Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses
Abstract
:1. Introduction
1.1. Background
1.2. Viral Migration during Infection and the Blood–Brain Barrier
2. Blood–Brain-Barrier Crossing
2.1. Mechanisms of Permeabilizing the BBB
2.2. Mechanisms of Passing through an Intact BBB
2.3. Trojan horse
2.4. Mechanisms of Bypassing the BBB Entirely
3. Neuropathogenesis
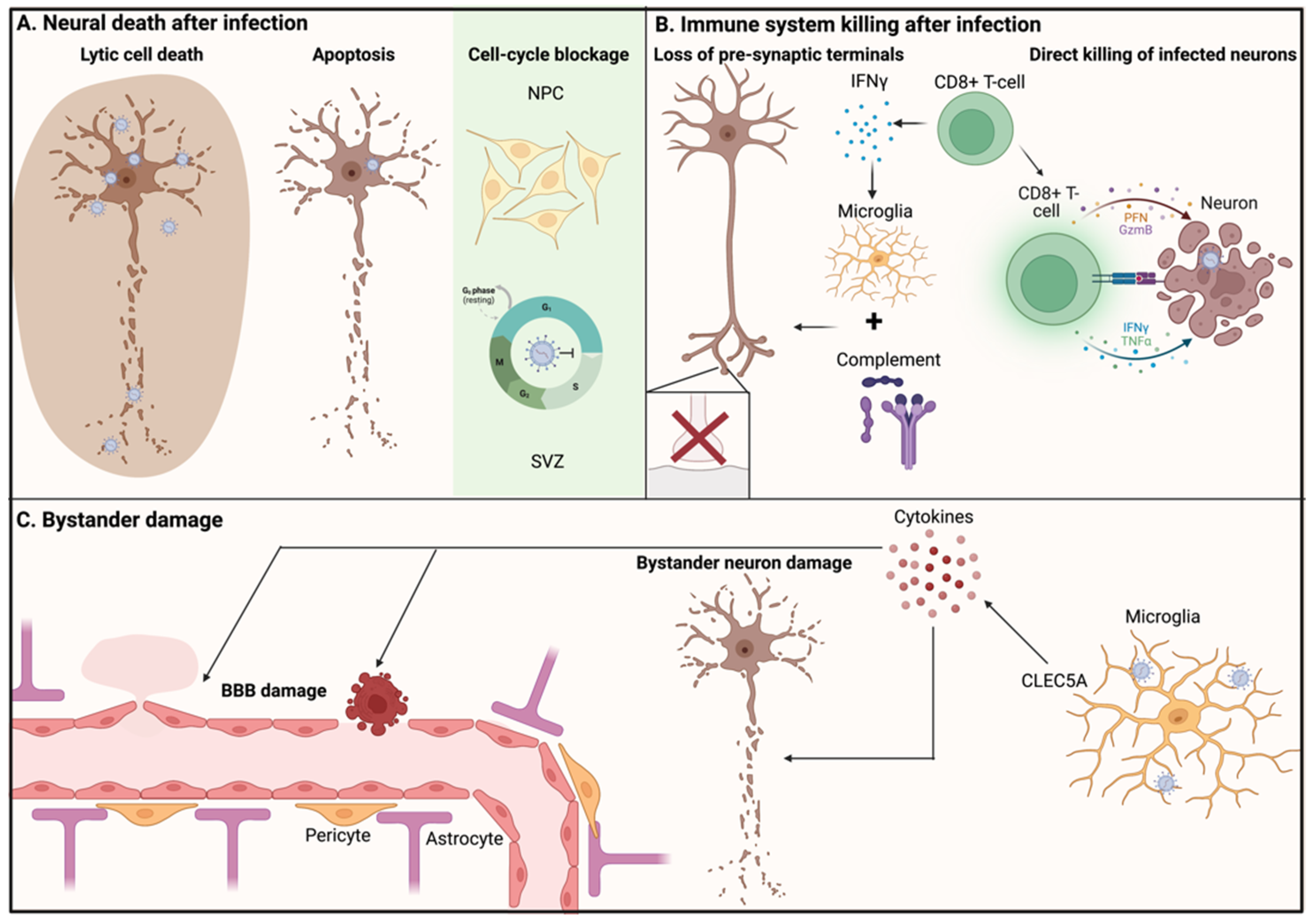
3.1. Neuronal Death after Infection
3.2. Immune System Killing Infected Neurons
3.3. Non-Infected Bystander Neurons
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| BFA | brefeldin A |
| BM | basement membrane |
| CNS | Central Nervous System |
| DC | dendritic cell |
| DENV | dengue virus |
| DHA | Docosahexaenoic acid |
| E | Envelope protein |
| JEV | Japanese encephalitis virus |
| MVEV | Murray Valley Encephalitis Virus |
| NHP | non-human primate |
| NPC | neural progenitor cell |
| ORF | open reading frame |
| POWV | Powassan Virus |
| prM | pre-membrane protein |
| SLEV | St Louis encephalitis Virus |
| SVZ | sub ventricular zone |
| TBEV | Tick-Borne Encephalitis virus |
| TJ | Tight junction |
| USV | Usutu virus |
| WNV | West Nile Virus |
| YFV | yellow fever Virus |
| ZIKV | Zika virus |
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2014, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Whitehorn, J.; Simmons, C.P. The pathogenesis of dengue. Vaccine 2011, 29, 7221–7228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sips, G.J.; Wilschut, J.; Smit, J.M. Neuroinvasive flavivirus infections. Rev. Med. Virol. 2011, 22, 69–87. [Google Scholar] [CrossRef]
- Monath, T.P.; Barrett, A.D. Pathogenesis and Pathophysiology of Yellow Fever. Adv. Virus Res. 2003, 60, 343–395. [Google Scholar] [CrossRef]
- Sulaiman, W.A.W.; Mat, L.N.I.; Hashim, H.Z.; Hoo, F.K.; Ching, S.M.; Vasudevan, R.; Mohamed, M.H.; Basri, H. Acute disseminated encephalomyelitis in dengue viral infection. J. Clin. Neurosci. 2017, 43, 25–31. [Google Scholar] [CrossRef]
- Prabhat, N.; Ray, S.; Chakravarty, K.; Kathuria, H.; Saravana, S.; Singh, D.; Rebello, A.; Lakhanpal, V.; Goyal, M.K.; Lal, V. Atypical neurological manifestations of dengue fever: A case series and mini review. Postgrad. Med. J. 2020, 96, 759–765. [Google Scholar] [CrossRef]
- Jang, H.; Boltz, D.A.; Webster, R.G.; Smeyne, R.J. Viral parkinsonism. Biochim. et Biophys. Acta BBA-Mol. Basis Dis. 2009, 1792, 714–721. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, R. Tick-Borne Encephalitis. Infect. Dis. Clin. N. Am. 2008, 22, 561–575. [Google Scholar] [CrossRef]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Liang, B.; Guida, J.P.; Costa Do Nascimento, M.L.; Mysorekar, I.U. Host and viral mechanisms of congenital Zika syndrome. Virulence 2019, 10, 768–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miner, J.J.; Diamond, M.S. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibovitch, E.C.; Jacobson, S. Vaccinations for Neuroinfectious Disease: A Global Health Priority. Neurotherapeutics 2016, 13, 562–570. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, M.R. Historical Perspectives on Flavivirus Research. Viruses 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, F.; Das, S.; Mukherjee, D.; Mal, S.; Ray, U. Insight into the Tropism of Dengue Virus in Humans. Viruses 2019, 11, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricklin, M.E.; Garcìa-Nicolàs, O.; Brechbühl, D.; Python, S.; Zumkehr, B.; Posthaus, H.; Oevermann, A.; Summerfield, A. Japanese encephalitis virus tropism in experimentally infected pigs. Veter-Res. 2016, 47, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazurek, R.; Dave, J.; Chandran, R.; Misra, A.; Sheikh, A.; Greif, D. Vascular Cells in Blood Vessel Wall Development and Disease. Dvances Pharmacol. 2016, 78, 323–350. [Google Scholar] [CrossRef] [Green Version]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.-A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [Green Version]
