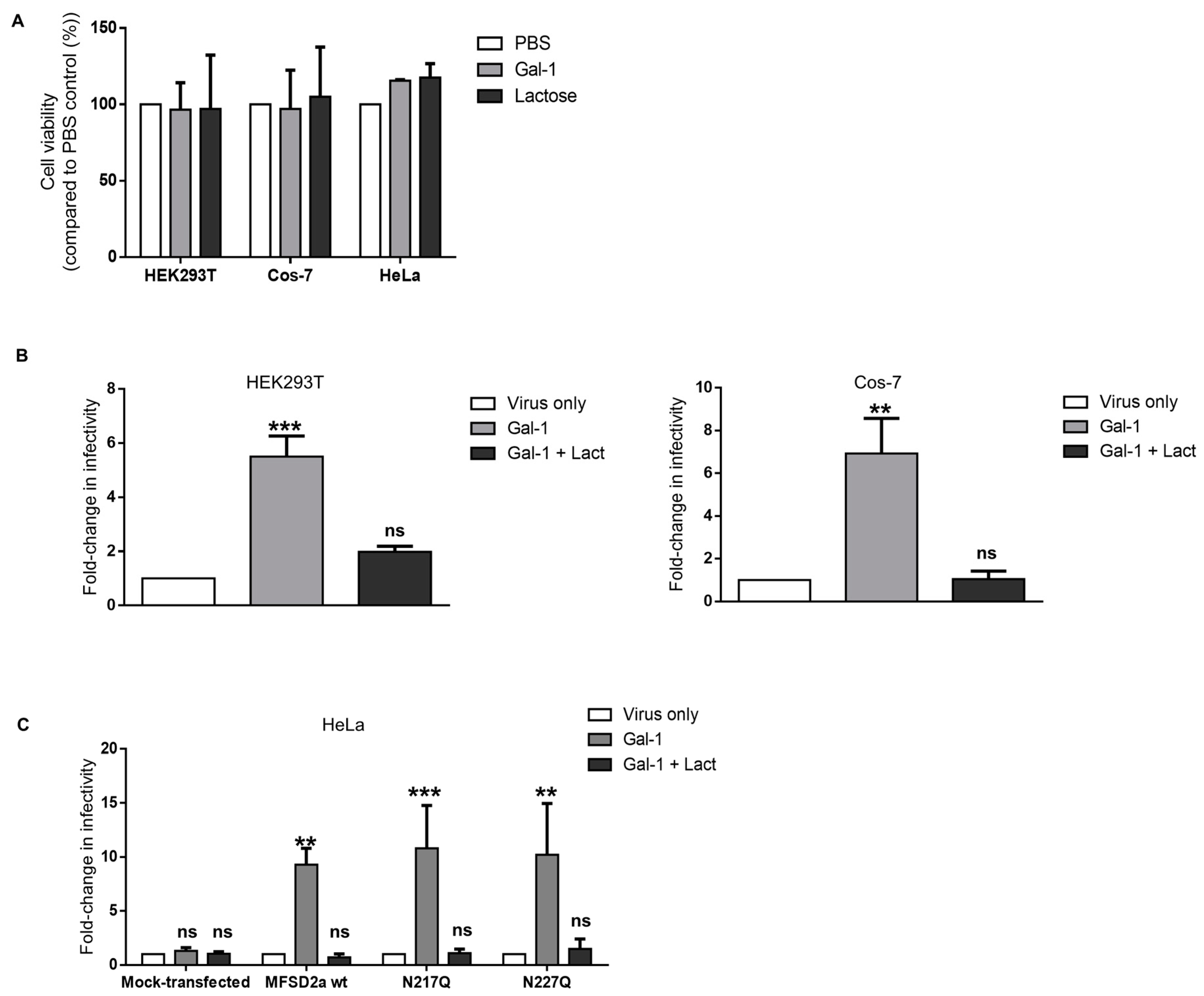
3.3. Increase in the Infectivity of Syncytin-2-Pseudotyped Viruses by Galectin-1 in Different Cell Lines Is MFSD2a-Dependent
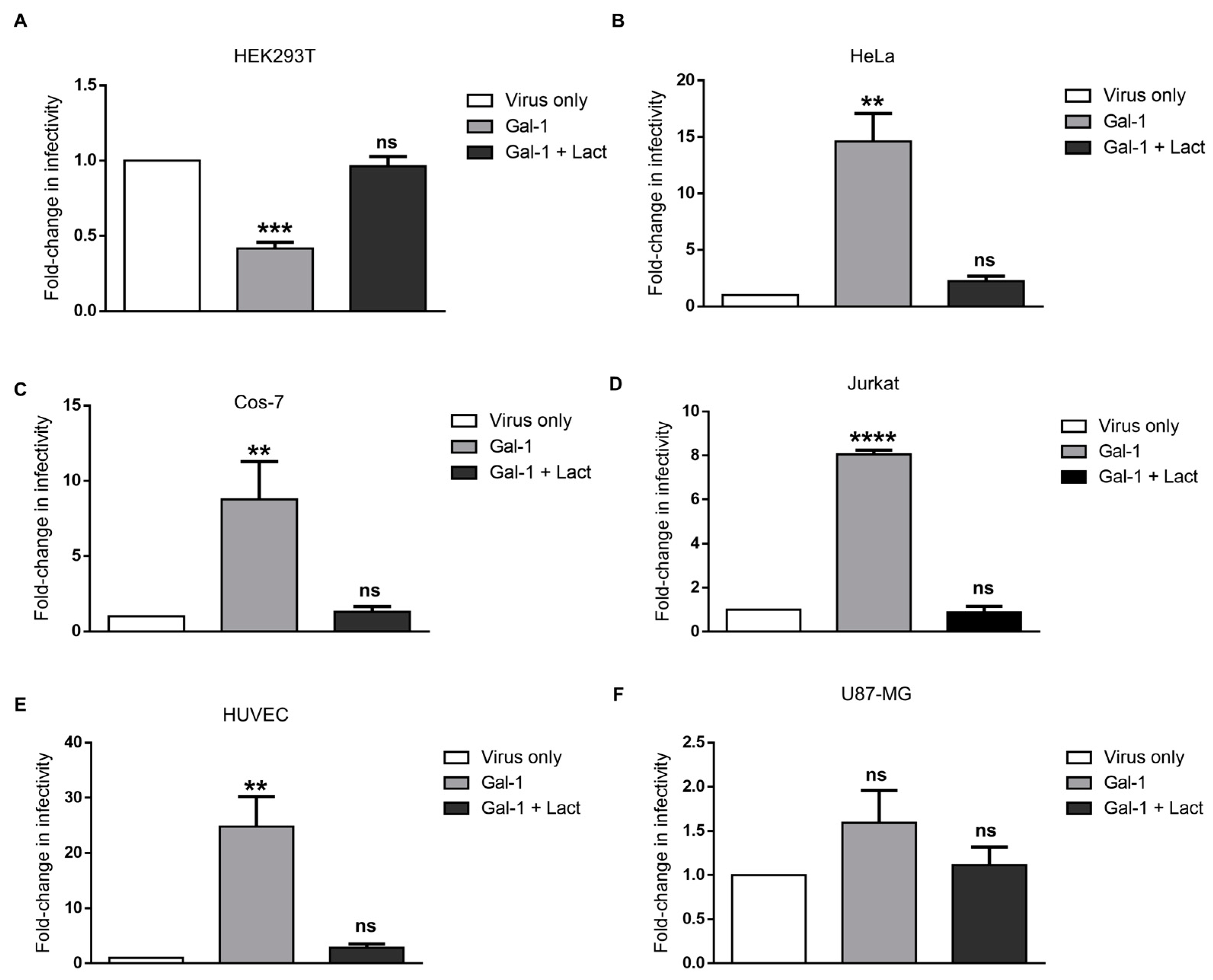
3.4. Galectin-1-Dependent Increase in the Infectivity of Syncytin-2-Pseudotyped Viruses Is Reproduced on Human Cell Lines of Different Types
3.5. Response of the Infectivity of Syncytin-1-Pseudotyped Viruses to Galectin-1 Is Dose-Dependent and Varies in Different Cell Lines
3.6. Galectin-3 Does Not Increase the Infectivity of Syncytin-2-Pseudotyped Viruses but Affects Syncytin-1-Pseudotyped Viruses in Certain Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Dupressoir, A.; Lavialle, C.; Heidmann, T. From ancestral infectious retroviruses to bona fide cellular genes: Role of the captured syncytins in placentation. Placenta 2012, 33, 663–671. [Google Scholar] [CrossRef] [PubMed]
- de Parseval, N.; Lazar, V.; Casella, J.F.; Benit, L.; Heidmann, T. Survey of human genes of retroviral origin: Identification and transcriptome of the genes with coding capacity for complete envelope proteins. J. Virol. 2003, 77, 10414–10422. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lee, X.; Li, X.; Veldman, G.M.; Finnerty, H.; Racie, L.; LaVallie, E.; Tang, X.Y.; Edouard, P.; Howes, S.; et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 2000, 403, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Blond, J.L.; Lavillette, D.; Cheynet, V.; Bouton, O.; Oriol, G.; Chapel-Fernandes, S.; Mandrand, B.; Mallet, F.; Cosset, F.L. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 2000, 74, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Blaise, S.; de Parseval, N.; Benit, L.; Heidmann, T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 13013–13018. [Google Scholar] [CrossRef]
- Malassine, A.; Handschuh, K.; Tsatsaris, V.; Gerbaud, P.; Cheynet, V.; Oriol, G.; Mallet, F.; Evain-Brion, D. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta 2005, 26, 556–562. [Google Scholar] [CrossRef]
- Malassine, A.; Blaise, S.; Handschuh, K.; Lalucque, H.; Dupressoir, A.; Evain-Brion, D.; Heidmann, T. Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta 2007, 28, 185–191. [Google Scholar] [CrossRef]
- Mallet, F.; Bouton, O.; Prudhomme, S.; Cheynet, V.; Oriol, G.; Bonnaud, B.; Lucotte, G.; Duret, L.; Mandrand, B. The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc. Natl. Acad. Sci. USA 2004, 101, 1731–1736. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Handwerger, S. A placenta-specific enhancer of the human syncytin gene. Biol. Reprod. 2005, 73, 500–509. [Google Scholar] [CrossRef]
- Chen, C.P.; Chen, L.F.; Yang, S.R.; Chen, C.Y.; Ko, C.C.; Chang, G.D.; Chen, H. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 2008, 79, 815–823. [Google Scholar] [CrossRef]
