Immunogenicity of Wild Type and Mutant Hepatitis B Surface Antigen Virus-like Particles (VLPs) in Mice with Pre-Existing Immunity against the Wild Type Vector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
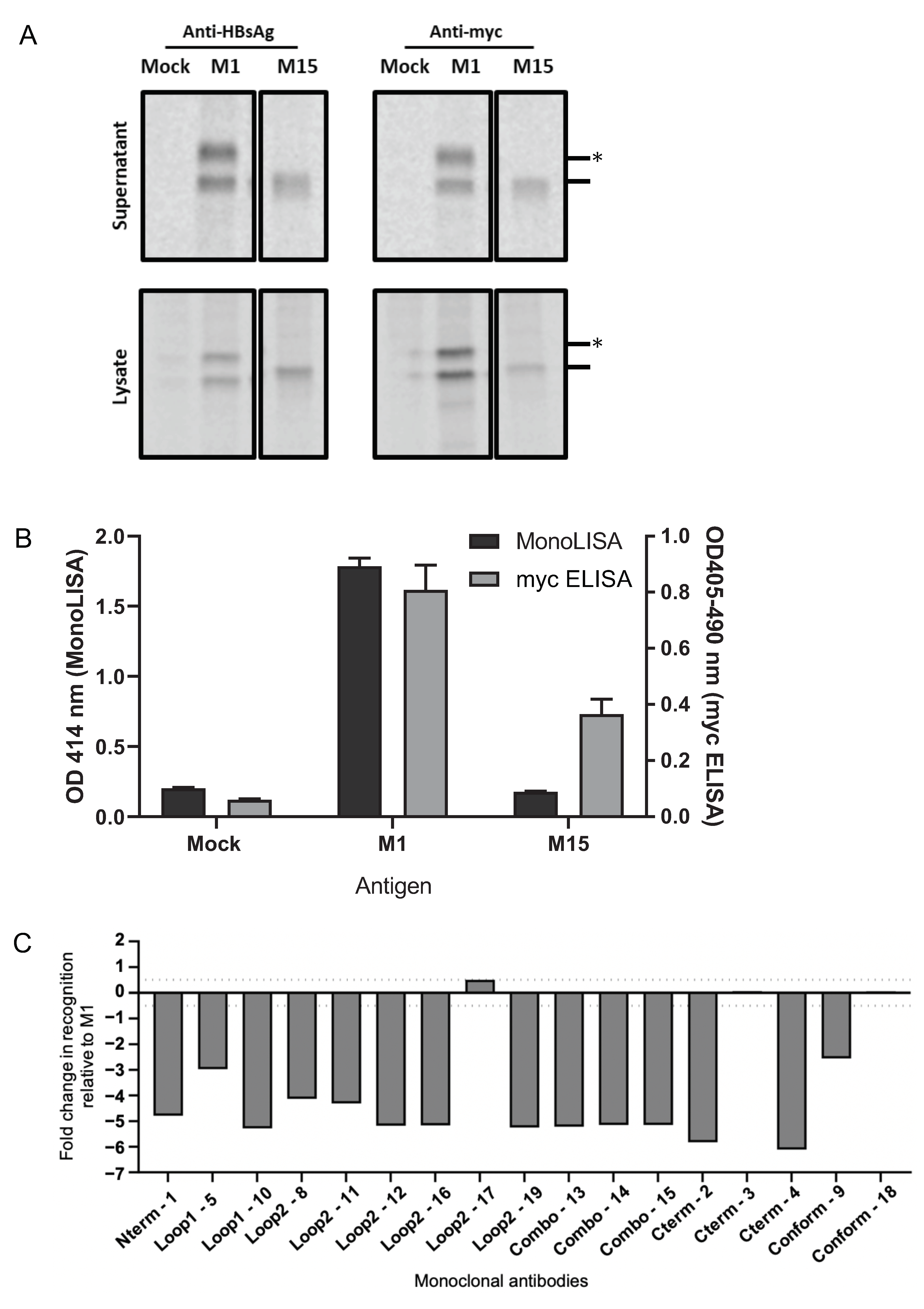
2.2. Immunoanalyses of HBsAgS Proteins
2.3. Multiplex Immunoassay
2.4. Large-Scale HBsAgS VLP Production
2.5. Immunization and Assessment of Antibody Responses
3. Results
3.1. Generation of Antigenically Distinct HBsAg VLPs
3.2. Determination of the Immunogenicity of Myc-Tagged wt M1 and Myc-Tagged Mutant M15 VLPs
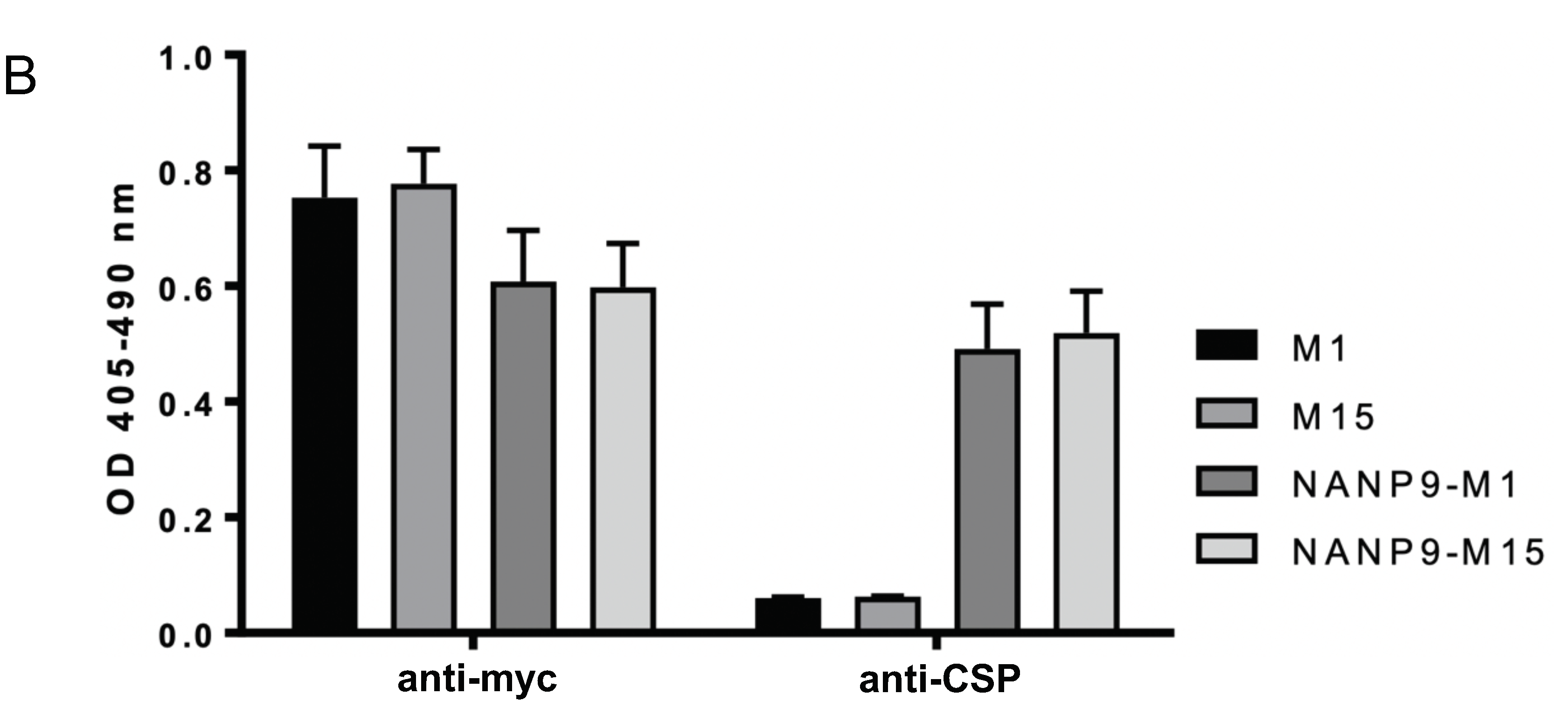
3.3. Generation of Chimeric M1- and M15-HBsAgS VLPs with Inserted Foreign NANP9 Antigenic Sequence
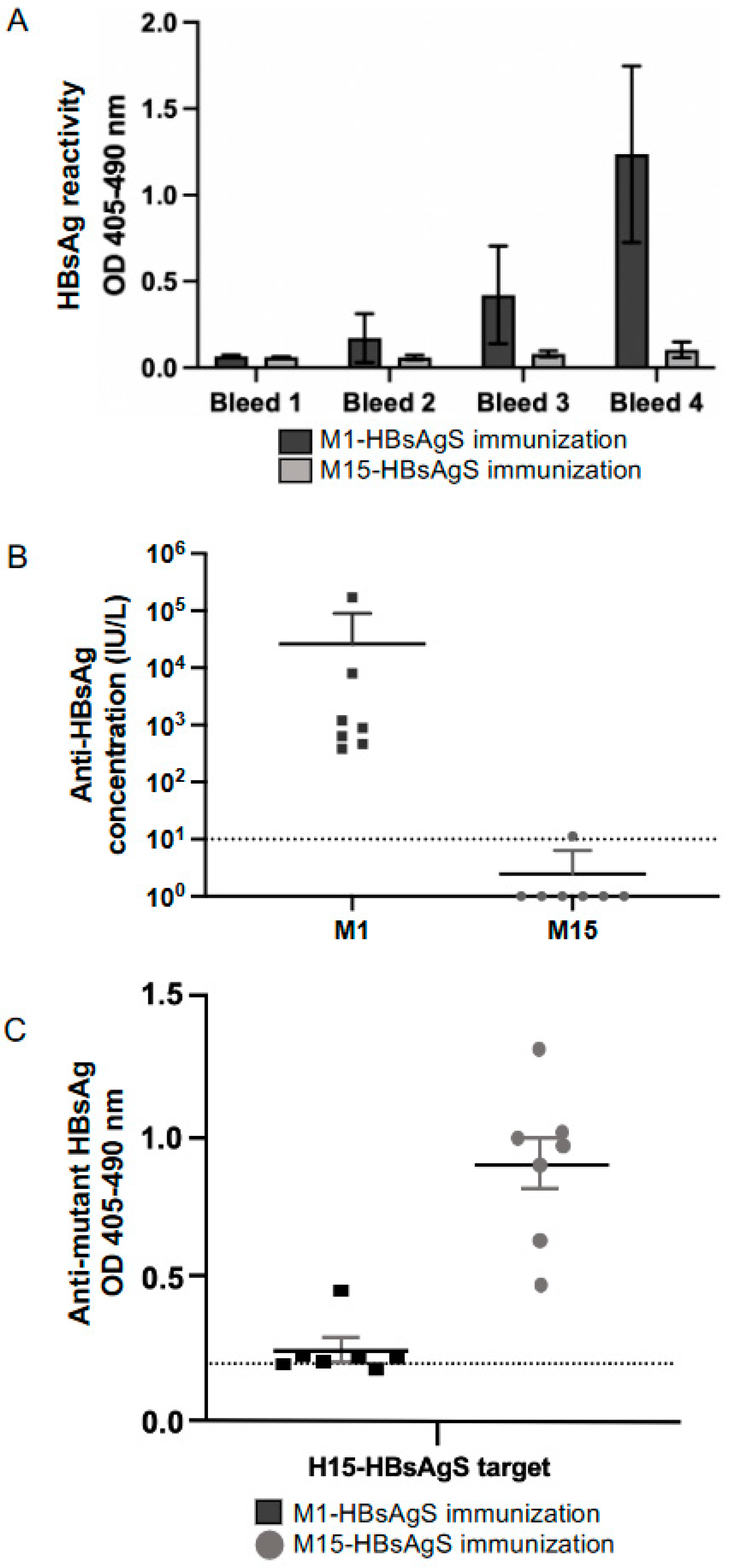
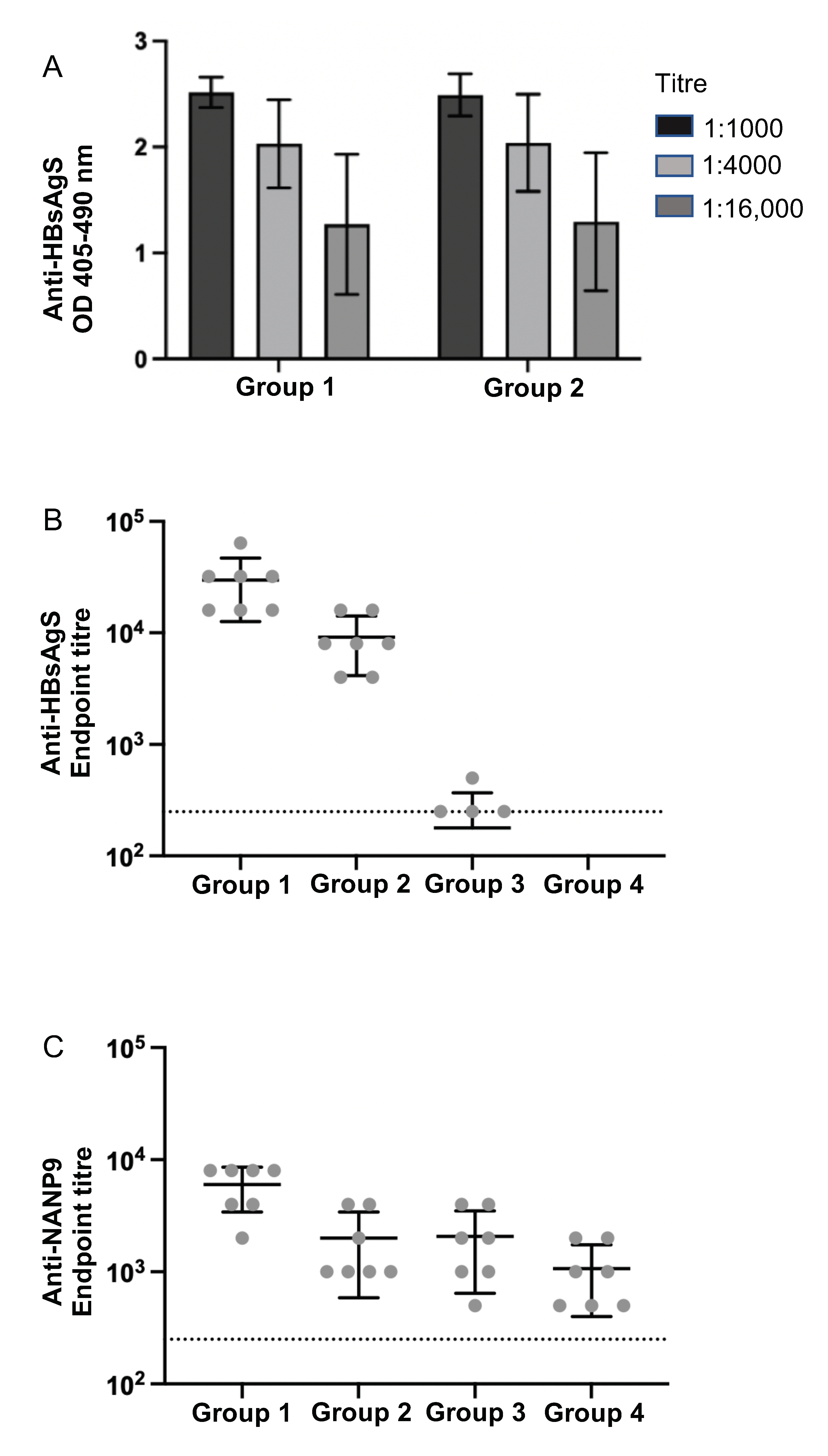
3.4. Immunogenicity of M1- and M15 HBsAgS Platforms in the Presence and Absence of Pre-Existing Anti-HBsAgS Antibodies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennings, G.T.; Bachmann, M.F. Immunodrugs: Therapeutic VLP-based vaccines for chronic diseases. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Van, T.T.H.; Baird, F.J.; Coloe, P.J.; Smooker, P.M. Pre-existing immunity against vaccine vectors—Friend or foe? Microbiology 2013, 159, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors. Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Bradt, V.; Malafa, S.; von Braun, A.; Jarmer, J.; Tsouchnikas, G.; Medits, I.; Wanke, K.; Karrer, U.; Stiasny, K.; Heinz, F.X. Pre-existing yellow fever immunity impairs and modulates the antibody response to tick-borne encephalitis vaccination. NPJ Vaccines 2019, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Schutze, M.-P.; Leclerc, C.; Jolivet, M.; Audibert, F.; Chedid, L. Carrier-induced epitope suppression, a major issue for future synthetic vaccines. J. Immunol. 1985, 135, 2319–2322. [Google Scholar] [CrossRef]