- Simmons, C.P.; Farrar, J.J.; van Vinh Chau, N.; Wills, B. Dengue. Current concepts: Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef]
- MacGibeny, M.A.; Koyuncu, O.O.; Wirblich, C.; Schnell, M.; Enquist, L.W. Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. PLOS Pathog. 2018, 14, e1007188. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, R.; Miranda-Saksena, M.; Douglas, M.W.; Cunningham, A.L. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2007, 18, 35–51. [Google Scholar] [CrossRef]
- Spindler, K.R.; Hsu, T.-H. Viral disruption of the blood–brain barrier. Trends Microbiol. 2012, 20, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, M.; Dutta, K.; Saheb, S.K.; Basu, A. Understanding the molecular mechanism of blood–brain barrier damage in an experimental model of Japanese encephalitis: Correlation with minocycline administration as a therapeutic agent. Neurochem. Int. 2009, 55, 717–723. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, M.; Gurjav, U.; Lum, S.; Nerurkar, V.R. Reversal of West Nile virus-induced blood–brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 2010, 397, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood?brain barrier dysfunction. J. Neurochem. 2006, 101, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Roe, K.; Kumar, M.; Lum, S.; Orillo, B.; Nerurkar, V.R.; Verma, S. West Nile virus-induced disruption of the blood–brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J. Gen. Virol. 2012, 93, 1193–1203. [Google Scholar] [CrossRef]
- Tung, W.-H.; Tsai, H.-W.; Lee, I.-T.; Hsieh, H.-L.; Chen, W.-J.; Chen, Y.-L.; Yang, C.-M. Japanese encephalitis virus induces matrix metalloproteinase-9 in rat brain astrocytes via NF-κB signalling dependent on MAPKs and reactive oxygen species. Br. J. Pharmacol. 2010, 161, 1566–1583. [Google Scholar] [CrossRef] [Green Version]
- Luplerdlop, N.; Missé, D.; Bray, D.; Deleuze-Marquès, V.; Gonzalez, J.-P.; Leardkamolkarn, V.; Yssel, H.; Veas, F. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006, 7, 1176–1181. [Google Scholar] [CrossRef]
- Shen, J.; Devery, J.M.; King, N.J. Adherence status regulates the primary cellular activation responses to the flavivirus West Nile. Immunology 1995, 84, 254–264. [Google Scholar]
- Wang, P.; Dai, J.; Bai, F.; Kong, K.-F.; Wong, S.J.; Montgomery, R.R.; Madri, J.A.; Fikrig, E. Matrix Metalloproteinase 9 Facilitates West Nile Virus Entry into the Brain. J. Virol. 2008, 82, 8978–8985. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Huitinga, I.; Lustig, S.; van Rooijen, N.; Kobiler, D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 1996, 141, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hossain, F.M.A.; Patil, A.M.; Choi, J.Y.; Kim, S.B.; Uyangaa, E.; Park, S.-Y.; Lee, J.-H.; Kim, B.; Kim, K.; et al. Ablation of CD11c hi dendritic cells exacerbates Japanese encephalitis by regulating blood-brain barrier permeability and altering tight junction/adhesion molecules. Comp. Immunol. Microbiol. Infect. Dis. 2016, 48, 22–32. [Google Scholar] [CrossRef]
- Chiu, C.-F.; Chu, L.-W.; Liao, I.-C.; Simanjuntak, Y.; Lin, Y.-L.; Juan, C.-C.; Ping, Y.-H. The Mechanism of the Zika Virus Crossing the Placental Barrier and the Blood-Brain Barrier. Front. Microbiol. 2020, 11, 214. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Steinhoff, U.; Reis, L.F.L.; Hemmi, S.; Pavlovic, J.; Zinkernagel, R.M.; Aguet, M. Functional Role of Type I and Type II Interferons in Antiviral Defense. Science 1994, 264, 1918–1921. [Google Scholar] [CrossRef]
- Han, Y.W.; Choi, J.Y.; Uyangaa, E.; Kim, S.B.; Kim, J.H.; Kim, B.S.; Kim, K.; Eo, S.K. Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways. PLOS Pathog. 2014, 10, e1004319. [Google Scholar] [CrossRef] [Green Version]