- Vargas, A.; Moreau, J.; Landry, S.; LeBellego, F.; Toufaily, C.; Rassart, E.; Lafond, J.; Barbeau, B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol. 2009, 392, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, R.; Zhu, C.; Wang, H.; Lin, H.Y.; Gu, Y.; Cross, J.C.; Wang, H. Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Rep. 2017, 21, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Esnault, C.; Cornelis, G.; Heidmann, O.; Heidmann, T. Differential evolutionary fate of an ancestral primate endogenous retrovirus envelope gene, the EnvV syncytin, captured for a function in placentation. PLoS Genet. 2013, 9, e1003400. [Google Scholar] [CrossRef] [PubMed]
- Lavialle, C.; Cornelis, G.; Dupressoir, A.; Esnault, C.; Heidmann, O.; Vernochet, C.; Heidmann, T. Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philos. Trans. R. Soc. Lond B Biol. Sci. 2013, 368, 20120507. [Google Scholar] [CrossRef]
- Frendo, J.L.; Olivier, D.; Cheynet, V.; Blond, J.L.; Bouton, O.; Vidaud, M.; Rabreau, M.; Evain-Brion, D.; Mallet, F. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol. Cell. Biol. 2003, 23, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A. Scrutinising the regulators of syncytialization and their expression in pregnancy-related conditions. Mol. Cell. Endocrinol. 2016, 420, 180–193. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef]
- Lavillette, D.; Marin, M.; Ruggieri, A.; Mallet, F.; Cosset, F.L.; Kabat, D. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J. Virol. 2002, 76, 6442–6452. [Google Scholar] [CrossRef]
- Esnault, C.; Priet, S.; Ribet, D.; Vernochet, C.; Bruls, T.; Lavialle, C.; Weissenbach, J.; Heidmann, T. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc. Natl. Acad. Sci. USA 2008, 105, 17532–17537. [Google Scholar] [CrossRef] [PubMed]
- Toufaily, C.; Vargas, A.; Lemire, M.; Lafond, J.; Rassart, E.; Barbeau, B. MFSD2a, the Syncytin-2 receptor, is important for trophoblast fusion. Placenta 2013, 34, 85–88. [Google Scholar] [CrossRef]
- Lee, X.; Keith, J.C., Jr.; Stumm, N.; Moutsatsos, I.; McCoy, J.M.; Crum, C.P.; Genest, D.; Chin, D.; Ehrenfels, C.; Pijnenborg, R.; et al. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta 2001, 22, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Wang, K.G.; Chen, C.Y.; Yu, C.; Chuang, H.C.; Chen, H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG 2006, 113, 152–158. [Google Scholar] [CrossRef]
- Vargas, A.; Toufaily, C.; LeBellego, F.; Rassart, E.; Lafond, J.; Barbeau, B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod. Sci. 2011, 18, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Thiery, M.; Lafond, J.; Barbeau, B. Transcriptional and functional studies of Human Endogenous Retrovirus envelope EnvP(b) and EnvV genes in human trophoblasts. Virology 2012, 425, 1–10. [Google Scholar] [CrossRef]
- Blaise, S.; de Parseval, N.; Heidmann, T. Functional characterization of two newly identified Human Endogenous Retrovirus coding envelope genes. Retrovirology 2005, 2, 19. [Google Scholar] [CrossRef]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental syncytins: Genetic disjunction between the fusogenic and immunosuppressive activity of retroviral envelope proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef]
