Discovery of a Closterovirus Infecting Jujube Plants Grown at Aksu Area in Xinjiang of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Source and Sample Collection
2.2. Amplification of the Viral Genome
2.3. RT-PCR Detection of PAmpV-Ju
2.4. Sequence Analysis
3. Results
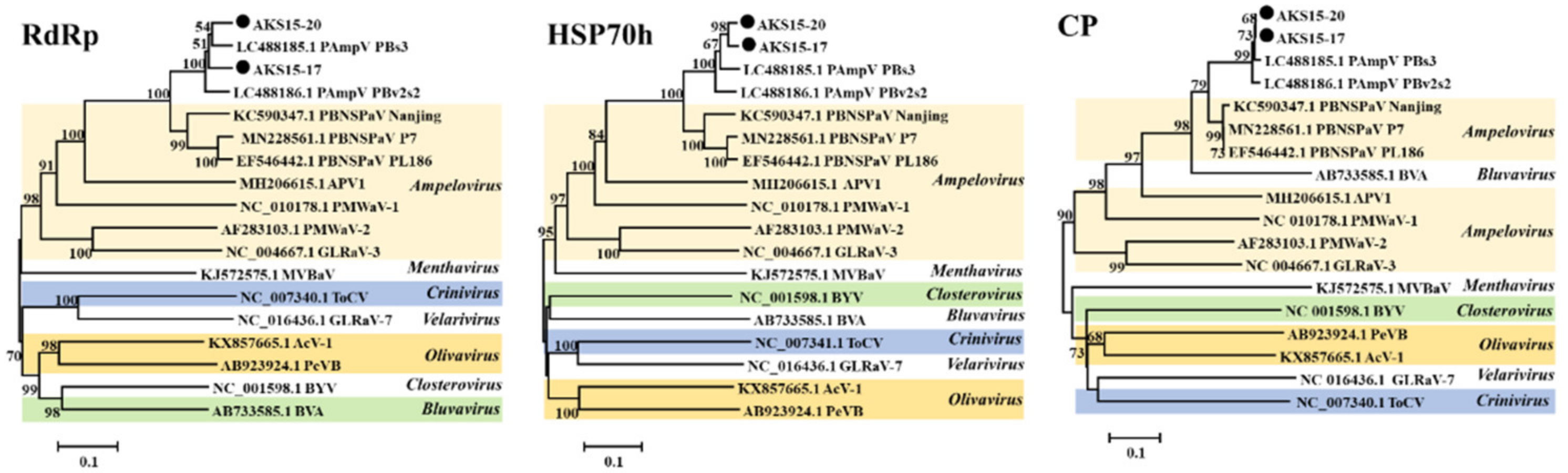
3.1. Sequences of PAmpV-Ju Identified by RNA-Seq Analysis
3.2. Amplification and Analysis of the Genomic Sequence of PAmpV-Ju
3.3. Characterization of PAmpV-Ju Genome
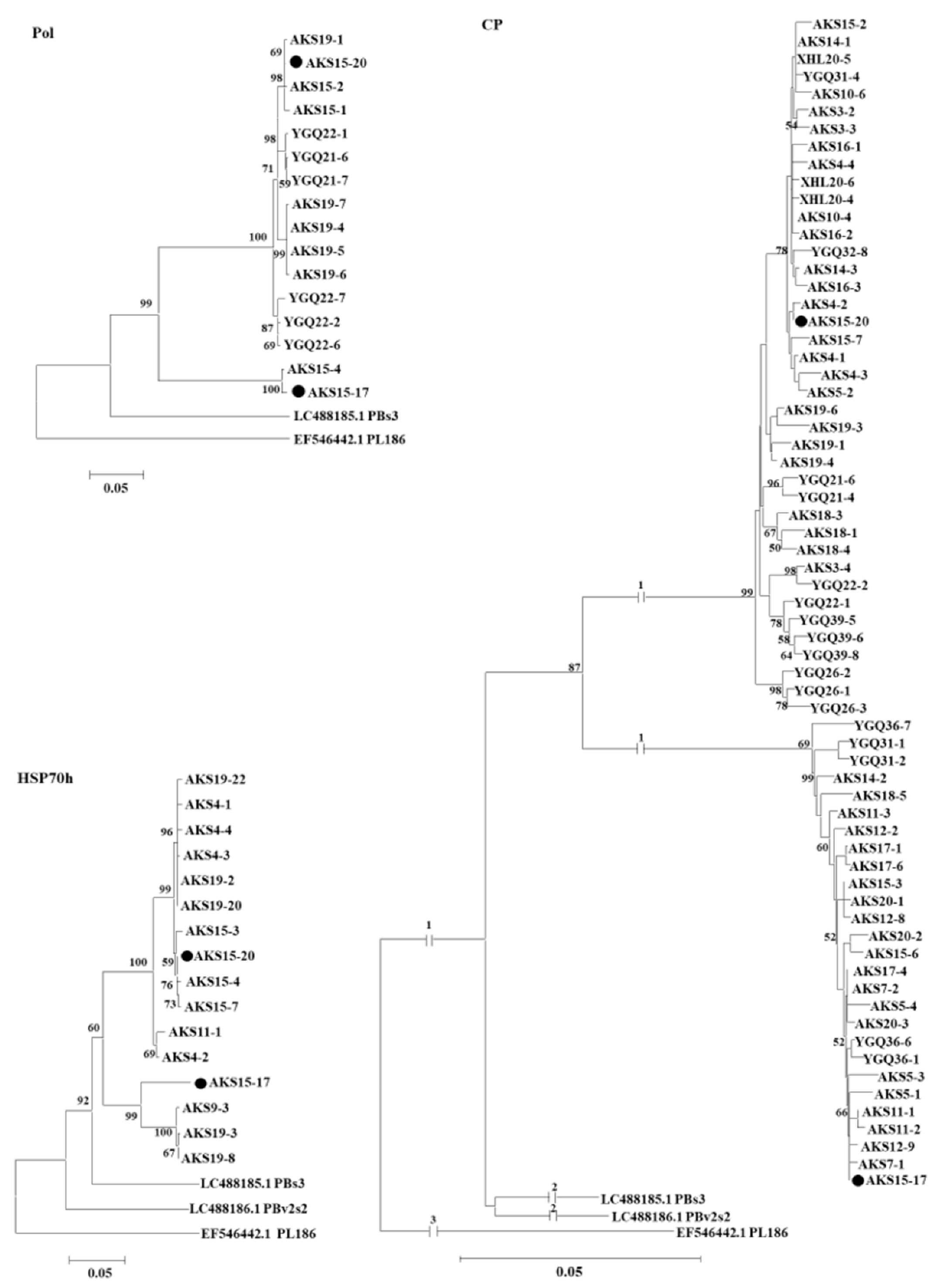
3.4. RT-PCR Detection of PAmpV-Ju in Jujube Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J.; King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Ruiz-García, A.B.; Candresse, T.; Canales, C.; Morán, F.; Machado de Oliveira, C.; Bertolini, E.; Olmos, A. Molecular characterization of the complete coding sequence of olive leaf yellowing-associated virus. Plants 2020, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Blouin, A.G.; Biccheri, R.; Khalifa, M.E.; Pearson, M.N.; Poggi Pollini, C.; Hamiaux, C.; Cohen, D.; Ratti, C. Characterization of Actinidia virus 1, a new member of the family Closteroviridae encoding a thaumatin-like protein. Arch. Virol. 2018, 163, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sato, A.; Suzaki, K. An assemblage of divergent variants of a novel putative closterovirus from American persimmon. Virus Genes 2015, 51, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Isogai, M.; Muramatu, S.; Watanabe, M.; Yoshikawa, N. Complete nucleotide sequence and latency of a novel blueberry-infecting closterovirus. J. Gen. Plant Pathol. 2013, 79, 123–127. [Google Scholar] [CrossRef]
- Tzanetakis, I.E.; Postman, J.D.; Martin, R.R. A member of the Closteroviridae from mint with similarities to all three genera of the family. Plant Dis. 2005, 89, 654–658. [Google Scholar] [CrossRef]
- Ito, T.; Sato, A. Three novel viruses detected from Japanese persimmon ‘Reigyoku’ associated with graft-transmissible stunt. Eur. J. Plant Pathol. 2020, 158, 163–175. [Google Scholar] [CrossRef]
- Martelli, G.P.; Abou Ghanem-Sabanadzovic, N.; Agranovsky, A.A.; Al Rwahnih, M.; Dolja, V.V.; Dovas, C.I.; Fuchs, M.; Gugerli, P.; Hu, J.S.; Jelkmann, W.; et al. Taxonomic revision of the family Closteroviridae with special reference to the grapevine leafroll-associated members of the genus Ampelovirus and the putative species unassigned to the family. J. Plant Pathol. 2012, 94, 7–19. [Google Scholar]