- Daffis, S.; Samuel, M.A.; Suthar, M.S.; Gale, M.; Diamond, M.S. Toll-Like Receptor 3 Has a Protective Role against West Nile Virus Infection. J. Virol. 2008, 82, 10349–10358. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.-Y.; Stuart, C.; Takeda, K.; D’Agnillo, F.; Golding, B. Poly(I:C) Induces Human Lung Endothelial Barrier Dysfunction by Disrupting Tight Junction Expression of Claudin-5. PLoS ONE 2016, 11, e0160875. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef]
- Arjona, A.; Ledizet, M.; Anthony, K.; Bonafé, N.; Modis, Y.; Town, T.; Fikrig, E. West Nile Virus Envelope Protein Inhibits dsRNA-Induced Innate Immune Responses. J. Immunol. 2007, 179, 8403–8409. [Google Scholar] [CrossRef] [PubMed]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafá, Y.M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Desse, S.; Martinez, A.; Worthen, R.J.; Jope, R.S.; Beurel, E. TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 2018, 69, 556–567. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Fischer, R.; Torres-Badillo, C.C.; Davies, H.A.; Logan, K.; Pfizenmaier, K.; Male, D.K.; Sharrack, B.; Romero, I.A. Role of Caspases in Cytokine-Induced Barrier Breakdown in Human Brain Endothelial Cells. J. Immunol. 2012, 189, 3130–3139. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Mehta, V.K.; Garg, R.K.; Malhotra, H.S.; Sharma, P.K.; Jain, A. Study of interleukin-6 and interleukin-8 levels in patients with neurological manifestations of dengue. J. Postgrad. Med. 2017, 63, 11–15. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ou, Y.-C.; Li, J.-R.; Chang, C.-Y.; Pan, H.-C.; Lai, C.-Y.; Liao, S.-L.; Raung, S.-L.; Chang, C.-J. Infection of Pericytes In Vitro by Japanese Encephalitis Virus Disrupts the Integrity of the Endothelial Barrier. J. Virol. 2014, 88, 1150–1161. [Google Scholar] [CrossRef] [Green Version]
- Lazear, H.M.; Daniels, B.P.; Pinto, A.K.; Huang, A.C.; Vick, S.C.; Doyle, S.E.; Gale, M., Jr.; Klein, R.S.; Diamond, M.S. Interferon-λ restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med. 2015, 7, 284ra59. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.A.; Diamond, M.S. Alpha/Beta Interferon Protects against Lethal West Nile Virus Infection by Restricting Cellular Tropism and Enhancing Neuronal Survival. J. Virol. 2005, 79, 13350–13361. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, R.; Mundt, F.; Gilthorpe, J.D.; Wölfel, S.; Gekara, N.O.; Kröger, A.; Överby, A.K. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflamm. 2016, 13, 277. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, B. Molecular mechanisms involved in T cell migration across the blood–brain barrier. J. Neural Transm. 2006, 113, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, P.; Bai, F.; Town, T.; Fikrig, E. ICAM-1 Participates in the Entry of West Nile Virus into the Central Nervous System. J. Virol. 2008, 82, 4164–4168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clé, M.; Desmetz, C.; Barthelemy, J.; Martin, M.-F.; Constant, O.; Maarifi, G.; Foulongne, V.; Bolloré, K.; Glasson, Y.; De Bock, F.; et al. Zika Virus Infection Promotes Local Inflammation, Cell Adhesion Molecule Upregulation, and Leukocyte Recruitment at the Blood-Brain Barrier. Mbio 2020, 11, e01183-20. [Google Scholar] [CrossRef] [PubMed]
- Roe, K.; Orillo, B.; Verma, S. West Nile Virus-Induced Cell Adhesion Molecules on Human Brain Microvascular Endothelial Cells Regulate Leukocyte Adhesion and Modulate Permeability of the In Vitro Blood-Brain Barrier Model. PLoS ONE 2014, 9, e102598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grygorczuk, S.; Świerzbińska, R.; Kondrusik, M.; Dunaj, J.; Czupryna, P.; Moniuszko, A.; Siemieniako, A.; Pancewicz, S. The intrathecal expression and pathogenetic role of Th17 cytokines and CXCR2-binding chemokines in tick-borne encephalitis. J. Neuroinflammation 2018, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.T.; Rathore, A.P.S.; Soundarajan, G.; John, A.L.S. Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat. Commun. 2019, 10, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syenina, A.; Saron, W.A.A.; Jagaraj, C.J.; Bibi, S.; Arock, M.; Gubler, D.J.; Rathore, A.P.S.; Abraham, S.N.; John, A.L.S. Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability. Viruses 2020, 12, 1379. [Google Scholar] [CrossRef]