- Tolosa, J.M.; Schjenken, J.E.; Clifton, V.L.; Vargas, A.; Barbeau, B.; Lowry, P.; Maiti, K.; Smith, R. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012, 33, 933–941. [Google Scholar] [CrossRef]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elisseeff, X.; Vargas, A.; Rassart, E.; Lafond, J.; Leduc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous retrovirus-encoded Syncytin-2 contributes to exosome-mediated immunosuppression of T cells. Biol. Reprod. 2020, 102, 185–198. [Google Scholar] [CrossRef]
- Aagaard, L.; Villesen, P.; Kjeldbjerg, A.L.; Pedersen, F.S. The approximately 30-million-year-old ERVPb1 envelope gene is evolutionarily conserved among hominoids and Old World monkeys. Genomics 2005, 86, 685–691. [Google Scholar] [CrossRef]
- Bjerregaard, B.; Lemmen, J.G.; Petersen, M.R.; Ostrup, E.; Iversen, L.H.; Almstrup, K.; Larsson, L.I.; Ziebe, S. Syncytin-1 and its receptor is present in human gametes. J. Assist. Reprod. Genet. 2014, 31, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Bjerregard, B.; Ziomkiewicz, I.; Schulz, A.; Larsson, L.I. Syncytin-1 in differentiating human myoblasts: Relationship to caveolin-3 and myogenin. Cell Tissue Res. 2014, 357, 355–362. [Google Scholar] [CrossRef]
- Soe, K.; Andersen, T.L.; Hobolt-Pedersen, A.S.; Bjerregaard, B.; Larsson, L.I.; Delaisse, J.M. Involvement of human endogenous retroviral syncytin-1 in human osteoclast fusion. Bone 2011, 48, 837–846. [Google Scholar] [CrossRef]
- Frese, S.; Ruebner, M.; Suhr, F.; Konou, T.M.; Tappe, K.A.; Toigo, M.; Jung, H.H.; Henke, C.; Steigleder, R.; Strissel, P.L.; et al. Long-Term Endurance Exercise in Humans Stimulates Cell Fusion of Myoblasts along with Fusogenic Endogenous Retroviral Genes In Vivo. PLoS ONE 2015, 10, e0132099. [Google Scholar] [CrossRef]
- Redelsperger, F.; Raddi, N.; Bacquin, A.; Vernochet, C.; Mariot, V.; Gache, V.; Blanchard-Gutton, N.; Charrin, S.; Tiret, L.; Dumonceaux, J.; et al. Genetic Evidence That Captured Retroviral Envelope syncytins Contribute to Myoblast Fusion and Muscle Sexual Dimorphism in Mice. PLoS Genet. 2016, 12, e1006289. [Google Scholar] [CrossRef]
- Diaz-Carballo, D.; Acikelli, A.H.; Klein, J.; Jastrow, H.; Dammann, P.; Wyganowski, T.; Guemues, C.; Gustmann, S.; Bardenheuer, W.; Malak, S.; et al. Therapeutic potential of antiviral drugs targeting chemorefractory colorectal adenocarcinoma cells overexpressing endogenous retroviral elements. J. Exp. Clin. Cancer Res. 2015, 34, 81. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Trejbalova, K.; Kovarova, D.; Vernerova, Z.; Hron, T.; Kucerova, D.; Hejnar, J. DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas. Retrovirology 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J.; Zhu, F. Human Endogenous Retroviral Envelope Protein Syncytin-1 and Inflammatory Abnormalities in Neuropsychological Diseases. Front. Psychiatry 2018, 9, 422. [Google Scholar] [CrossRef]