- Shah, S.; Raghupathy, R.; Singh, O.; Talwar, G.P.; Sodhi, A. Prior immunity to a carrier enhances antibody responses to hCG in recipients of an hCG-carrier conjugate vaccine. Vaccine 1999, 17, 3116–3123. [Google Scholar] [CrossRef]
- Pobre, K.; Tashani, M.; Ridda, I.; Rashid, H.; Wong, M.; Booy, R. Carrier priming or suppression: Understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine 2014, 32, 1423–1430. [Google Scholar] [CrossRef]
- Borrow, R.; Dagan, R.; Zepp, F.; Hallander, H.; Poolman, J. Glycoconjugate vaccines and immune reactions, and implications for vaccination schedules. Exp. Rev. Vaccines 2011, 10, 1621–1631. [Google Scholar] [CrossRef]
- Schutze, M.-P.; Deriaud, E.; Przewlocki, G.; LeClerc, C. Carrier-induced epitopic suppression is initiated through clonal dominance. J. Immunol. 1989, 142, 2635–2640. [Google Scholar] [CrossRef] [PubMed]
- LeClerc, C.; Schutze, M.P.; Deriaud, E.; Przewlocki, G. The in vivo elimination of CD4+ T cells prevents the induction but not the expression of carrier-induced epitopic suppression. J. Immunol. 1990, 145, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, A.; Wiesel, M.; Dietmeier, K.; Zabel, F.; Gatto, D.; Saudan, P.; Bachmann, M.F. Carrier-induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine 2010, 28, 5503–5512. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Evans, D.M.; Zhang, N.; Benoit, M.; McElhiney, S.P.; Unnithan, M.; DeMarco, S.C.; Clay, B.; Huber, C.; Deora, A.; et al. The effect of preexisting anti-carrier immunity on subsequent responses to CRM197 or Qb-VLP conjugate vaccines. Immunopharmacol. Immunotoxicol. 2016, 38, 184–196. [Google Scholar] [CrossRef]