- Sahu, A.K.; Aggarwal, P.; Ekka, M.; Nayer, J.; Bhoi, S.; Kumar, A.; Luthra, K. Assessing the serum chymase level as an early predictor of dengue severity. J. Med. Virol. 2020, 93, 3330–3337. [Google Scholar] [CrossRef]
- Kim, K.S. Mechanisms of microbial traversal of the blood–brain barrier. Nat. Rev. Genet. 2008, 6, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Velandia-Romero, M.L.; Calderón-Peláez, M.-A.; Castellanos, J.E. In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium. PLoS ONE 2016, 11, e0157786. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.; Martín-Tapia, D.; Valdespino-Vázquez, Y.; Alarcón, L.; Espejel-Nuñez, A.; Guzmán-Huerta, M.; Muñoz-Medina, J.E.; Shibayama, M.; Chávez-Munguía, B.; Estrada-Gutiérrez, G.; et al. Syncytiotrophoblast of Placentae from Women with Zika Virus Infection Has Altered Tight Junction Protein Expression and Increased Paracellular Permeability. Cells 2019, 8, 1174. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Yu, L.; Cao, S.; Wang, K.; Yuan, J.; Wang, C.; Wang, K.; Cui, M.; Fu, Z.F. Viral Infection of the Central Nervous System and Neuroinflammation Precede Blood-Brain Barrier Disruption during Japanese Encephalitis Virus Infection. J. Virol. 2015, 89, 5602–5614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papa, M.P.; Meuren, L.M.; Coelho, S.V.A.; de Oliveira Lucas, C.G.; Mustafá, Y.M.; Matassoli, F.L.; Silveira, P.P.; Frost, P.S.; Pezzuto, P.; Ribeiro, M.R.; et al. Zika Virus Infects, Activates, and Crosses Brain Microvascular Endothelial Cells, without Barrier Disruption. Front. Microbiol. 2017, 8, 2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Huang, Y.; Shi, Y.; Bai, X.; Yang, P.; Chen, Q. Tembusu Virus Entering the Central Nervous System Caused Nonsuppurative Encephalitis without Disrupting the Blood-Brain Barrier. J. Virol. 2021, 95, e02191-20. [Google Scholar] [CrossRef] [PubMed]
- Patabendige, A.; Michael, B.; Craig, A.; Solomon, T. Brain microvascular endothelial-astrocyte cell responses following Japanese encephalitis virus infection in an in vitro human blood-brain barrier model. Mol. Cell. Neurosci. 2018, 89, 60–70. [Google Scholar] [CrossRef]
- Panganiban, A.T.; Blair, R.V.; Hattler, J.B.; Bohannon, D.G.; Bonaldo, M.C.; Schouest, B.; Maness, N.J.; Kim, W. A Zika virus primary isolate induces neuroinflammation, compromises the blood-brain barrier and upregulates CXCL12 in adult macaques. Brain Pathol. 2020, 30, 1017–1027. [Google Scholar] [CrossRef]
- Ayloo, S.; Gu, C. Transcytosis at the blood–brain barrier. Curr. Opin. Neurobiol. 2019, 57, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Vacca, F.; Gruenberg, J. Endosome maturation, transport and functions. Semin. Cell Dev. Biol. 2014, 31, 2–10. [Google Scholar] [CrossRef]
- Cornford, E.M.; Hyman, S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. Neurorx 2005, 2, 27–43. [Google Scholar] [CrossRef]
- Liou, M.-L.; Hsu, C.-Y. Japanese encephalitis virus is transported across the cerebral blood vessels by endocytosis in mouse brain. Cell Tissue Res. 1998, 293, 389–394. [Google Scholar] [CrossRef]