- Laufer, G.; Mayer, J.; Mueller, B.F.; Mueller-Lantzsch, N.; Ruprecht, K. Analysis of transcribed human endogenous retrovirus W env loci clarifies the origin of multiple sclerosis-associated retrovirus env sequences. Retrovirology 2009, 6, 37. [Google Scholar] [CrossRef]
- Antony, J.M.; van Marle, G.; Opii, W.; Butterfield, D.A.; Mallet, F.; Yong, V.W.; Wallace, J.L.; Deacon, R.M.; Warren, K.; Power, C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 2004, 7, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Garson, J.; Creange, A.; Dolei, A.; Ferrante, P.; Jouvin-Marche, E.; Marche, P.N.; Rieger, F.; Ruprecht, K.; Saresella, M.; Sotgiu, S.; et al. MSRV, Syncytin and the role of endogenous retroviral proteins in demyelination. Mult. Scler. 2005, 11, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.M.; Izad, M.; Bar-Or, A.; Warren, K.G.; Vodjgani, M.; Mallet, F.; Power, C. Quantitative analysis of human endogenous retrovirus-W env in neuroinflammatory diseases. AIDS Res. Hum. Retrovir. 2006, 22, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.M.; Ellestad, K.K.; Hammond, R.; Imaizumi, K.; Mallet, F.; Warren, K.G.; Power, C. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: A role for endoplasmic reticulum chaperones in astrocytes. J. Immunol. 2007, 179, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Dolei, A.; Serra, C.; Mameli, G.; Pugliatti, M.; Sechi, G.; Cirotto, M.C.; Rosati, G.; Sotgiu, S. Multiple sclerosis-associated retrovirus (MSRV) in Sardinian MS patients. Neurology 2002, 58, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, B.; Holck, S.; Christensen, I.J.; Larsson, L.I. Syncytin is involved in breast cancer-endothelial cell fusions. Cell. Mol. Life Sci. 2006, 63, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Strick, R.; Ackermann, S.; Langbein, M.; Swiatek, J.; Schubert, S.W.; Hashemolhosseini, S.; Koscheck, T.; Fasching, P.A.; Schild, R.L.; Beckmann, M.W.; et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral Syncytin-1 and regulated by TGF-beta. J. Mol. Med. 2007, 85, 23–38. [Google Scholar] [CrossRef]
- Larsen, J.M.; Christensen, I.J.; Nielsen, H.J.; Hansen, U.; Bjerregaard, B.; Talts, J.F.; Larsson, L.I. Syncytin immunoreactivity in colorectal cancer: Potential prognostic impact. Cancer Lett. 2009, 280, 44–49. [Google Scholar] [CrossRef]
- Aagaard, L.; Bjerregaard, B.; Kjeldbjerg, A.L.; Pedersen, F.S.; Larsson, L.I.; Rossi, J.J. Silencing of endogenous envelope genes in human choriocarcinoma cells shows that envPb1 is involved in heterotypic cell fusions. J. Gen. Virol. 2012, 93, 1696–1699. [Google Scholar] [CrossRef]
- Yan, T.L.; Wang, M.; Xu, Z.; Huang, C.M.; Zhou, X.C.; Jiang, E.H.; Zhao, X.P.; Song, Y.; Song, K.; Shao, Z.; et al. Up-regulation of syncytin-1 contributes to TNF-alpha-enhanced fusion between OSCC and HUVECs partly via Wnt/beta-catenin-dependent pathway. Sci. Rep. 2017, 7, 40983. [Google Scholar] [CrossRef]