- Fuchs, M.; Bar-Joseph, M.; Candresse, T.; Maree, H.J.; Martelli, G.P.; Melzer, M.J.; Menzel, W.; Minafra, A.; Sabanadzovic, S.; Consortium, I.R. ICTV Virus Taxonomy Profile: Closteroviridae. J. Gen. Virol. 2020, 101, 364–365. [Google Scholar] [CrossRef]
- Karasev, A.V. Genetic diversity and evolution of Closteroviruses. Annu. Rev. Microbiol. 2000, 38, 293–324. [Google Scholar] [CrossRef]
- Rubio, L.; Ayllón, M.A.A.; Kong, P.; Fernández, A.; Polek, M.; Guerri, J.; Moreno, P.; Falk, B.W. Genetic variation of citrus tristeza virus isolates from California and Spain: Evidence for mixed infections and recombination. J. Virol. 2001, 75, 8054–8062. [Google Scholar] [CrossRef]
- Bar-Joseph, M.; Mawassi, M. The defective RNAs of Closteroviridae. Front. Microbiol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, T.; Gowda, S.; Boyko, V.P.; Albiach-Marti, M.R.; Mawassi, M.; Navas-Castillo, J.; Karasev, A.V.; Dolja, V.; Hilf, M.E.; Lewandowski, D.J.; et al. An engineered closterovirus RNA replicon and analysis of heterologous terminal sequences for replication. Proc. Natl. Acad. Sci. USA 1999, 96, 7433–7438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, S.; Mei, S.; Zhou, Y.; Wang, J.; Han, G.-Z.; Chen, L.; Zhou, C.; Cao, M. Viromics unveils extraordinary genetic diversity of the family Closteroviridae in wild citrus. PLoS Pathog. 2021, 17, e1009751. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Barthelson, R.; Gowda, S.; Hilf, M.E.; Dawson, W.O.; Galbraith, D.W.; Xiong, Z. Persistent infection and promiscuous recombination of multiple genotypes of an RNA virus within a single host generate extensive diversity. PLoS ONE 2007, 2, e917. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.J. Citrus tristeza virus: Evolution of complex and varied genotypic groups. Front. Microbiol. 2013, 4, 93. [Google Scholar] [CrossRef]
- Richardson, J.E.; Fay, M.F.; Cronk, Q.C.B.; Chase, M.W. A revision of the tribal classification of Rhamnaceae. Kew Bull. 2000, 55, 311–340. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Wang, L.; Liu, P.; Zhao, J.; Zhao, Z.; Yao, S.; Stanica, F.; Liu, Z.; Wang, L.; et al. The historical and current research progress on jujube-a superfruit for the future. Hortic. Res. 2020, 7, 119. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Wang, G.; Hong, J.; Yang, Z.; Bai, J.; Hong, N. Molecular characteristics of jujube yellow mottle-associated virus infecting jujube (Ziziphus jujuba Mill.) grown at Aksu in Xinjiang of China. Viruses 2021, 13, 25. [Google Scholar] [CrossRef]