- Hasebe, R.; Suzuki, T.; Makino, Y.; Igarashi, M.; Yamanouchi, S.; Maeda, A.; Horiuchi, M.; Sawa, H.; Kimura, T. Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein. BMC Microbiol. 2010, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chi, X.; Cheng, M.; Huang, X.; Liu, X.; Fan, J.; Xu, H.; Lin, T.; Shi, L.; Qin, C.; et al. Zika virus degrades the ω-3 fatty acid transporter Mfsd2a in brain microvascular endothelial cells and impairs lipid homeostasis. Sci. Adv. 2019, 5, eaax7142. [Google Scholar] [CrossRef] [Green Version]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood–brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, E.; Kato, F.; Tajima, S.; Ogawa, S.; Yan, K.; Takahashi, K.; Sato, Y.; Suzuki, T.; Kawai, Y.; Inagaki, T.; et al. Neuroinvasiveness of the MR766 strain of Zika virus in IFNAR-/- mice maps to prM residues conserved amongst African genotype viruses. PLOS Pathog. 2021, 17, e1009788. [Google Scholar] [CrossRef] [PubMed]
- Mladinich, M.C.; Schwedes, J.; Mackow, E.R. Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells. Mbio 2017, 8, e00952-17. [Google Scholar] [CrossRef] [Green Version]
- Clé, M.; Constant, O.; Barthelemy, J.; Desmetz, C.; Martin, M.F.; Lapeyre, L.; Cadar, D.; Savini, G.; Teodori, L.; Monaco, F.; et al. Differential neurovirulence of Usutu virus lineages in mice and neuronal cells. J. Neuroinflammation 2021, 18, 11. [Google Scholar] [CrossRef]
- Palus, M.; Vancova, M.; Sirmarova, J.; Elsterova, J.; Perner, J.; Ruzek, D. Tick-borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity. Virology 2017, 507, 110–122. [Google Scholar] [CrossRef]
- Conde, J.N.; Sanchez-Vicente, S.; Saladino, N.; Gorbunova, E.E.; Schutt, W.R.; Mladinich, M.C.; Himmler, G.E.; Benach, J.; Kim, H.K.; Mackow, E.R. Powassan Viruses Spread Cell to Cell during Direct Isolation from Ixodes Ticks and Persistently Infect Human Brain Endothelial Cells and Pericytes. J. Virol. 2022, 96, e0168221. [Google Scholar] [CrossRef]
- Hussmann, K.L.; Fredericksen, B.L. Differential induction of CCL5 by pathogenic and non-pathogenic strains of West Nile virus in brain endothelial cells and astrocytes. J. Gen. Virol. 2014, 95, 862–867. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.I. Pharmacological Inhibition of Endocytic Pathways: Is It Specific Enough to Be Useful? Methods Mol. Biol. 2008, 440, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Oda, K.; Yokota, S.; Takatsuki, A.; Ikehara, Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1988, 263, 18545–18552. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, G.C.; Borget, M.-Y.; Bernier, S.; Garneau, D.; Duarte, A.J.D.S.; Dumais, N. RAGE and CCR7 mediate the transmigration of Zika-infected monocytes through the blood-brain barrier. Immunobiology 2019, 224, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.M.; Acharya, D.; Duty, L.; Thompson, E.A.; Le, L.; Stokic, D.S.; Leis, A.A.; Bai, F. Osteopontin facilitates West Nile virus neuroinvasion via neutrophil “Trojan horse” transport. Sci. Rep. 2017, 7, 4722. [Google Scholar] [CrossRef]
- Zou, S.-S.; Zou, Q.-C.; Xiong, W.-J.; Cui, N.-Y.; Wang, K.; Liu, H.-X.; Lou, W.-J.; Higazy, D.; Zhang, Y.-G.; Cui, M. Brain Microvascular Endothelial Cell-Derived HMGB1 Facilitates Monocyte Adhesion and Transmigration to Promote JEV Neuroinvasion. Front. Cell. Infect. Microbiol. 2021, 11, 701820. [Google Scholar] [CrossRef]
- McDonald, E.M.; Anderson, J.; Wilusz, J.; Ebel, G.D.; Brault, A.C. Zika Virus Replication in Myeloid Cells during Acute Infection Is Vital to Viral Dissemination and Pathogenesis in a Mouse Model. J. Virol. 2020, 94, e00838-20. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ward, M.F.; Sama, A.E.; Wang, H. Extracellular HMGB1 as a Proinflammatory Cytokine. J. Interf. Cytokine Res. 2004, 24, 329–333. [Google Scholar] [CrossRef]
- Maximova, O.A.; Bernbaum, J.G.; Pletnev, A.G. West Nile Virus Spreads Transsynaptically within the Pathways of Motor Control: Anatomical and Ultrastructural Mapping of Neuronal Virus Infection in the Primate Central Nervous System. PLOS Neglected Trop. Dis. 2016, 10, e0004980. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.A.; Wang, H.; Siddharthan, V.; Morrey, J.D.; Diamond, M.S. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. USA 2007, 104, 17140–17145. [Google Scholar] [CrossRef] [Green Version]
