Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethic Committee Approval
2.2. Patient Recruitment
2.3. Collection of Blood Samples
2.4. Processing of Samples
2.5. Radiologic Evaluation
2.6. Statistical Analyses
3. Results
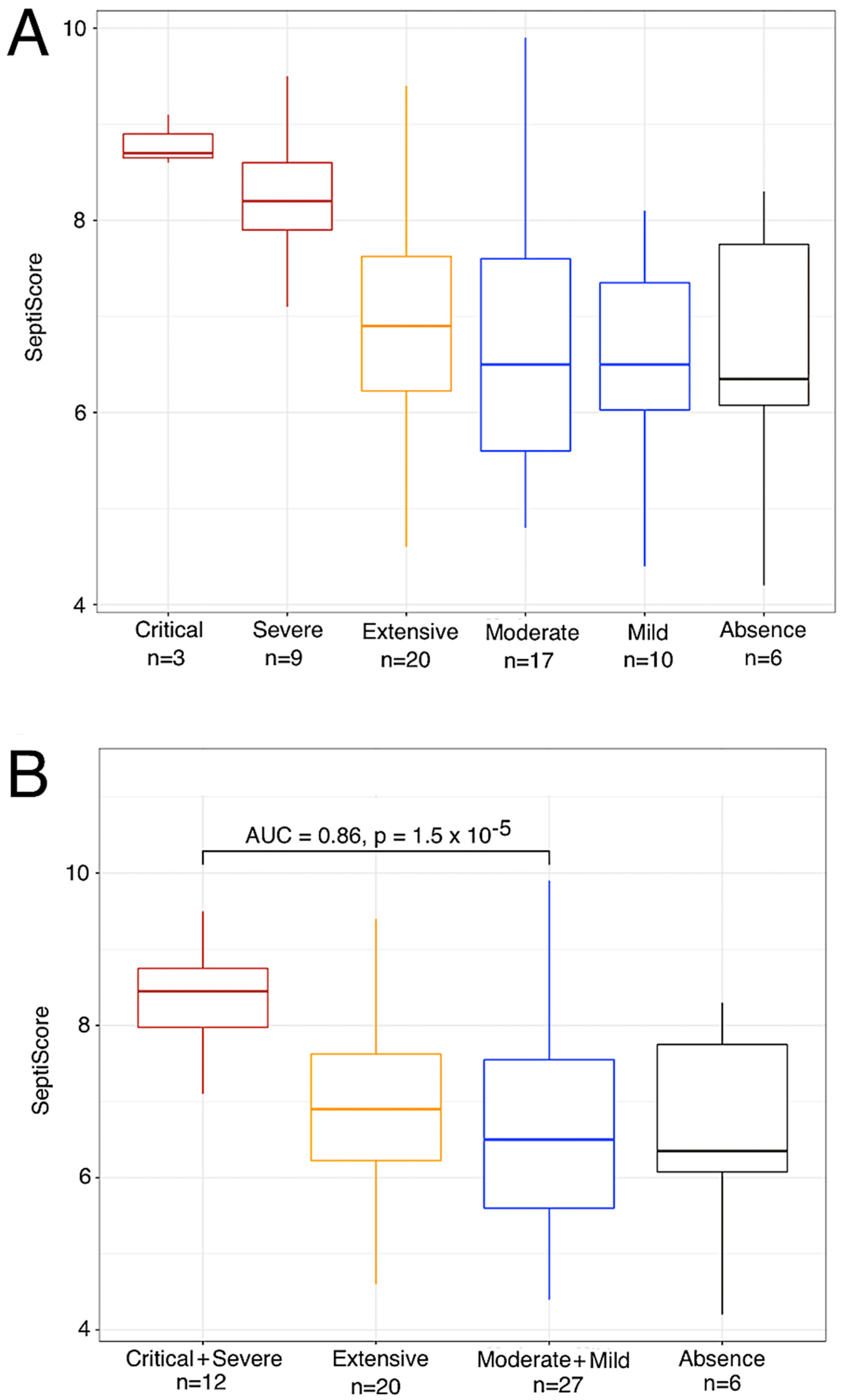
3.1. Variations of SeptiScore and Other Biological Markers According to Patient Group
3.2. Variation in SeptiScore, According to Lung Injury Observed in CT Scan
3.3. Elevated SeptiScore Is Predictive of ICU Admission in Patients with Extensive Lung Injury
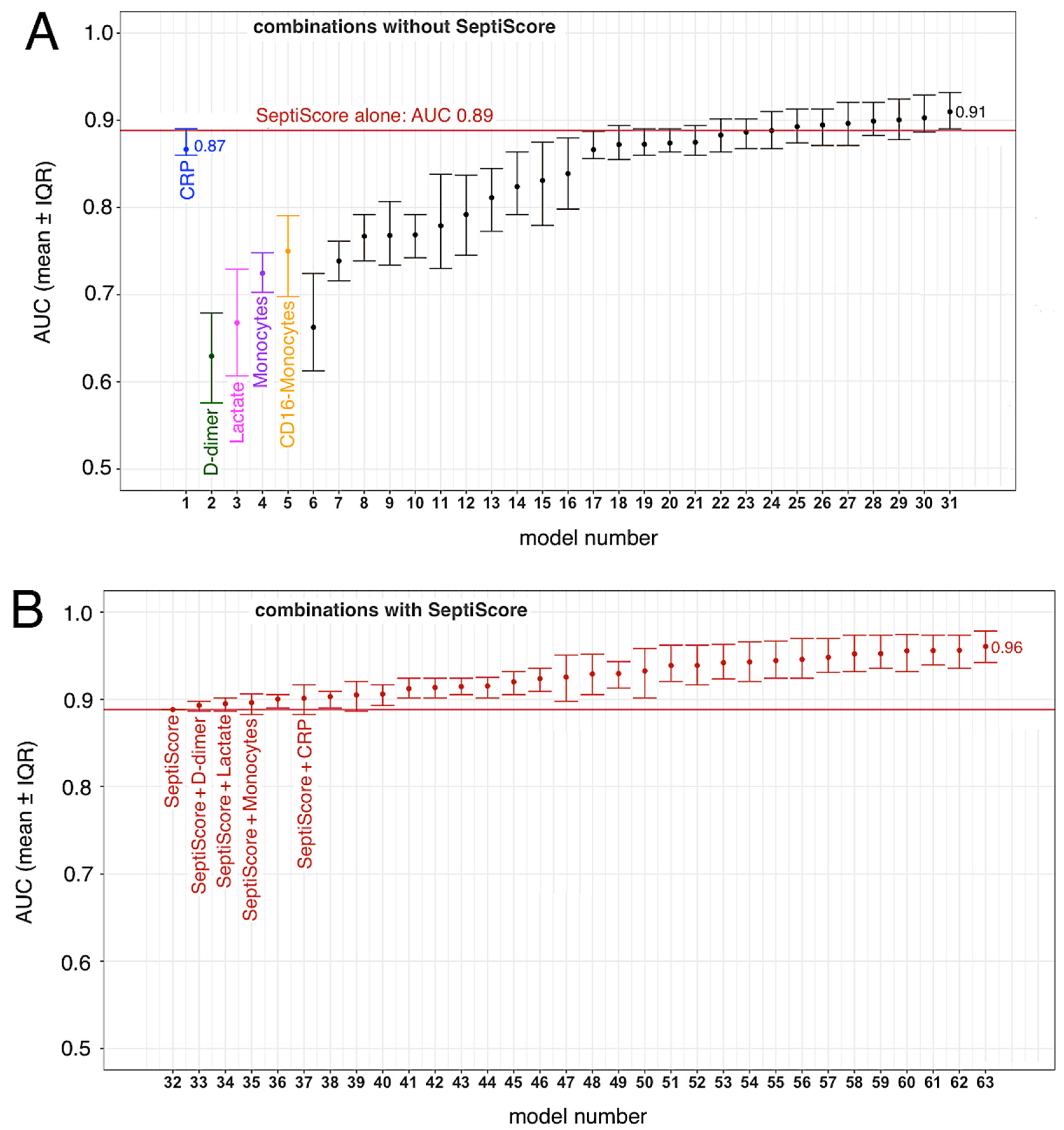
3.4. SeptiCyte RAPID, Compared to/Combined with Other Clinical Variables
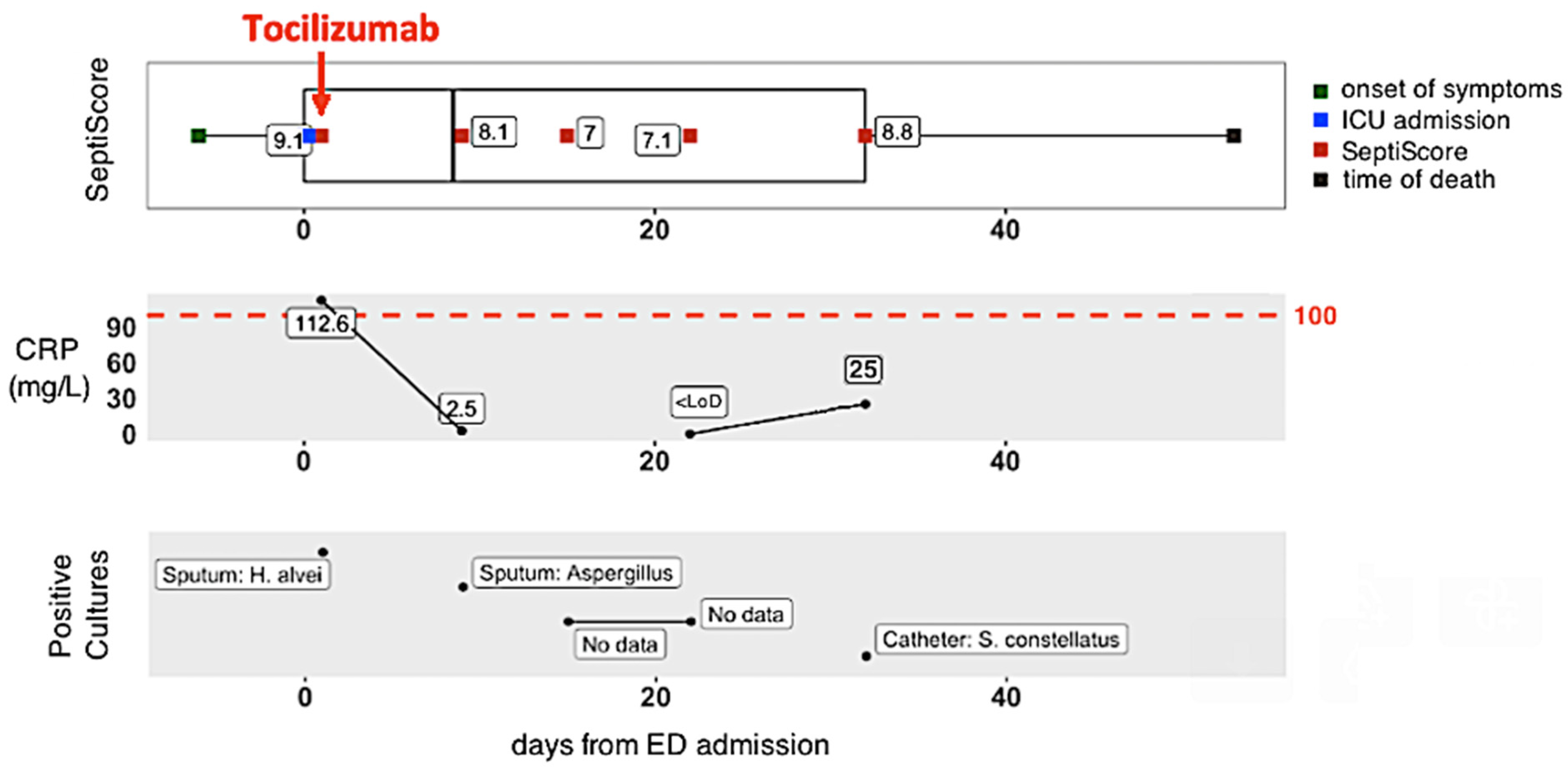
3.5. Longitudinal Monitoring of Patient Trajectories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Shappell, C.N.; Klompas, M.M.; Kanjilal, S.M.; Chan, C.M.; Rhee, C.M. Prevalence, Clinical Characteristics, and Outcomes of Sepsis Caused by Severe Acute Respiratory Syndrome Coronavirus 2 Versus Other Pathogens in Hospitalized Patients with COVID-19. Crit. Care Explor. 2022, 4, e0703. [Google Scholar] [CrossRef]