- Yang, F.; Wang, G.; Xu, W.; Hong, N. A rapid silica spin column-based method of RNA extraction from fruit trees for RT-PCR detection of viruses. J. Virol. Methods. 2017, 247, 61–67. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Machleder, E.M.; Chenchik, A.; Li, R.; Siebert, P.D. Reverse transcriptase template switching: A SMART™ approach for full-length cDNA library construction. Biotechniques 2001, 30, 892–897. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, 222–226. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Agranovsky, A.A.; Lesemann, D.E.; Maiss, E.; Hull, R.; Atabekov, J.G. “Rattlesnake” structure of a filamentous plant RNA virus built of two capsid proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 2470–2473. [Google Scholar] [CrossRef]
- Adams, I.P.; Glover, R.H.; Monger, W.A.; Mumford, R.; Jackeviciene, E.; Navalinskiene, M.; Samuitiene, M.; Boonham, N. Next-generation sequencing and metagenomic analysis: A universal diagnostic tool in plant virology. Mol. Plant Pathol. 2009, 10, 537–545. [Google Scholar] [CrossRef]
- Kreuze, J.F.; Perez, A.; Untiveros, M.; Quispe, D.; Fuentes, S.; Barker, I.; Simon, R. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: A generic method for diagnosis, discovery and sequencing of viruses. Virology 2009, 388, 1–7. [Google Scholar] [CrossRef]
- Barba, M.; Czosnek, H.; Hadidi, A. Historical perspective, development and applications of next-generation sequencing in plant virology. Viruses 2014, 6, 106–136. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, S.-W.; Zhang, Y.; Zhu, S. Identification of viruses and viroids by next-generation sequencing and homology-dependent and homology-independent algorithms. Annu. Rev. Phytopathol. 2015, 53, 425–444. [Google Scholar] [CrossRef]
- Hadidi, A.; Flores, R.; Candresse, T.; Barba, M. Next-generation sequencing and genome editing in plant virology. Front. Microbiol. 2016, 7, 1325. [Google Scholar] [CrossRef]
- Villamor, D.E.V.; Ho, T.; Al Rwahnih, M.; Martin, R.R.; Tzanetakis, I.E. High throughput sequencing for plant virus detection and discovery. Phytopathology 2019, 109, 716–725. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, W.; Zhang, Y.; Wu, Z.; Wang, Q.-C.; Wu, Y. Occurrence and molecular variability of kiwifruit viruses in Actinidia deliciosa Xuxiang’ in the Shaanxi Province of China. Plant Dis. 2019, 103, 1309–1318. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, L.; Wen, S.; Yang, Z.; Wang, G.; Hong, N. Molecular characterization of a novel emaravrius infecting Actinidia spp. in China. Virus Res. 2020, 275, 197736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, L.; Ma, L.; Tian, X.; Li, R.; Zhou, C.; Cao, M. Virome of Camellia japonica: Discovery of and molecular characterization of new viruses of different taxa in Camellias. Front. Microbiol. 