- Ohka, S.; Sakai, M.; Bohnert, S.; Igarashi, H.; Deinhardt, K.; Schiavo, G.; Nomoto, A. Receptor-Dependent and -Independent Axonal Retrograde Transport of Poliovirus in Motor Neurons. J. Virol. 2009, 83, 4995–5004. [Google Scholar] [CrossRef] [Green Version]
- Gluska, S.; Zahavi, E.E.; Chein, M.; Gradus, T.; Bauer, A.; Finke, S.; Perlson, E. Rabies Virus Hijacks and Accelerates the p75NTR Retrograde Axonal Transport Machinery. PLOS Pathog. 2014, 10, e1004348. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Dias, P.; Holzbaur, E.L.F. Axonal transport: Driving synaptic function (80-). Science 2019, 366, eaaw9997. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Siddharthan, V.; Hall, J.O.; Morrey, J.D. West Nile virus preferentially transports along motor neuron axons after sciatic nerve injection of hamsters. J. NeuroVirology 2009, 15, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Leis, A.A.; Stokic, D.S. Neuromuscular Manifestations of West Nile Virus Infection. Front. Neurol. 2012, 3, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darman, J.; Backovic, S.; Dike, S.; Maragakis, N.J.; Krishnan, C.; Rothstein, J.D.; Irani, D.N.; Kerr, U.A. Viral-Induced Spinal Motor Neuron Death Is Non-Cell-Autonomous and Involves Glutamate Excitotoxicity. J. Neurosci. 2004, 24, 7566–7575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Bhattacharyya, S.; Sharma, K.B.; Chauhan, S.; Asthana, S.; Abdin, M.Z.; Vrati, S.; Kalia, M. Japanese encephalitis virus activates autophagy through XBP1 and ATF6 ER stress sensors in neuronal cells. J. Gen. Virol. 2017, 98, 1027–1039. [Google Scholar] [CrossRef]
- Bílý, T.; Palus, M.; Eyer, L.; Elsterová, J.; Vancová, M.; Růžek, D. Electron Tomography Analysis of Tick-Borne Encephalitis Virus Infection in Human Neurons. Sci. Rep. 2015, 5, 10745. [Google Scholar] [CrossRef] [Green Version]
- Hunsperger, E.A.; Roehrig, J.T. Nocodazole delays viral entry into the brain following footpad inoculation with West Nile virus in mice. J. NeuroVirology 2009, 15, 211–218. [Google Scholar] [CrossRef]
- Li, M.Y.; Naik, T.S.; Siu, L.Y.L.; Acuto, O.; Spooner, E.; Wang, P.; Yang, X.; Lin, Y.; Bruzzone, R.; Ashour, J.; et al. Lyn kinase regulates egress of flaviviruses in autophagosome-derived organelles. Nat. Commun. 2020, 11, 5189. [Google Scholar] [CrossRef]
- Raung, S.-L.; Kuo, M.-D.; Wang, Y.-M.; Chen, C.-J. Role of reactive oxygen intermediates in Japanese encephalitis virus infection in murine neuroblastoma cells. Neurosci. Lett. 2001, 315, 9–12. [Google Scholar] [CrossRef]
- Swarup, V.; Das, S.; Ghosh, S.; Basu, A. Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J. Neurochem. 2007, 103, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Parquet, M.D.C.; Kumatori, A.; Hasebe, F.; Morita, K.; Igarashi, A. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 2001, 500, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-S.; Liao, C.-L.; Tsao, C.-H.; Chen, M.-C.; Liu, C.-I.; Chen, L.-K.; Lin, Y.-L. Membrane Permeabilization by Small Hydrophobic Nonstructural Proteins of Japanese Encephalitis Virus. J. Virol. 1999, 73, 6257–6264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-Juárez, M.; Martínez-Castillo, M.; Shrivastava, G.; García-Cordero, J.; Villegas-Sepulveda, N.; Mondragón-Castelán, M.; Mondragón-Flores, R.; Cedillo-Barrón, L. Recombinant Dengue virus protein NS2B alters membrane permeability in different membrane models. Virol. J. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzek, D.; Vancová, M.; Tesařová, M.; Ahantarig, A.; Kopecký, J.; Grubhoffer, L. Morphological changes in human neural cells following tick-borne encephalitis virus infection. J. Gen. Virol. 2009, 90, 1649–1658. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, A.; Wang, M.; Yin, Z.; Jia, R. The Dual Regulation of Apoptosis by Flavivirus. Front. Microbiol. 2021, 12, 654494. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Gottlieb, D.; Diamond, M.S. Infection and Injury of Neurons by West NileEncephalitisVirus. J. Virol. 2003, 77, 13203–13213. [Google Scholar] [CrossRef] [Green Version]