- Bruse, N.; Kooistra, E.J.; Jansen, A.; van Amstel, R.B.E.; de Keizer, N.F.; Kennedy, J.N.; Seymour, C.; van Vught, L.A.; Pickkers, P.; Kox, M. Clinical sepsis phenotypes in critically ill COVID-19 patients. Crit. Care 2022, 26, 1–6. [Google Scholar] [CrossRef]
- Vincent, J.-L. COVID-19: It’s all about sepsis. Futur. Microbiol. 2021, 16, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Olwal, C.O.; Nganyewo, N.N.; Tapela, K.; Djomkam, L.A.; Owoicho, O.; Bediako, Y.; Duodu, S. Parallels in Sepsis and COVID-19 Conditions: Implications for Managing Severe COVID-19. Front. Immunol. 2021, 12, 602848. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Pallardó, F.V.; Ferreres, J.; Rodríguez, M.; Mulet, S.; Ferrando-Sánchez, C.; Carbonell, N.; García-Giménez, J.L. Sepsis and Coronavirus Disease 2019: Common Features and Anti-Inflammatory Therapeutic Approaches. Crit. Care Med. 2020, 48, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Abate, S.M.; Ali, S.A.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef]
- Hirabara, S.M.; Serdan, T.D.A.; Gorjao, R.; Masi, L.N.; Pithon-Curi, T.C.; Covas, D.T.; Curi, R.; Durigon, E.L. SARS-CoV-2 Variants: Differences and Potential of Immune Evasion. Front. Cell. Infect. Microbiol. 2022, 11, 781429. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Kmiec, D.; Koepke, L.; Zech, F.; Jacob, T.; Sparrer, K.M.J.; Kirchhoff, F. Omicron: What Makes the Latest SARS-CoV-2 Variant of Concern So Concerning? J. Virol. 2022, 96, e0207721. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Dhama, K.; Agoramoorthy, G. A comprehensive analysis of the mutational landscape of the newly emerging Omicron (B.1.1.529) variant and comparison of mutations with VOCs and VOIs. Geroscience 2022, 44, 2393–2425. [Google Scholar] [CrossRef]
- Dhawan, M.; Saied, A.A.; Mitra, S.; Alhumaydhi, F.A.; Bin Emran, T.; Wilairatana, P. Omicron variant (B.1.1.529) and its sublineages: What do we know so far amid the emergence of recombinant variants of SARS-CoV-2? Biomed. Pharmacother. 2022, 154, 113522. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Lan, W.; Wu, X.; Zhao, T.; Duan, B.; Yang, P.; Ren, Y.; Quan, L.; Zhao, W.; Seto, D.; et al. Tracking SARS-CoV-2 Omicron diverse spike gene mutations identifies multiple inter-variant recombination events. Signal Transduct. Target. Ther. 2022, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.G.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 2020, 30, 4381–4389. [Google Scholar] [CrossRef]