2020, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, S.; Han, T.; Fu, J.; Di Serio, F.; Cao, M. Identification and characterization of a novel Emaravirus associated with jujube (Ziziphus jujuba Mill.) yellow mottle disease. Front. Microbiol. 2019, 10, 1417. [Google Scholar]
- Du, K.; Liu, S.; Chen, Z.; Fan, Z.; Wang, H.; Tian, G.; Zhou, T. Full genome sequence of jujube mosaic-associated virus, a new member of the family Caulimoviridae. Arch. Virol. 2017, 162, 3221–3224. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.Z.; Li, C.H.; Goszczynski, D.E.; Gonsalves, D. Genome sequences and structures of two biologically distinct strains of grapevine leafroll-associated virus 2 and sequence analysis. Virus Genes 2005, 31, 31–41. [Google Scholar] [CrossRef]
- Thompson, J.R.; Fuchs, M.; Perry, K.L. Genomic analysis of grapevine leafroll associated virus-5 and related viruses. Virus Res. 2012, 163, 19–27. [Google Scholar] [CrossRef]
- Wen, S.; Wang, G.; Yang, Z.; Wang, Y.; Rao, M.; Lu, Q.; Hong, N. Next-generation sequencing combined with conventional sanger sequencing reveals high molecular diversity in actinidia virus 1 populations from kiwifruit grown in China. Front. Microbiol. 2020, 11, 602039. [Google Scholar] [CrossRef]
- Mine, A.; Hyodo, K.; Tajima, Y.; Kusumanegara, K.; Taniguchi, T.; Kaido, M.; Mise, K.; Taniguchi, H.; Okuno, T. Differential roles of Hsp70 and Hsp90 in the assembly of the replicase complex of a positive-strand RNA plant virus. J. Virol. 2012, 86, 12091–12104. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Huot, O.B.; Martin, K.M.; Kondo, H.; Dietzgen, R.G. Plant rhabdoviruses-their origins and vector interactions. Curr. Opin. Virol. 2018, 33, 198–207. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Bejerman, N.E.; Goodin, M.M.; Higgins, C.M.; Huot, O.B.; Kondo, H.; Martin, K.M.; Whitfield, A.E. Diversity and epidemiology of plant rhabdoviruses. Virus Res. 2020, 281, 197942. [Google Scholar] [CrossRef]
- Pinheiro-Lima, B.; Pereira-Carvalho, R.C.; Alves-Freitas, D.M.T.; Kitajima, E.W.; Vidal, A.H.; Lacorte, C.; Godinho, M.T.; Fontenele, R.S.; Faria, J.C.; Abreu, E.F.M.; et al. Transmission of the bean-associated cytorhabdovirus by the Whitefly Bemisia tabaci MEAM1. Viruses 2020, 12, 1028. [Google Scholar] [CrossRef]
