Obesity Induces an Impaired Placental Antiviral Immune Response in Pregnant Women Infected with Zika Virus
Abstract
:1. Introduction
2. Materials and Methods
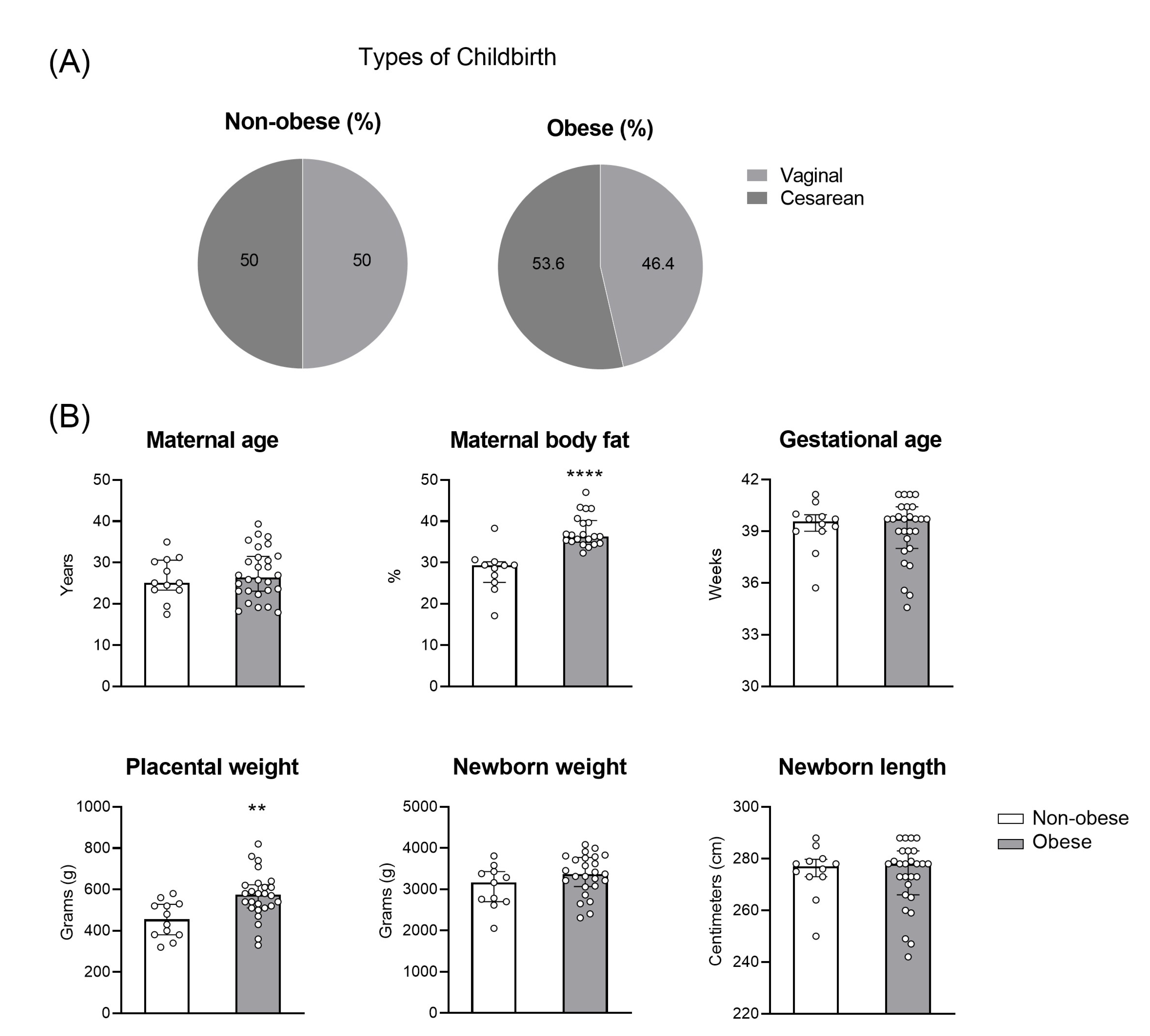
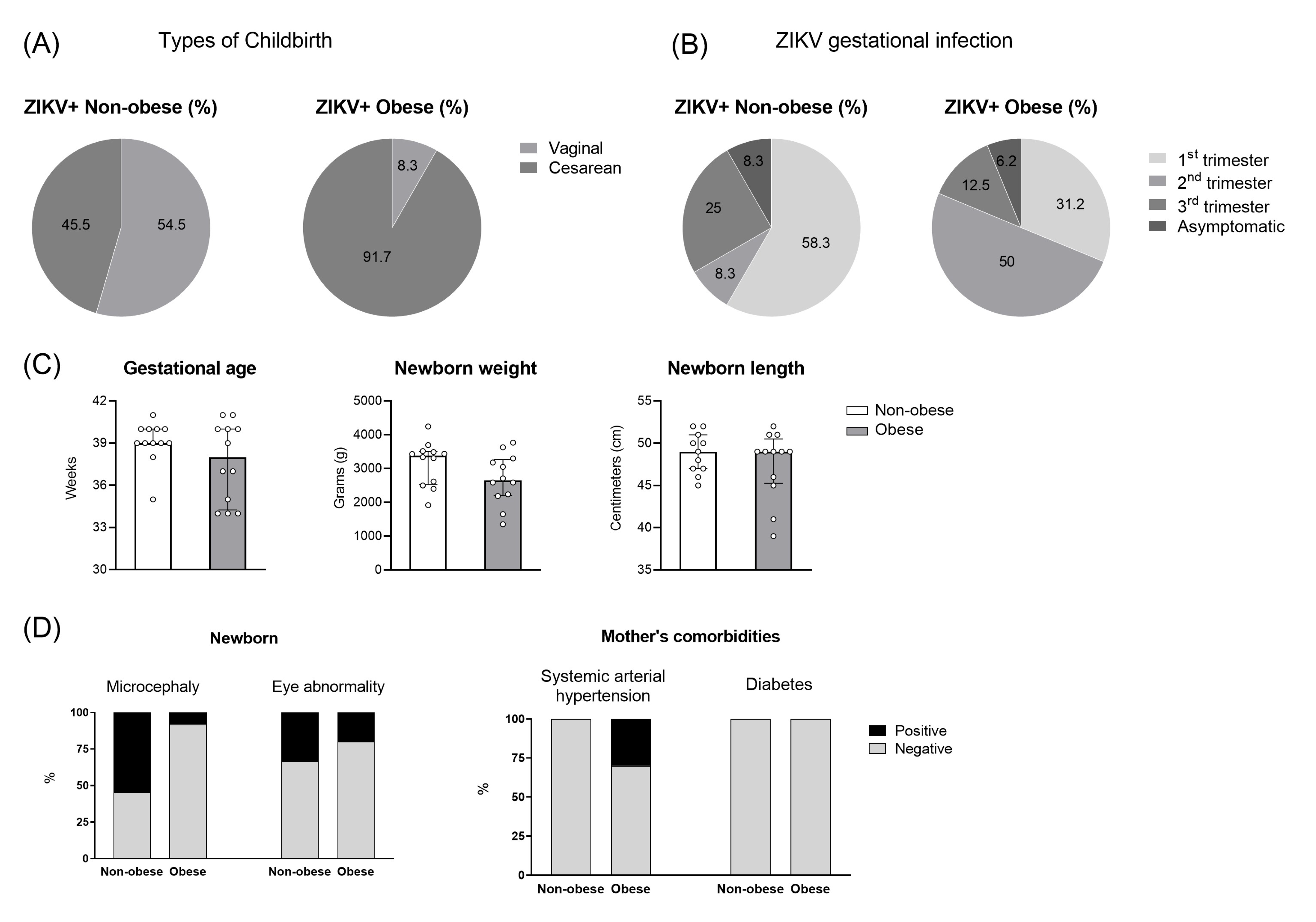