- Brandon, R.; Kirk, J.; Yager, T.; Cermelli, S.; Davis, R.; Sampson, D.; Sillekens, P.; Keuleers, I.; Vanhoey, T. Clinical performance of a rapid sepsis test on a near-patient molecular testing platform. Abstract # P481, ISICEM 2020 - 40th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium. Crit Care 2020, 24 (Suppl 1), 87. [Google Scholar] [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440. [Google Scholar] [CrossRef]
- Gravrand, V.; Mellot, F.; Ackermann, F.; Ballester, M.-C.; Zuber, B.; Kirk, J.; Yager, T.; Navalkar, K.; Pascreau, T.; Farfour, E.; et al. SeptitiCyte RAPID in COVID-19 Severity Stratification and Triage. Abstract # 04729, ECCMID 2022. In Proceedings of the European Congress of Clinical Microbiology and Infectious Diseases, Lisbon, Portugal, 23–26 April 2022; Available online: https://2022.eccmid.org/ (accessed on 6 January 2023).
- Vasse, M.; Zuber, B.; Goubeau, L.; Ballester, M.-C.; Roumier, M.; Delcominette, F.; Habarou, F.; Jolly, E.; Ackermann, F.; Cerf, C.; et al. A low level of CD16pos monocytes in SARS-CoV-2 infected patients is a marker of severity. Clin. Chem. Lab. Med. 2021, 59, 1315–1322. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 1 November 2021)ISBN 3-900051-07-0.