- Hirano, M.; Yoshii, K.; Sakai, M.; Hasebe, R.; Ichii, O.; Kariwa, H. Tick-borne flaviviruses alter membrane structure and replicate in dendrites of primary mouse neuronal cultures. J. Gen. Virol. 2014, 95, 849–861. [Google Scholar] [CrossRef]
- Samuel, M.A.; Morrey, J.D.; Diamond, M.S. Caspase 3-Dependent Cell Death of Neurons Contributes to the Pathogenesis of West Nile Virus Encephalitis. J. Virol. 2007, 81, 2614–2623. [Google Scholar] [CrossRef] [Green Version]
- Swarup, V.; Ghosh, J.; Das, S.; Basu, A. Tumor necrosis factor receptor-associated death domain mediated neuronal death contributes to the glial activation and subsequent neuroinflammation in Japanese encephalitis. Neurochem. Int. 2008, 52, 1310–1321. [Google Scholar] [CrossRef]
- García-Verdugo, J.M.; Alvarez-Buylla, A.; Gil-Perotín, S. Identification and characterization of neural progenitor cells in the adult mammalian brain. Adv. Anat. Embryol. Cell Biol. 2009, 203, 1–101. [Google Scholar] [CrossRef]
- Das, S.; Basu, A. Japanese encephalitis virus infects neural progenitor cells and decreases their proliferation. J. Neurochem. 2008, 106, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larena, M.; Regner, M.; Lee, E.; Lobigs, M. Pivotal Role of Antibody and Subsidiary Contribution of CD8 + T Cells to Recovery from Infection in a Murine Model of Japanese Encephalitis. J. Virol. 2011, 85, 5446–5455. [Google Scholar] [CrossRef] [Green Version]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Stonedahl, S.; Leser, J.S.; Clarke, P.; Tyler, K.L. Depletion of Microglia in an Ex Vivo Brain Slice Culture Model of West Nile Virus Infection Leads to Increased Viral Titers and Cell Death. Microbiol. Spectr. 2022, 10, e0068522. [Google Scholar] [CrossRef]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Perez-Torres, C.; Frouin, A.; Wilton, D.K.; et al. A complement–microglial axis drives synapse loss during virus-induced memory impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, T.; Wulff, P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience 2015, 309, 1–16. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
- Garber, C.; Soung, A.; Vollmer, L.L.; Kanmogne, M.; Last, A.; Brown, J.; Klein, R.S. T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses. Nat. Neurosci. 2019, 22, 1276–1288. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.M.L.; Lee, E.; Müllbacher, A.; Blanden, R.V.; Langman, R.; Lobigs, M. Lack of both Fas Ligand and Perforin Protects from Flavivirus-Mediated Encephalitis in Mice. J. Virol. 2002, 76, 3202–3211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, N.; Oswal, N.; Chawla, A.S.; Agrawal, T.; Biswas, M.; Vrati, S.; Rath, S.; George, A.; Bal, V.; Medigeshi, G.R. CD8 T cells protect adult naive mice from JEV-induced morbidity via lytic function. PLOS Neglected Trop. Dis. 2017, 11, e0005329. [Google Scholar] [CrossRef] [Green Version]
- Růžek, D.; Salát, J.; Palus, M.; Gritsun, T.S.; Gould, E.A.; Dyková, I.; Skallová, A.; Jelínek, J.; Kopecký, J.; Grubhoffer, L. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 2008, 384, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gelpi, E.; Preusser, M.; Garzuly, F.; Holzmann, H.; Heinz, F.X.; Budka, H. Visualization of Central European Tick-Borne Encephalitis Infection in Fatal Human Cases. J. Neuropathol. Exp. Neurol. 2005, 64, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Cattaneo, E. Neural stem cell therapy for neurological diseases: Dreams and reality. Nat. Rev. Neurosci. 2002, 3, 401–409. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Chen, S.-T.; Liu, R.-S.; Wu, M.-F.; Lin, Y.-L.; Chen, S.-Y.; Tan, D.T.-W.; Chou, T.-Y.; Tsai, I.-S.; Li, L.; Hsieh, S.-L. CLEC5A Regulates Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality. PLOS Pathog. 2012, 8, e1002655. [Google Scholar] [CrossRef] [Green Version]