2.1. Human Subjects
2.2. Gene Expression by Real-Time PCR
2.3. Morphological Analysis of the Placenta
2.4. Antiviral Factors’ Expression by Immunohistochemistry
2.5. Statistical Analyses
3. Results
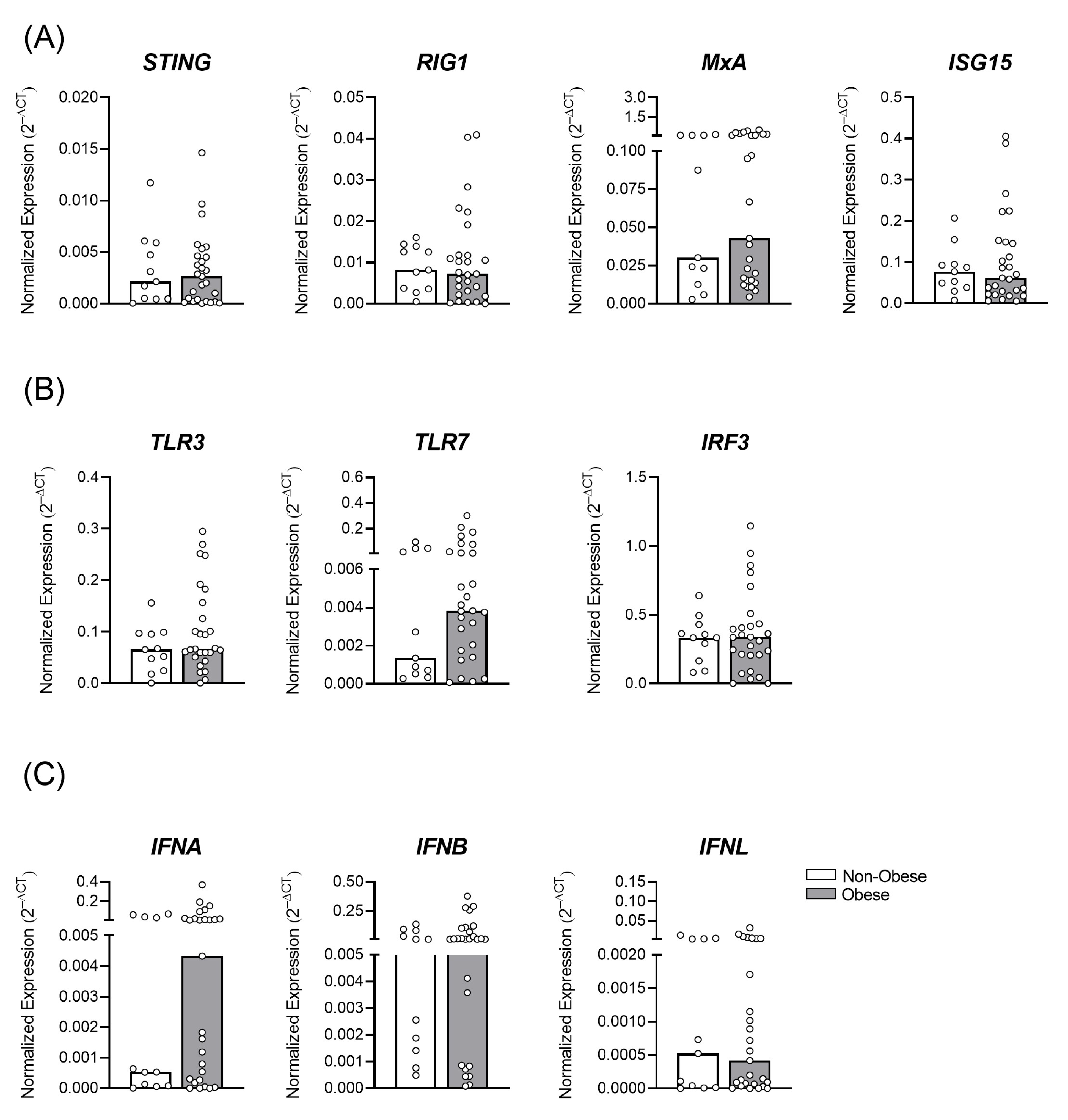