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Honaker, J.; King, G.; Blackwell, M. AmeliaII: A Program for Missing Data. J. Stat. Softw. 2011, 45, 1–47. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.F.F.; Miranda, J.R.A.; Reis, F.; Oliveira, A.A.; Souza, S.A.S.; Fortaleza, C.M.C.B.; Tanni, S.E.; Castro, J.T.S.; Pina, D.R. Automatic algorithm for quantifying lung involvement in patients with chronic obstructive pulmonary disease, infection with SARS-CoV-2, paracoccidioidomycosis and no lung disease patients. PLoS ONE 2021, 16, e0251783. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Abedi, A.; Balakrishnan, S.; Gholamrezanezhad, A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. Am. J. Roentgenol. 2020, 215, 87–93. [Google Scholar] [CrossRef]
- Ali, N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J. Med Virol. 2020, 92, 2409–2411. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Varshney, S.; Dutt, N.; Munikumar, M.; Asfahan, S.; Kulkarni, P.P.; Gupta, P. D-dimer, disease severity, and deaths (3D-study) in patients with COVID-19: A systematic review and meta-analysis of 100 studies. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Correia, B.S.B.; Ferreira, V.G.; Piagge, P.M.F.D.; Almeida, M.B.; Assunção, N.A.; Raimundo, J.R.S.; Fonseca, F.L.A.; Carrilho, E.; Cardoso, D.R. 1H qNMR-Based Metabolomics Discrimination of Covid-19 Severity. J. Proteome Res. 2022, 21, 1640–1653. [Google Scholar] [CrossRef]
- Knoll, R.; Schultze, J.L.; Schulte-Schrepping, J. Monocytes and Macrophages in COVID-19. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Payen, D.; Cravat, M.; Maadadi, H.; Didelot, C.; Prosic, L.; Dupuis, C.; Losser, M.-R.; Bittencourt, M.D.C. A Longitudinal Study of Immune Cells in Severe COVID-19 Patients. Front. Immunol. 2020, 11, 580250. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Keske, Ş.; Tekin, S.; Sait, B.; Irkören, P.; Kapmaz, M.; Çimen, C.; Uğur, S.; Çelebi, I.; Bakır, V.O.; Palaoğlu, E.; et al. Appropriate use of tocilizumab in COVID-19 infection. Int. J. Infect. Dis. 2020, 99, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xie, M.-H.; Yang, J.; Chao, S.-W.; Xu, E.-L. Clinical efficacy of tocilizumab treatment in severe and critical COVID-19 patients. World J. Clin. Cases 2020, 8, 3763–3773. [Google Scholar] [CrossRef]