- Roy, A.; Brlansky, R.H. Population dynamics of a Florida citrus tristeza virus Isolate and Aphid-transmitted subisolates: Identification of three genotypic groups and recombinants after Aphid transmission. Phytopathology 2009, 99, 1297–1306. [Google Scholar] [PubMed]
- Rubio, L.; Guerri, J.; Moreno, P. Genetic variability and evolutionary dynamics of viruses of the family Closteroviridae. Front. Microbiol. 2013, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Blaisdell, G.K.; Zhang, S.; Rowhani, A.; Klaassen, V.; Cooper, M.L.; Daane, K.M.; Almeida, R.P.P. Trends in vector-borne transmission efficiency from coinfected hosts: Grapevine leafroll-associated virus-3 and grapevine virus A. Eur. J. Plant Pathol. 2020, 156, 1163–1167. [Google Scholar] [CrossRef]
- Nemchinov, L.; Hadidi, A.; Foster, J.A.; Candresse, T.; Verderevskaya, T. Sensitive detection of apple chlorotic leaf spot virus from infected apple or peach tissue using RT-PCR, IC-RT-PCR, or multiplex IC-RT-PCR. Acta Hort. 1995, 386, 51–62. [Google Scholar] [CrossRef]
- Nemchinov, L.; Hadidi, A.; Faggioli, F. PCR-detection of apple stem pitting virus from pome fruit hosts and sequence variability among viral isolates. Acta Hort. 1998, 472, 67–73. [Google Scholar] [CrossRef]
- Malinowski, T. Potential problems with the reliability of PCR based diagnostic methods related to plant viruses sequence variation. Phytopathol. Polon. 2005, 35, 125–139. [Google Scholar]
- Komorowska, B.; Malinowski, T.; Michalczuk, L. Evaluation of several RT-PCR primer pairs for the detection of apple stem pitting virus. J. Virol. Methods. 2010, 168, 242–247. [Google Scholar] [CrossRef]
- Lu, Y.; Mcgavin, W.; Cock, P.J.; Schnettler, E.; Macfarlane, S. Newly identified RNAs of raspberry leaf blotch virus encoding a related group of proteins. J. Gen. Virol. 2015, 96, 3432–3439. [Google Scholar] [CrossRef]


| Primer Name | Sequence a (5′-3′) | Location (nt) b | Size (bp) | Target Sequence |
|---|---|---|---|---|
| Pol-F | AAACTCAAGGGTCGTAGAGC | 1220–2162 | 942 | contig17; contig20 |
| Pol-R | CGCCCATCAACGTGTTTCATCCA | |||
| HSP70-F | GGCTAARGGTTGTACTGAAGAGTC | 9097–9628 | 531 | contig17; contig20 |
| HSP70-R | GTACCTCCTCCAAAATCATAGAC | |||
| CP-F1 | TACCGAGGCATACATGTTA | 11,933–13,322 | 1389 | contig17 |
| CP-R1 | CGCCTAATCGCAACGTCAC | |||
| CP-F2 | TCCACTCGTATTATCGCATGA | 12,018–13,340 | 1322 | contig20 |
| CP-R2 | TGTACCCATTACTCCGCTTCAC | |||
| CP-F | GATGARTGGGTACCYGGGGATT | 12,357–13,334 | 977 | contig17; contig20 |
| CP-R | CGCGCAAKACAACAGAAGACAT |