3.1. Gestational Obesity and the Profile of Antiviral Immune Transcripts in the Placental Tissue
3.2. Decreased Antiviral Immune Factors Expression in Placenta due to Gestational Obesity in ZIKV Infection
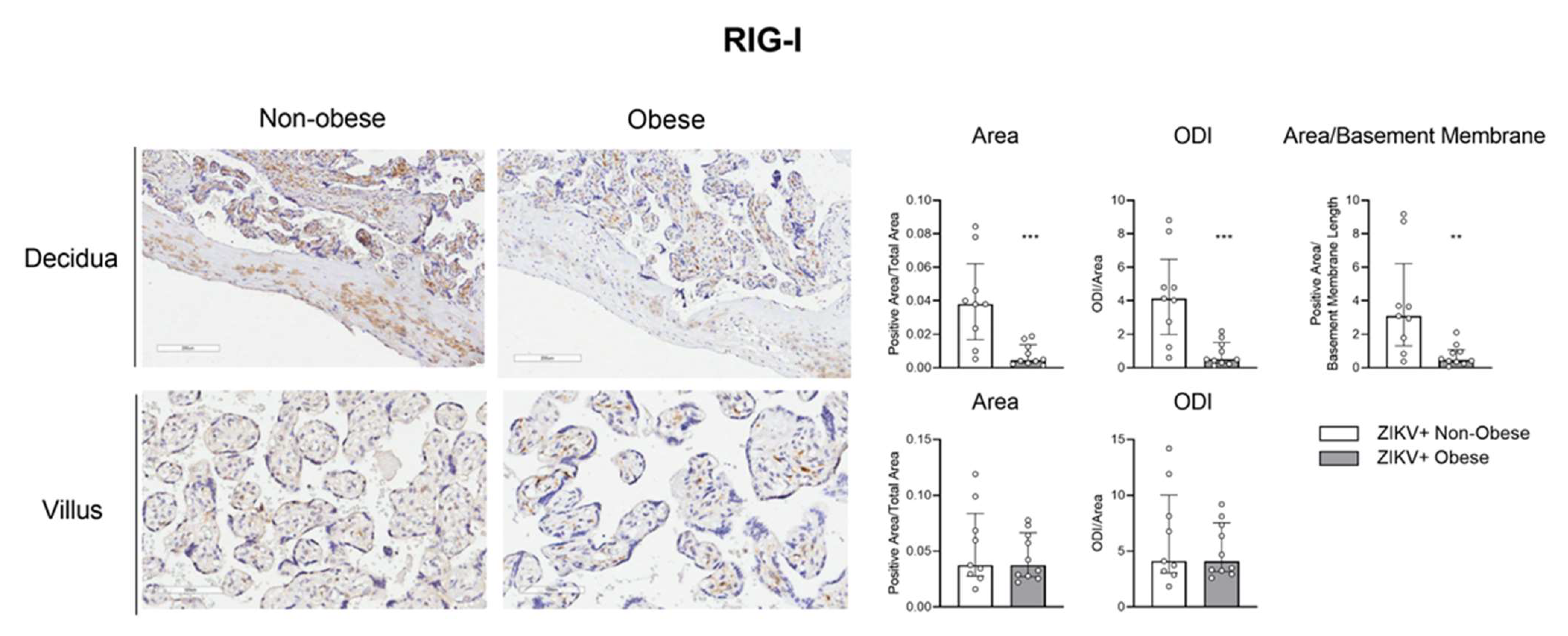