- Hofmaenner, D.A.; Garcia, P.D.W.; Ganter, C.C.; Brugger, S.D.; Buehler, P.K.; David, S. What every intensivist should know about Tocilizumab. Crit. Care 2021, 25, 262. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Yoshizaki, K.; Uno, K.; Kitajima, H.; Arai, T.; Tamura, Y.; Morishita, H.; Matsuoka, H.; Han, Y.; Minamoto, S.; et al. Prompt Reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1 and a Relatively Low Basal Ratio of Ferritin/CRP Is Possibly Associated with the Efficacy of Tocilizumab Monotherapy in Severely to Critically Ill Patients with COVID-19. Front. Med. 2021, 8, 734838. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.M.; Bruse, N.; Frenzel, T.; Berg, M.J.W.V.D.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit. Care 2021, 25, 281. [Google Scholar] [CrossRef] [PubMed]
- Hafez, W.; Ziade, M.A.; Arya, A.; Saleh, H.; Abdelshakor, M.; Alla, O.F.; Agrawal, P.; Ali, S.; Rao, S.R.; Gupta, S.; et al. Treatment Outcomes of Tocilizumab in Critically-Ill COVID-19 Patients, Single-Centre Retrospective Study. Antibiotics 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Bari, S.F.; Khan, A.; Lawson, T. C reactive protein may not be reliable as a marker of severe bacterial infection in patients receiving tocilizumab. BMJ Case Rep. 2013, 2013, bcr2013010423. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Perez, L. Acute phase protein response to viral infection and vaccination. Arch. Biochem. Biophys. 2019, 671, 196–202. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Belati, A.; Tulone, O.; Diella, L.; Bavaro, D.F.; Bonica, R.; Genna, V.; Smith, L.; Trott, M.; Bruyere, O.; et al. Incidence of long COVID-19 in people with previous SARS-Cov2 infection: A systematic review and meta-analysis of 120,970 patients. Intern. Emerg. Med. 2022, 1–9. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Bonica, R.; Cotugno, S.; Tulone, O.; Camporeale, M.; Smith, L.; Trott, M.; Bruyere, O.; Mirarchi, L.; Rizzo, G.; et al. Interventions for Improving Long COVID-19 Symptomatology: A Systematic Review. Viruses 2022, 14, 1863. [Google Scholar] [CrossRef]
- Veldhoen, M.; Simas, J.P. Endemic SARS-CoV-2 will maintain post-pandemic immunity. Nat. Rev. Immunol. 2021, 21, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Mullin, S.; Wyk, B.V.; Asher, J.L.; Compton, S.R.; Allore, H.G.; Zeiss, C.J. Modeling pandemic to endemic patterns of SARS-CoV-2 transmission using parameters estimated from animal model data. PNAS Nexus 2022, 1, pgac096. [Google Scholar] [CrossRef] [PubMed]
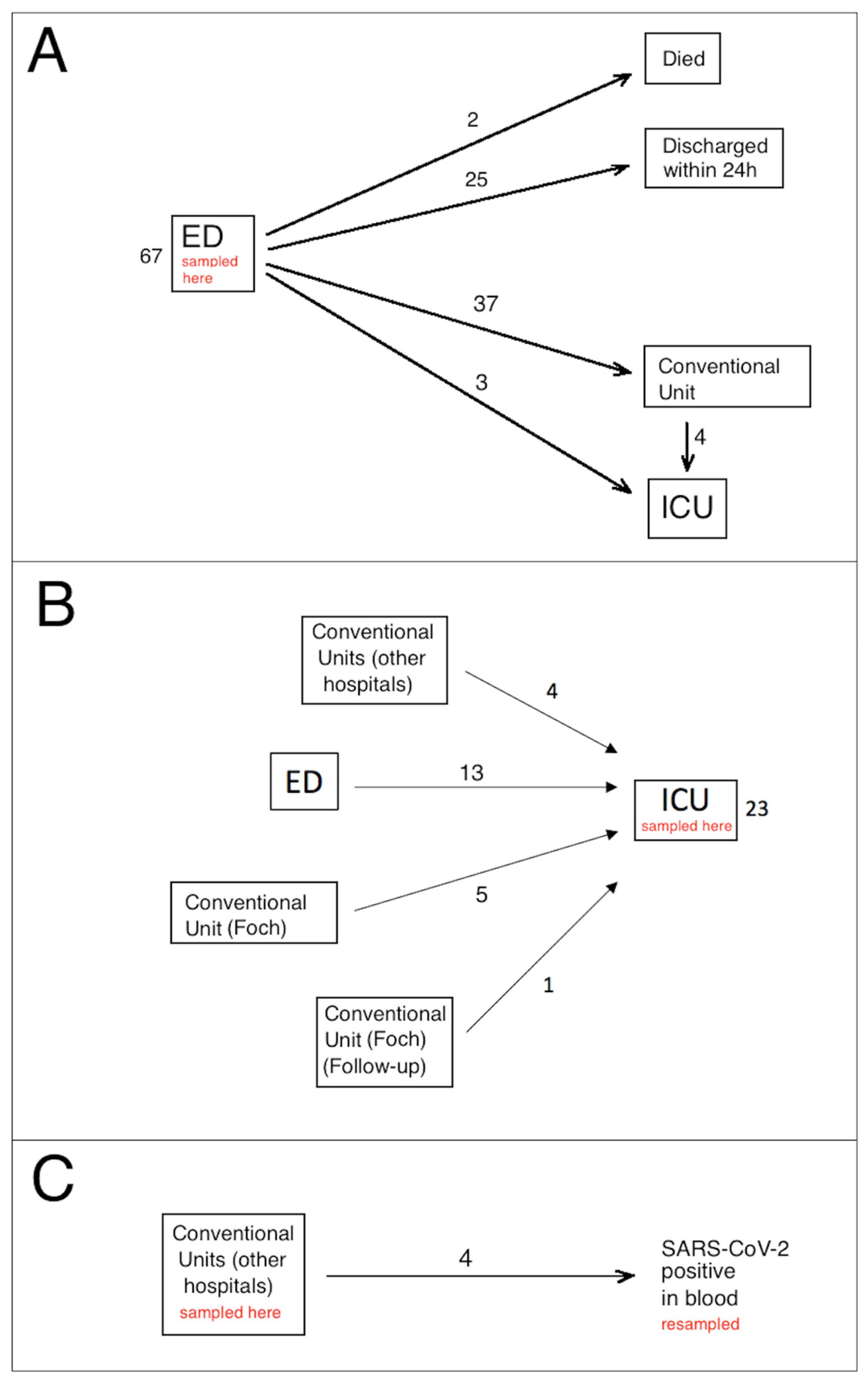