- Vargas, A.; Zhou, S.; Ethier-Chiasson, M.; Flipo, D.; Lafond, J.; Gilbert, C.; Barbeau, B. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 2014, 28, 3703–3719. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Bayer, A.; Chu, T.; Tyurin, V.A.; Kagan, V.E.; Morelli, A.E.; Coyne, C.B.; Sadovsky, Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta 2016, 47, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.G.; Toudic, C.; Barbeau, B. Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses 2014, 6, 4609–4627. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Ilarregui, J.M.; Tometten, M.; Garcia, M.; Orsal, A.S.; Cordo-Russo, R.; Toscano, M.A.; Bianco, G.A.; Kobelt, P.; Handjiski, B.; et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 2007, 13, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- D’Haene, N.; Sauvage, S.; Maris, C.; Adanja, I.; Le Mercier, M.; Decaestecker, C.; Baum, L.; Salmon, I. VEGFR1 and VEGFR2 involvement in extracellular galectin-1- and galectin-3-induced angiogenesis. PLoS ONE 2013, 8, e67029. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, E.P.; Spring, E.L.; Baron, J.H.; de Bono, D.P. Galectin 1 modulates attachment, spreading and migration of cultured vascular smooth muscle cells via interactions with cellular receptors and components of extracellular matrix. J. Vasc. Res. 1999, 36, 47–58. [Google Scholar] [CrossRef]
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef]
- Poirier, F.; Timmons, P.M.; Chan, C.T.; Guenet, J.L.; Rigby, P.W. Expression of the L14 lectin during mouse embryogenesis suggests multiple roles during pre- and post-implantation development. Development 1992, 115, 143–155. [Google Scholar] [CrossRef]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in innate immunity: Dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [CrossRef]
- Vasta, G.R.; Feng, C.; Gonzalez-Montalban, N.; Mancini, J.; Yang, L.; Abernathy, K.; Frost, G.; Palm, C. Functions of galectins as ‘self/non-self’-recognition and effector factors. Pathog. Dis. 2017, 75, ftx046. [Google Scholar] [CrossRef]
- Elola, M.T.; Blidner, A.G.; Ferragut, F.; Bracalente, C.; Rabinovich, G.A. Assembly, organization and regulation of cell-surface receptors by lectin-glycan complexes. Biochem. J. 2015, 469, 1–16. [Google Scholar] [CrossRef]
- Vicovac, L.; Jankovic, M.; Cuperlovic, M. Galectin-1 and -3 in cells of the first trimester placental bed. Hum. Reprod. 1998, 13, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Kolundzic, N.; Bojic-Trbojevic, Z.; Kovacevic, T.; Stefanoska, I.; Kadoya, T.; Vicovac, L. Galectin-1 is part of human trophoblast invasion machinery—A functional study in vitro. PLoS ONE 2011, 6, e28514. [Google Scholar] [CrossRef]
- Fischer, I.; Redel, S.; Hofmann, S.; Kuhn, C.; Friese, K.; Walzel, H.; Jeschke, U. Stimulation of syncytium formation in vitro in human trophoblast cells by galectin-1. Placenta 2010, 31, 825–832. [Google Scholar] [CrossRef]
- Barrientos, G.; Freitag, N.; Tirado-Gonzalez, I.; Unverdorben, L.; Jeschke, U.; Thijssen, V.L.; Blois, S.M. Involvement of galectin-1 in reproduction: Past, present and future. Hum. Reprod. Update 2014, 20, 175–193. [Google Scholar] [CrossRef]