- Rollier, C.S.; Reyes-Sandoval, A.; Cottingham, M.G.; Ewer, K.; Hill, A.V.S. Viral vectors as vaccine platforms: Deployment in sight. Curr. Opin. Immunol. 2011, 23, 377–382. [Google Scholar] [CrossRef]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.E.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.K.T.; Jeevan-Raj, B.; Netter, H.J. Hepatitis B virus (HBV) subviral particles as protective vaccines and vaccine platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuckerman, J.N. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J. Med. Virol. 2006, 78, 169–177. [Google Scholar] [CrossRef]
- Jilg, W.; Lorbeer, B.; Schmidt, M.; Wilske, B.; Zoulek, G.; Deinhardt, F. Clinical evaluation of a recombinant hepatitis B vaccine. Lancet 1984, 2, 1174–1175. [Google Scholar] [CrossRef]
- Carman, W.F. Vaccine-associated mutants of hepatitis B virus. In Viral Hepatitis and Liver Disease; Nishioka, K., Suzuki, H., Mishiro, S., Oda, T., Eds.; Springer: Tokyo, Japan, 1994; pp. 243–247. [Google Scholar]
- Mangold, C.M.T.; Unckell, F.; Werr, M.; Streeck, R.E. Analysis of intermolecular disulfide bonds and free sufhydryl groups in hepatitis B surface antigen particles. Arch. Virol. 1997, 142, 2257–2267. [Google Scholar] [CrossRef]
- Gordon, D.M.; McGovern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; LaChance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 1995, 171, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B. RTS,S/AS01 vaccine (Mosquirix™): An overview. Hum. Vaccines Immunother. 2020, 16, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Delpeyroux, F.; Chenciner, N.; Lim, A.; Malpièce, Y.; Blondel, B.; Crainic, R.; van der Werf, S.; Streeck, R.E. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science 1986, 233, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Netter, H.J.; Macnaughton, T.B.; Woo, W.P.; Tindle, R.; Gowans, E.J. Antigenicity and immunogenicity of novel chimeric hepatitis B surface antigen particles with exposed hepatitis C virus epitopes. J. Virol. 