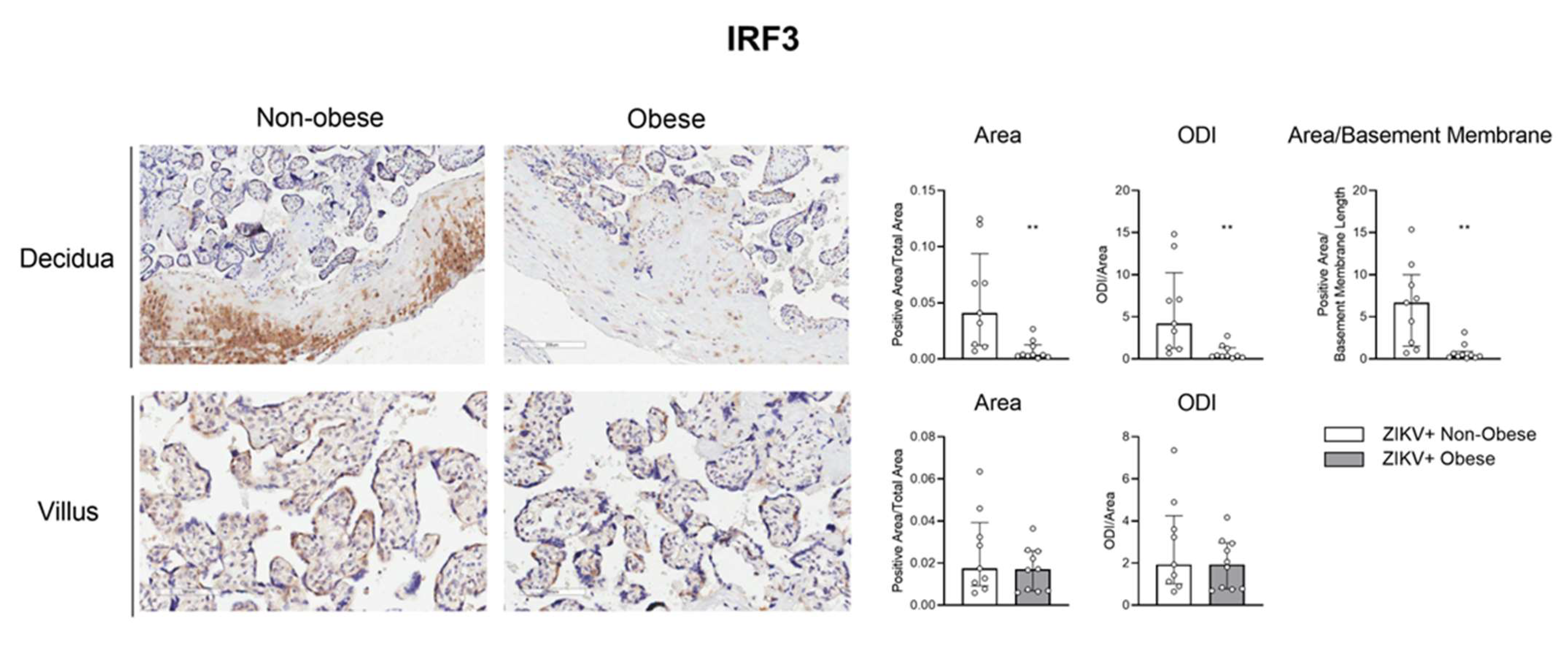
3.3. The IRF-3/RIG-1 Axis Is Altered in Pregnant Women with Obesity in ZIKV Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2019).
- Brasil. Ministério da Saúde Brasileiro—Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico. 2017. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/publicacoes-svs/vigitel/vigitel_brasil_2017_vigilancia_fatores_risco_1ed_rev.pdf/view (accessed on 20 February 2019).