- Ouellet, M.; Mercier, S.; Pelletier, I.; Bounou, S.; Roy, J.; Hirabayashi, J.; Sato, S.; Tremblay, M.J. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 2005, 174, 4120–4126. [Google Scholar] [CrossRef]
- Gauthier, S.; Pelletier, I.; Ouellet, M.; Vargas, A.; Tremblay, M.J.; Sato, S.; Barbeau, B. Induction of galectin-1 expression by HTLV-I Tax and its impact on HTLV-I infectivity. Retrovirology 2008, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Mercier, S.; St-Pierre, C.; Pelletier, I.; Ouellet, M.; Tremblay, M.J.; Sato, S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 2008, 371, 121–129. [Google Scholar] [CrossRef]
- Toudic, C.; Vargas, A.; Xiao, Y.; St-Pierre, G.; Bannert, N.; Lafond, J.; Rassart, E.; Sato, S.; Barbeau, B. Galectin-1 interacts with the human endogenous retroviral envelope protein syncytin-2 and potentiates trophoblast fusion in humans. FASEB J 2019, 33, 12873. [Google Scholar] [CrossRef]
- Spinola, M.; Falvella, F.S.; Colombo, F.; Sullivan, J.P.; Shames, D.S.; Girard, L.; Spessotto, P.; Minna, J.D.; Dragani, T.A. MFSD2A is a novel lung tumor suppressor gene modulating cell cycle and matrix attachment. Mol. Cancer 2010, 9, 62. [Google Scholar] [CrossRef]
- Audiffred, J.F.; De Leo, S.E.; Brown, P.K.; Hale-Donze, H.; Monroe, W.T. Characterization and applications of serum-free induced adhesion in Jurkat suspension cells. Biotechnol. Bioeng. 2010, 106, 784–793. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, C.; Manya, H.; Ouellet, M.; Clark, G.F.; Endo, T.; Tremblay, M.J.; Sato, S. Host-soluble galectin-1 promotes HIV-1 replication through a direct interaction with glycans of viral gp120 and host CD4. J. Virol. 2011, 85, 11742–11751. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.H.; Charron, M.J.; Silver, D.L. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS ONE 2012, 7, e50629. [Google Scholar] [CrossRef] [PubMed]
- Reiling, J.H.; Clish, C.B.; Carette, J.E.; Varadarajan, M.; Brummelkamp, T.R.; Sabatini, D.M. A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc. Natl. Acad. Sci. USA 2011, 108, 11756–11765. [Google Scholar] [CrossRef]
- Lange, F.; Brandt, B.; Tiedge, M.; Jonas, L.; Jeschke, U.; Pohland, R.; Walzel, H. Galectin-1 induced activation of the mitochondrial apoptotic pathway: Evidence for a connection between death-receptor and mitochondrial pathways in human Jurkat T lymphocytes. Histochem. Cell Biol. 2009, 132, 211–223. [Google Scholar] [CrossRef]
- Jouve, N.; Despoix, N.; Espeli, M.; Gauthier, L.; Cypowyj, S.; Fallague, K.; Schiff, C.; Dignat-George, F.; Vely, F.; Leroyer, A.S. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J. Biol. Chem. 2013, 288, 2571–2579. [Google Scholar] [CrossRef]