- Kalita, J.; Srivastava, R.; Mishra, M.K.; Basu, A.; Misra, U.K. Cytokines and chemokines in viral encephalitis: A clinicoradiological correlation. Neurosci. Lett. 2010, 473, 48–51. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Cummins, P.M. The blood–brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef]
- Kim, S.Y.; Buckwalter, M.; Soreq, H.; Vezzani, A.; Kaufer, D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia 2012, 53 (Suppl. S6), 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bar-Klein, G.; Lublinsky, S.; Kamintsky, L.; Noyman, I.; Veksler, R.; Dalipaj, H.; Senatorov, V.V., Jr.; Swissa, E.; Rosenbach, D.; Elazary, N.; et al. Imaging blood–brain barrier dysfunction as a biomarker for epileptogenesis. Brain 2017, 140, 1692–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood–Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Leow, C.Y.; Majeed, A.B.A. Flavivirus infection—A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef] [PubMed]
- Hunsperger, E.A.; Roehrig, J. Temporal analyses of the neuropathogenesis of a West Nile virus infection in mice. J. NeuroVirology 2006, 12, 129–139. [Google Scholar] [CrossRef]
- Davies, J.S.; Thompson, H.L.; Pulko, V.; Torres, J.P.; Nikolich-Žugich, J. Role of Cell-Intrinsic and Environmental Age-Related Changes in Altered Maintenance of Murine T Cells in Lymphoid Organs. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2017, 73, 1018–1026. [Google Scholar] [CrossRef] [Green Version]
- Angenvoort, J.; Brault, A.; Bowen, R.; Groschup, M. West Nile viral infection of equids. Veter-Microbiol. 2013, 167, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Seligman, S.J. Single nucleotide polymorphisms in human genes and increased susceptibility to West Nile Virus disease. J. Infect. Dis. 2006, 193, 1187–1188. [Google Scholar] [CrossRef] [Green Version]
- Depla, J.A.; Mulder, L.A.; de Sá, R.V.; Wartel, M.; Sridhar, A.; Evers, M.M.; Wolthers, K.C.; Pajkrt, D. Human Brain Organoids as Models for Central Nervous System Viral Infection. Viruses 2022, 14, 634. [Google Scholar] [CrossRef]
- Cheng, Y.; Medina, A.; Yao, Z.; Basu, M.; Natekar, J.P.; Lang, J.; Sanchez, E.; Nkembo, M.B.; Xu, C.; Qian, X.; et al. Intrinsic antiviral immunity of barrier cells revealed by an iPSC-derived blood-brain barrier cellular model. Cell Rep. 2022, 39, 110885. [Google Scholar] [CrossRef]
- Khou, C.; Díaz-Salinas, M.A.; da Costa, A.; Préhaud, C.; Jeannin, P.; Afonso, P.V.; Vignuzzi, M.; Lafon, M.; Pardigon, N. Comparative analysis of neuroinvasion by Japanese encephalitis virulent and vaccine viral strains in an in vitro model of human blood-brain barrier. PLoS ONE 2021, 16, e0252595. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.M.; Koopmans, M.P.G.; Rockx, B. A Journey to the Central Nervous System: Routes of Flaviviral Neuroinvasion in Human Disease. Viruses 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vries, L.; Harding, A.T. Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses. Viruses 2023, 15, 261. https://doi.org/10.3390/v15020261
de Vries L, Harding AT. Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses. Viruses. 2023; 15(2):261. https://doi.org/10.3390/v15020261
Chicago/Turabian Stylede Vries, Liset, and Alfred T. Harding. 2023. "Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses" Viruses 15, no. 2: 261. https://doi.org/10.3390/v15020261
APA Stylede Vries, L., & Harding, A. T. (2023). Mechanisms of Neuroinvasion and Neuropathogenesis by Pathologic Flaviviruses. Viruses, 15(2), 261. https://doi.org/10.3390/v15020261