| Virus | Isolate (Variant) a | Genome | ORF1a | ORF1b | ORF2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Polyprotein) | (RdRp) | (P6) | ||||||||||
| nt | nt% | nt | nt% | aa% | nt | nt% | aa% | nt | nt% | aa% | ||
| PAmpV | AKS15-17 | 14,209 | 7002 | 1587 | 180 | |||||||
| AKS15-20 | 14,209 | 83.7 | 7002 | 80.1 | 85.5 | 1587 | 86.2 | 93.2 | 180 | 87.2 | 98.3 | |
| PAmpV | PBs3 | 14,262 | 79.1/79.0 | 7071 | 74.5/75.4 | 79.1/79.5 | 1584 | 80.3/83.0 | 91.3/93.2 | 180 | 75.6/80.9 | 89.8/91.5 |
| PBv2s2 | 7070 | - | - | - | - | 1584 | 80.7/80.8 | 90.2/90.1 | 180 | 79.0/80.7 | 88.1/89.8 | |
| PBNSPaV | PL186 | 14,214 | 67.6/67.4 | 7032 | 64.8/64.6 | 55.7/55.7 | 1575 | 73.0/72.7 | 77.5/78.1 | 174 | 65.8/65.4 | 64.3/64.3 |
| Nanjing | 14,234 | 67.0/67.1 | 7035 | 61.8/55.8 | 33.3/36.7 | 1578 | 72.6/73.0 | 79.7/78.9 | 174 | 55.9/66.0 | 60.7/60.7 | |
| ORF3 | ORF4 | ORF5 | ORF6 | |||||||||
| (HSP70h) | (P61) | (CP) | (CPm) | |||||||||
| nt | nt% | aa% | nt | nt% | aa% | nt | nt% | aa% | nt | nt% | aa% | |
| 1590 | 1641 | 984 | 654 | |||||||||
| 1590 | 85.9 | 95.8 | 1641 | 84.6 | 96.3 | 984 | 85.9 | 96.3 | 654 | 92.4 | 93.5 | |
| 1590 | 82.6/81.2 | 93.2/92.6 | 1641 | 82.9/81.8 | 94.3/92.7 | 984 | 83.9/83.1 | 93.3/92.0 | 654 | 87.2/88.1 | 88.9/90.8 | |
| 1590 | 79.3/78.9 | 92.2/92.1 | 1641 | 79.8/78.8 | 88.8/89.0 | 984 | 81.4/82.7 | 93.3/91.7 | 654 | 86.5/87.8 | 86.6/88.9 | |
| 1590 | 71.4/70.8 | 76.9/77.3 | 1641 | 70.3/70.3 | 75.8/75.5 | 978 | 68.1/68.2 | 69.3/70.2 | 672 | 66.3/66.7 | 58.3/60.7 | |
| 1590 | 70.0/70.8 | 78.4/78.4 | 1641 | 70.6/70.5 | 73.8/73.8 | 978 | 68.3/69.4 | 69.3/70.5 | 657 | 63.5/63.8 | 57.0/56.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Wang, G.; Yang, Z.; Wang, Y.; Yang, B.; Bai, J.; Hong, N. Discovery of a Closterovirus Infecting Jujube Plants Grown at Aksu Area in Xinjiang of China. Viruses 2023, 15, 267. https://doi.org/10.3390/v15020267
Lu Q, Wang G, Yang Z, Wang Y, Yang B, Bai J, Hong N. Discovery of a Closterovirus Infecting Jujube Plants Grown at Aksu Area in Xinjiang of China. Viruses. 2023; 15(2):267. https://doi.org/10.3390/v15020267
Chicago/Turabian StyleLu, Qian, Guoping Wang, Zuokun Yang, Yanxiang Wang, Buchen Yang, Jianyu Bai, and Ni Hong. 2023. "Discovery of a Closterovirus Infecting Jujube Plants Grown at Aksu Area in Xinjiang of China" Viruses 15, no. 2: 267. https://doi.org/10.3390/v15020267
APA StyleLu, Q., Wang, G., Yang, Z., Wang, Y., Yang, B., Bai, J., & Hong, N. (2023). Discovery of a Closterovirus Infecting Jujube Plants Grown at Aksu Area in Xinjiang of China. Viruses, 15(2), 267. https://doi.org/10.3390/v15020267