- Blois, S.M.; Barrientos, G. Galectin signature in normal pregnancy and preeclampsia. J. Reprod. Immunol. 2014, 101–102, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.; Christian, H.C.; Redman, C.W.; Sargent, I.L.; Boyd, C.A. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells--implication for placental cell fusion. FEBS J. 2007, 274, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.H.; Lee, A.B.; Phillips, E.B.; Roberts, J.K.; Weitlauf, H.M. Spatio-temporal pattern for expression of galectin-3 in the murine utero-placental complex: Evidence for differential regulation. Biol. Reprod. 1998, 58, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Garner, O.B.; Aguilar, H.C.; Fulcher, J.A.; Levroney, E.L.; Harrison, R.; Wright, L.; Robinson, L.R.; Aspericueta, V.; Panico, M.; Haslam, S.M.; et al. Endothelial galectin-1 binds to specific glycans on nipah virus fusion protein and inhibits maturation, mobility, and function to block syncytia formation. PLoS Pathog. 2010, 6, e1000993. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.L.; Chen, Y.H.; Wang, S.W.; Huang, Y.J.; Leu, C.H.; Yeh, N.C.; Chu, C.Y.; Lin, C.C.; Shieh, G.S.; Chen, Y.L.; et al. Galectin-1 binds to influenza virus and ameliorates influenza virus pathogenesis. J. Virol. 2011, 85, 10010–10020. [Google Scholar] [CrossRef]
- Toledo, K.A.; Fermino, M.L.; Andrade Cdel, C.; Riul, T.B.; Alves, R.T.; Muller, V.D.; Russo, R.R.; Stowell, S.R.; Cummings, R.D.; Aquino, V.H.; et al. Galectin-1 exerts inhibitory effects during DENV-1 infection. PLoS ONE 2014, 9, e112474. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, H.; Lu, X.; Wang, R.; Zheng, R.; Li, Y.; Yang, X.; Jia, W.T.; Zhao, Y.; Wang, Y.; et al. Effects of individually silenced N-glycosylation sites and non-synonymous single-nucleotide polymorphisms on the fusogenic function of human syncytin-2. Cell Adh. Migr. 2016, 10, 39–55. [Google Scholar] [CrossRef]
- Earl, P.L.; Doms, R.W.; Moss, B. Multimeric CD4 binding exhibited by human and simian immunodeficiency virus envelope protein dimers. J. Virol. 1992, 66, 5610–5614. [Google Scholar] [CrossRef]
- Holst, S.; Belo, A.I.; Giovannetti, E.; van Die, I.; Wuhrer, M. Profiling of different pancreatic cancer cells used as models for metastatic behaviour shows large variation in their N-glycosylation. Sci. Rep. 2017, 7, 16623. [Google Scholar] [CrossRef]
- Gioia, L.; Siddique, A.; Head, S.R.; Salomon, D.R.; Su, A.I. A genome-wide survey of mutations in the Jurkat cell line. BMC Genom. 2018, 19, 334. [Google Scholar] [CrossRef]
- Croset, A.; Delafosse, L.; Gaudry, J.P.; Arod, C.; Glez, L.; Losberger, C.; Begue, D.; Krstanovic, A.; Robert, F.; Vilbois, F.; et al. Differences in the glycosylation of recombinant proteins expressed in HEK and CHO cells. J. Biotechnol. 2012, 161, 336–348. [Google Scholar] [CrossRef]
- Garcia Caballero, G.; Kaltner, H.; Kutzner, T.J.; Ludwig, A.K.; Manning, J.C.; Schmidt, S.; Sinowatz, F.; Gabius, H.J. How galectins have become multifunctional proteins. Histol. Histopathol. 2020, 35, 509–539. [Google Scholar] [CrossRef] [PubMed]