2001, 75, 2130–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vietheer, P.T.K.; Boo, I.; Drummer, H.E.; Netter, H.J. Immunizations with chimeric hepatitis B virus-like particles to induce potential anti-hepatitis C virus neutralizing antibodies. Antivir. Ther. 2007, 12, 477–487. [Google Scholar] [CrossRef]
- Wei, S.; Lei, Y.; Yang, J.; Wang, X.; Fang, S.; Wei, X.; Lin, F.; Li, B.; Cui, Y.; Zhang, H.; et al. Neutralization effects of antibody elicited by chimeric HBV S antigen viral-like particles presenting HCV neutralization epitopes. Vaccine 2018, 36, 2273–2281. [Google Scholar] [CrossRef]
- Kotiw, M.; Johnson, M.; Pandey, M.; Fry, S.; Hazell, S.L.; Netter, H.J.; Good, M.F.; Olive, C. Immunological response to parenteral vaccination with recombinant hepatitis B virus surface antigen virus-like particles expressing Helicobacter pylori KatA epitopes in a murine H. pylori challenge model. Clin. Vaccine Immunol. 2011, 19, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Kingston, N.J.; Kurtovic, L.; Walsh, R.; Joe, C.; Lovrecz, G.; Locarnini, S.; Beeson, J.; Netter, H.J. Hepatitis B virus-like particles expressing Plasmodium falciparum epitopes induce complement-fixing antibodies against the circumsporozoite protein. Vaccine 2019, 37, 1674–1684. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Fact Sheet. Hepatitis, B., 27 July 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Hyakumura, M.; Walsh, R.; Thaysen-Andersen, M.; Kingston, N.J.; La, M.; Lu, L.; Lovrecz, G.; Packer, N.; Locarnini, S.; Netter, H.J. Modification of asparagine-linked glycan density for the design of hepatitis B virus virus-like particles with enhanced immunogenicity. J. Virol. 2015, 89, 11312–11322. [Google Scholar] [CrossRef] [Green Version]