- James, W.P. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32 (Suppl. 7), S120–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Kompoti, M. Obesity and infection. Lancet Infect. Dis. 2006. 6, 438–446. [CrossRef]
- Rocha, D.M.; Caldas, A.P.; Oliveira, L.L.; Bressan, J.; Hermsdorff, H.H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O.; Bethea, C.; Roberts, C. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Poston, L.; Harthoorn, L.F.; Van Der Beek, E.M. Obesity in pregnancy: Implications for the mother and lifelong health of the child. A consensus statement. Pediatr. Res. 2011, 69, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, K.M.; Vyas, S.; Khazaezadeh, N.; Oteng-Ntim, E. Maternal obesity: A review of interventions. Int. J. Clin. Pract. 2009, 63, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrilho, T.R.B.; Rasmussen, K.M.; Hutcheon, J.A.; Alves, R.F.S.; Farias, D.R.; Freitas-Costa, N.C.; Gonzales, M.M.; Batalha, M.A.; Kac, G. Prevalence and temporal trends in prepregnancy nutritional status and gestational weight gain of adult women followed in the Brazilian Food and Nutrition Surveillance System from 2008 to 2018. Matern. Child. Nutr. 2022, 18, e13240. [Google Scholar] [CrossRef]
- Tan, V.P.K.; Ngim, C.F.; Lee, E.Z.; Ramadas, A.; Pong, L.Y.; Ng, J.I.; Hassan, S.S.; Ng, X.Y.; Dhanoa, A. The association between obesity and dengue virus (DENV) infection in hospitalised patients. PLoS ONE 2018, 13, e0200698. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Vandemaele, K.A.; Shinde, V.; Jaramillo-Gutierrez, G.; Koukounari, A.; Donnelly, C.; Carlino, L.O.; Owen, R.; Paterson, B.; Pelletier, L.; et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: A global pooled analysis. PLoS Med. 2011, 8, e1001053. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Julien, L.; Mathieu, D.; Pattou, F.; Verkindt, H. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, C.R.; Chuturgoon, A.; Maharaj, N. Maternal overweight and obesity and its associated factors and outcomes in human immunodeficiency virus (HIV)-infected and HIV-uninfected black South African pregnant women. J. Obstet. Gynaecol. Res. 2022, 48, 2697–2712. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Sáude Brasileiro—Monitoramento dos Casos de Arboviroses Urbanas Transmitidas Pelo Aedes (Dengue, Chikungunya e Zika) Até a Semana Epidemiológica 7 de 2019. 2019. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2022/boletim-epidemiologico-vol-53-no18 (accessed on 10 March 2019).
- Carod-Artal, F.J. Epidemiology and neurological complications of infection by the Zika virus: A new emerging neurotropic virus. Reply. Rev. Neurol. 2016, 63, 143–144. [Google Scholar]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasil. Ministério da Saúde Brasileiro—Situação Epidemiológica da Síndrome Congênita Associada à Infecção Pelo Vírus Zika: Brasil, 2015 a 2022. 2022. Available online: http://plataforma.saude.gov.br/anomalias-congenitas/boletim-epidemiologico-SVS-35-2022.pdf (accessed on 20 November 2022).