- Daroqui, C.M.; Ilarregui, J.M.; Rubinstein, N.; Salatino, M.; Toscano, M.A.; Vazquez, P.; Bakin, A.; Puricelli, L.; Bal de Kier Joffe, E.; Rabinovich, G.A. Regulation of galectin-1 expression by transforming growth factor beta1 in metastatic mammary adenocarcinoma cells: Implications for tumor-immune escape. Cancer Immunol. Immunother. 2007, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Barkan, B.; Shoji, H.; Aries, I.M.; Mathieu, V.; Deltour, L.; Hackeng, T.M.; Kiss, R.; Kloog, Y.; Poirier, F.; et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010, 70, 6216–6224. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Platt, J.L.; Zhou, X.; Lefferts, A.R.; Cascalho, M. Cell Fusion in the War on Cancer: A Perspective on the Inception of Malignancy. Int. J. Mol. Sci. 2016, 17, 1118. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Ruiz, D.; Van Hoesen, K.; Cohen, M. The Dark Side of Cell Fusion. Int. J. Mol. Sci. 2016, 17, 638. [Google Scholar] [CrossRef]
- Holder, B.S.; Tower, C.L.; Forbes, K.; Mulla, M.J.; Aplin, J.D.; Abrahams, V.M. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology 2012, 136, 184–191. [Google Scholar] [CrossRef]
- Cronqvist, T.; Tannetta, D.; Morgelin, M.; Belting, M.; Sargent, I.; Familari, M.; Hansson, S.R. Syncytiotrophoblast derived extracellular vesicles transfer functional placental miRNAs to primary human endothelial cells. Sci. Rep. 2017, 7, 4558. [Google Scholar] [CrossRef]
- Holder, B.S.; Tower, C.L.; Jones, C.J.; Aplin, J.D.; Abrahams, V.M. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol. Reprod. 2012, 86, 103. [Google Scholar] [CrossRef]
- Mincheva-Nilsson, L.; Baranov, V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: Immune modulation for pregnancy success. Am. J. Reprod Immunol. 2014, 72, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Postel, R.; Brandwijk, R.J.; Dings, R.P.; Nesmelova, I.; Satijn, S.; Verhofstad, N.; Nakabeppu, Y.; Baum, L.G.; Bakkers, J.; et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 2006, 103, 15975–15980. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toudic, C.; Maurer, M.; St-Pierre, G.; Xiao, Y.; Bannert, N.; Lafond, J.; Rassart, É.; Sato, S.; Barbeau, B. Galectin-1 Modulates the Fusogenic Activity of Placental Endogenous Retroviral Envelopes. Viruses 2023, 15, 2441. https://doi.org/10.3390/v15122441
Toudic C, Maurer M, St-Pierre G, Xiao Y, Bannert N, Lafond J, Rassart É, Sato S, Barbeau B. Galectin-1 Modulates the Fusogenic Activity of Placental Endogenous Retroviral Envelopes. Viruses. 2023; 15(12):2441. https://doi.org/10.3390/v15122441
Chicago/Turabian StyleToudic, Caroline, Maike Maurer, Guillaume St-Pierre, Yong Xiao, Norbert Bannert, Julie Lafond, Éric Rassart, Sachiko Sato, and Benoit Barbeau. 2023. "Galectin-1 Modulates the Fusogenic Activity of Placental Endogenous Retroviral Envelopes" Viruses 15, no. 12: 2441. https://doi.org/10.3390/v15122441
APA StyleToudic, C., Maurer, M., St-Pierre, G., Xiao, Y., Bannert, N., Lafond, J., Rassart, É., Sato, S., & Barbeau, B. (2023). Galectin-1 Modulates the Fusogenic Activity of Placental Endogenous Retroviral Envelopes. Viruses, 15(12), 2441. https://doi.org/10.3390/v15122441