- Walsh, R.; Hammond, R.; Yuen, L.; Deerain, J.; O’Donnell, T.; Leary, T.; Cloherty, G.; Gaggar, A.; Kitrinos, K.; Subramanian, M.; et al. Predicting HBsAg clearance in genotype A chronic hepatitis B using HBsAg epitope profiling: A biomarker for functional cure. Liver Int. 2019, 39, 2066–2076. [Google Scholar] [CrossRef]
- Torresi, J.; Earnest-Silveira, L.; Deliyannis, G.; Edgtton, K.; Zhuang, H.; Locarnini, S.A.; Fyfe, J.; Sozzi, T.; Jackson, D.C. Reduced antigenicity of the hepatitis B virus HBsAg protein arising as a consequence of sequence changes in the overlapping polymerase gene that are selected by Lamivudine therapy. Virology 2002, 293, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carman, W.F. The clinical significance of surface antigen variants of hepatitis B virus. J. Viral Hepat. 1997, 4, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, E.; Patient, R.; Hourioux, C.; Dimier-Poisson, I.; Roingeard, P. Chimeric hepatitis B virus/hepatitis C virus envelope proteins elicit broadly neutralizing antibodies and constitute a potential bivalent prophylactic vaccine. Hepatology 2013, 57, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Juarez, V.; Pasolli, H.A.; Hellwig, A.; Garbi, N.; Cid Arregui, A. Virus-like particles harbouring CCL19, IL-2 and HPV16 E7 elicit protective T cell responses in HLA-A2 transgenic mice. Open Virol. J. 2012, 6 (Suppl. 2:M13), 270–276. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.; Arora, U.; Shukla, R.; Poddar, A.; Shanmugam, R.K.; White, L.J.; Mattocks, M.M.; Raut, R.; Perween, A.; Tyagi, P.; et al. A tetravalent virus-like particle vaccine designed to display domain III of dengue envelope proteins induces multi-serotype neutralizing antibodies in mice and macaques which confer protection against antibody dependent enhancement in AG129 mice. PloS Negl. Trop. Dis. 2018, 12, e0006191-35. [Google Scholar] [CrossRef] [Green Version]