- Noorbakhsh, F.; Abdolmohammadi, K.; Fatahi, Y.; Dalili, H.; Rasoolinejad, M.; Rezaei, F.; Salehi-Vaziri, M.; Shafiei-Jandaghi, N.Z.; Gooshki, E.S.; Zaim, M.; et al. Zika Virus Infection, Basic and Clinical Aspects: A Review Article. Iran J. Public Health 2019, 48, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues de Sousa, J.; Azevedo, R.D.S.D.S.; Quaresma, J.A.S.; Vasconcelos, P.F.D.C. The innate immune response in Zika virus infection. Rev. Med. Virol. 2021, 31, e2166. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell. Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Azamor, T.; Azamor, T.; Cunha, D.P.; da Silva, A.M.V.; Bezerra, O.C.D.L.; Ribeiro-Alves, M.; Calvo, T.L.; Kehdy, F.D.S.G.; Manta, F.S.D.N.; Pinto, T.G.D.T.; et al. Congenital Zika Syndrome Is Associated With Interferon Alfa Receptor 1. Front. Immunol. 2021, 12, 764746. [Google Scholar] [CrossRef]
- Schwartz, D.A. Autopsy and Postmortem Studies Are Concordant: Pathology of Zika Virus Infection Is Neurotropic in Fetuses and Infants With Microcephaly Following Transplacental Transmission. Arch. Pathol. Lab. Med. 2017, 141, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Simoni, M.K.; Jurado, K.A.; Abrahams, V.M.; Fikrig, E.; Guller, S. Zika virus infection of Hofbauer cells. Am. J. Reprod. Immunol. 2017, 77, e12613. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, K.; De Souza, L.J.; Salomão, N.G.; Machado, L.N.; Pereira, P.G.; Portari, E.A.; Basílio-De-Oliveira, R.; Dos Santos, F.B.; Neves, L.D.; Morgade, L.F.; et al. Zika Induces Human Placental Damage and Inflammation. Front. Immunol. 2020, 11, 2146. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Meller, M.; Vadachkoria, S.; Luthy, D.; Williams, M. Evaluation of housekeeping genes in placental comparative expression studies. Placenta 2005, 26, 601–607. [Google Scholar] [CrossRef]
- Gaudet, L.; Ferraro, Z.M.; Wen, S.W.; Walker, M. Maternal obesity and occurrence of fetal macrosomia: A systematic review and meta-analysis. Biomed. Res. Int. 2014, 2014, 640291. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Pellielo, G.; Tognon, M.; Martini, F.; Rotondo, J.C. Significantly Low Levels of IgG Antibodies Against Oncogenic Merkel Cell Polyomavirus in Sera From Females Affected by Spontaneous Abortion. Front. Microbiol. 2021, 12, 789991. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, R.; Syrjänen, J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013, 37, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.F.; Merckx, J.; Fell, D.B.; Kuchenbecker, R.; Miranda, A.E.; de Oliveira, W.K.; Platt, R.W.; Antunes, L.; Silveira, M.F.; Barbieri, N.B. Characteristics and outcomes of pregnant women with SARS-CoV-2 infection and other severe acute respiratory infections (SARI) in Brazil from January to November 2020. Braz. J. Infect. Dis. 2021, 25, 101620. [Google Scholar] [CrossRef]
- Pereira, A.M.; Júnior, E.A.; Werner, H.; Monteiro, D.L.M. Zika Virus and Pregnancy: Association between Acute Infection and Microcephaly in Newborns in the State of Rio de Janeiro, Brazil. Geburtshilfe Frauenheilkd 2020, 80, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Diao, L.; Huang, C.; Li, Y.; Zeng, Y.; Kwak-Kim, J.Y. The role of decidual immune cells on human pregnancy. J. Reprod. Immunol. 2017, 124, 44–53. [Google Scholar] [CrossRef]
- Schilling, M.; Bridgeman, A.; Gray, N.; Hertzog, J.; Hublitz, P.; Kohl, A.; Rehwinkel, J. RIG-I Plays a Dominant Role in the Induction of Transcriptional Changes in Zika Virus-Infected Cells, which Protect from Virus-Induced Cell Death. Cells 2020, 9, 1476. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dong, X.; He, Z.; Wu, Y.; Zhang, S.; Lin, J.; Yang, Y.; Chen, J.; An, S.; Yin, Y.; et al. Zika virus antagonizes interferon response in patients and disrupts RIG-I-MAVS interaction through its CARD-TM domains. Cell Biosci. 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, H.E.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. RIG-I Deficiency Promotes Obesity-Induced Insulin Resistance. Pharmaceuticals 2021, 14, 1178. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | ZIKV+ Non-Obese (n = 8) | ZIKV+ Obese (n = 11) | ||||