- Cheong, W.S.; Reiseger, J.; Turner, S.J.; Boyd, R.; Netter, H.J. Chimeric virus-like particles for the delivery of an inserted conserved influenza A-specific CTL epitope. Antivir. Res. 2009, 81, 113–122. [Google Scholar] [CrossRef]
- Phogat, S.; Svehla, K.; Tang, M.; Spadaccini, A.; Muller, J.; Mascola, J.; Berkower, I.; Wyatt, R. Analysis of the human immunodeficiency virus type 1 gp41 membrane proximal external region arrayed on hepatitis B surface antigen particles. Virology 2008, 373, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Lone, Y.C.; Centlivre, M.; Roux, P.; Wain-Hobson, S.; Sala, M. Optimisation of secretion of recombinant HBsAg virus-like particles: Impact on the development of HIV-1/HBV bivalent vaccines. Vaccine 2007, 25, 1901–1911. [Google Scholar] [CrossRef]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef] [Green Version]
- Syed, Y.Y. RTS,S/AS01 malaria vaccine (Mosquirix®): A profile of its use. Drugs Ther. Perspect. 2022, 38, 373–381. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21, in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Netter, H.J.; Woo, W.-P.; Tindle, R.; Macfarlan, R.I.; Gowans, E.J. Immunogenicity of recombinant HBsAg/HCV particles in mice pre-immunised with hepatitis B virus-specific vaccine. Vaccine 2003, 21, 2692–2697. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, E.; Roingeard, P. Chimeric hepatitis B virus (HBV)/hepatitis C virus (HCV) subviral envelope particles induce efficient anti-HCV antibody production in animals pre-immunized with HBV vaccine. Vaccine 2015, 33, 973–976. [Google Scholar] [CrossRef]
- Lell, B.; Agnandji, S.; von Glasenapp, I.; Haertle, S.; Oyakhiromen, S.; Issifou, S.; Vekemans, J.; Leach, A.; Lievens, M.; Dubois, M.C.; et al. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabun. PLoS ONE 2009, 4, e7611. [Google Scholar] [CrossRef]