|---|---|---|---|---|---|---|
| Score | n/N | % | Score | n/N | % | |
| Trophoplast necrosis | + | (0/8) | 0 | + | (2/11) | 18.2 |
| + + | (7/8) | 87.5 | + + | (4/11) | 36.4 | |
| + + + | (1/8) | 12.5 | + + + | (5/11) | 45.4 | |
| Villitis | - | (7/8) | 87.5 | - | (8/11) | 72.7 |
| + | (1/8) | 12.5 | + | (3/11) | 27.3 | |
| Intervillositis | - | (8/8) | 100 | - | (10/11) | 90.9 |
| + | (0/8) | 0 | + | (1/11) | 9.1 | |
| Intervillous thrombi | - | (2/8) | 25 | - | (0/11) | 0 |
| + | (1/8) | 12.5 | + | 4/11) | 36.4 | |
| + + | (4/8) | 50 | + + | (6/11) | 54.5 | |
| + + + | (1/8) | 12.5 | + + + | (1/11) | 9.1 | |
| Hofbauer cell hyperplasia | + | (0/8) | 0 | + | (1/11) | 9.1 |
| + + | (6/8) | 75 | + + | (8/11) | 72.7 | |
| + + + | (2/8) | 25 | + + + | (2/11) | 18.2 | |
| Fibrinoid necrosis of villi | - | (0/8) | 0 | - | (2/11) | 18.2 |
| + | (2/8) | 25 | + | (2/11) | 18.2 | |
| + + | (6/8) | 75 | + + | (7/11) | 63.6 | |
| Villi Calcification | - | (0/8) | 0 | - | (2/11) | 18.2 |
| + | (5/8) | 62.5 | + | (3/11) | 27.3 | |
| + + | (0/8) | 0 | + + | (3/11) | 27.3 | |
| + + + | (3/8) | 37.5 | + + + | (3/11) | 27.3 | |
| Fetal surface changes | - | (8/8) | 100 | - | (11/11) | 100 |
| Syncytial knots | + + | (2/8) | 25 | + + | (6/11) | 54.5 |
| + + + | (6/8) | 75 | + + + | (5/11) | 45.4 | |
| Decidual inflammation | - | (6/8) | 75 | - | (7/11) | 63.6 |
| + | (2/8) | 25 | + | (4/11) | 36.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, A.C.C.C.; De Oliveira, E.A.; Pereira, N.Z.; Alberca, R.W.; Duarte-Neto, A.N.; Da Silva, L.F.F.; Luiz, F.G.; Pereira, N.V.; Sotto, M.N.; Dejani, N.N.; et al. Obesity Induces an Impaired Placental Antiviral Immune Response in Pregnant Women Infected with Zika Virus. Viruses 2023, 15, 320. https://doi.org/10.3390/v15020320
Branco ACCC, De Oliveira EA, Pereira NZ, Alberca RW, Duarte-Neto AN, Da Silva LFF, Luiz FG, Pereira NV, Sotto MN, Dejani NN, et al. Obesity Induces an Impaired Placental Antiviral Immune Response in Pregnant Women Infected with Zika Virus. Viruses. 2023; 15(2):320. https://doi.org/10.3390/v15020320
Chicago/Turabian StyleBranco, Anna Cláudia Calvielli Castelo, Emily Araujo De Oliveira, Nátalli Zanete Pereira, Ricardo Wesley Alberca, Amaro Nunes Duarte-Neto, Luiz Fernando Ferraz Da Silva, Fernanda Guedes Luiz, Naiura Vieira Pereira, Mirian Nacagami Sotto, Naiara Naiana Dejani, and et al. 2023. "Obesity Induces an Impaired Placental Antiviral Immune Response in Pregnant Women Infected with Zika Virus" Viruses 15, no. 2: 320. https://doi.org/10.3390/v15020320
APA StyleBranco, A. C. C. C., De Oliveira, E. A., Pereira, N. Z., Alberca, R. W., Duarte-Neto, A. N., Da Silva, L. F. F., Luiz, F. G., Pereira, N. V., Sotto, M. N., Dejani, N. N., Rondó, P. H. C., Avvad-Portari, E., De Vasconcelos, Z. F. M., Duarte, A. J. d. S., Azamor, T., & Sato, M. N. (2023). Obesity Induces an Impaired Placental Antiviral Immune Response in Pregnant Women Infected with Zika Virus. Viruses, 15(2), 320. https://doi.org/10.3390/v15020320