- Zahid, M.; Lünsdorf, H.; Rinas, U. Assessing stability and assembly of the hepatitis B surface antigen into virus-like particles during down-stream processing. Vaccine 2015, 33, 3739–3745. [Google Scholar] [CrossRef]
- Joe, C.C.D.; Chatterjee, S.; Lovrecz, G.; Adams, T.E.; Thaysen-Andersen, M.; Walsh, R.; Locarnini, S.A.; Smooker, P.; Netter, H.J. Glycoengineered hepatitis B virus-like particles with enhanced immunogenicity. Vaccine 2020, 38, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Al-Barwani, F.; Young, S.L.; Baird, M.A.; Larsen, D.S.; Ward, V.K. Mannosylation of virus-like particles enhances internalization by antigen presenting cells. PLoS ONE 2014, 9, e104523. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kingston, N.J.; Walsh, R.; Hammond, R.; Joe, C.C.D.; Lovrecz, G.; Locarnini, S.; Netter, H.J. Immunogenicity of Wild Type and Mutant Hepatitis B Surface Antigen Virus-like Particles (VLPs) in Mice with Pre-Existing Immunity against the Wild Type Vector. Viruses 2023, 15, 313. https://doi.org/10.3390/v15020313
Kingston NJ, Walsh R, Hammond R, Joe CCD, Lovrecz G, Locarnini S, Netter HJ. Immunogenicity of Wild Type and Mutant Hepatitis B Surface Antigen Virus-like Particles (VLPs) in Mice with Pre-Existing Immunity against the Wild Type Vector. Viruses. 2023; 15(2):313. https://doi.org/10.3390/v15020313
Chicago/Turabian StyleKingston, Natalie J., Renae Walsh, Rachel Hammond, Carina C. D. Joe, George Lovrecz, Stephen Locarnini, and Hans J. Netter. 2023. "Immunogenicity of Wild Type and Mutant Hepatitis B Surface Antigen Virus-like Particles (VLPs) in Mice with Pre-Existing Immunity against the Wild Type Vector" Viruses 15, no. 2: 313. https://doi.org/10.3390/v15020313