| Emergency Department (Figure 1A) (n = 67) | ICU (Figure 1B) | Conventional Unit, Viremia (Figure 1C) | Bacterial Sepsis (ICU) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-Up | Discharged (<24 h in ED) | Conventional Hospitalization | Delayed ICU Admission | Immediate ICU Admission | Deceased in ED | ||||
| n | 25 | 33 | 4 | 3 | 2 | 23 | 4 | 8 | |
| Sex (F/M) | 15/10 | 15/18 | 2/2 | 1/2 | 2/0 | 8/15 | 1/3 | 2/6 | 0.4 |
| Median age (years) | 60 [20–84] | 72 [23–96] | 66 [63–66] | 76 [69–78] | 89 [85–93] | 57 [23–78] | 56 [38–67] | 62 [35–90] | 0.001 |
| Median time between COVID-19 diagnosis and SeptiCyte result, days 1 | 0 [0–12] | 0 [0–7] | 0.5 [0–16] | 0 | 0 | 3 [0–25] | 8.5 [1–10] | NA | <0.001 |
| Median time between onset of symptoms and SeptiCyte result, days (symptomatic patients) | 5 (n = 21) [1–12] | 7 (n = 31) [0–30] | 6.5 (n = 4) [4–9] | 7 (n = 3) [4–10] | NA | 10.5 [4–42] | 14 [10–21] | 3 [1–10] | <0.001 |
| Diabetes, n (%) | 6 (24.0) | 11 (33.3) | 1 (25.0) | 2 (66.7) | 1 (50) | 10 (43.5) | 1 (25.0) | 5 (62.5) | 0.3 |
| Arterial Hypertension (AHT), n (%) | 9 (36.0) | 13 (39.4) | 2 (50.0) | 3 (100) | 1 (50) | 14 (60.9) | 0 (0) | 3 (37.5) | 0.2 |
| Diabetes and AHT, n (%) | 4 (16.0) | 4 (12.1) | 1 (25) | 2 (66.7) | 1 (50) | 8 (34.8) | 0 (0) | 3 (37.5) | 0.07 |
| CT scan performed, n (%) | 12 (48) | 30 (90.1) | 4 (100) | 2 (66.7) | 1 (50) | 15 (65.2) | 4 (100) | 0(0) | <0.001 |
| Alive, n (%) | 25 (100) | 27 (81.8) | 4 (100) | 3 (100) | 0 (0) | 15 (65.2) | 4 (100) | 7 (87.5] | <0.001 |
| Median length of hospitalization, days | 0 (100) | 7 [2–22] | 21 [6–37] | 20 [7–38] | 1 [1–1] | 24 [6–74] | 10 [3–13] | 10 [6–34] | <0.001 |
| Median delay to death (days) 2 | no cases | 8 [5–17] | no cases | no cases | 1 [1–1] | 24 [7–55] | no cases | 7 [7–7] | 0.005 |
| Emergency Department (Figure 1A) | ICU (Figure 1B) | Conventional Unit, Viremia (Figure 1C) | Bacterial Sepsis (ICU) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-Up | Discharged (<24 h in ED) | Conventional Hospitalization | Delayed ICU Admission | Immediate ICU Admission | Deceased in ED | ||||
| n | 25 | 33 | 4 | 3 | 2 | 23 | 4 | 8 | |
| SeptiCyte score (0–15 scale) | 6.4 [3.1–8.3] | 6.4 [3.5–9.9] | 8 [6.4–8.6] | 8.1 [7.7–8.2] | 7.2 [6.5–7.9] | 7.6 [5.3–9.5] | 6.2 [4.8–6.7] | 8.5 [2.9–11.1] | 0.003 |
| White blood cell (×109/L) | 5 [1.9–5.5] | 7.1 [2–24] | 5.3 [3.5–8.1] | 9.4 [7.5–10.7] | 12.6 [9.8–15.3] | 8.2 [2.2–39.7] | 6.3 [4.8–7.1] | 13.3 [4.4–24.3] | 0.020 |
| Platelets (×109/L) | 197 [97–708] | 198 [86–588] | 180 [117–311] | 316 [136–421] | 178 [139–217] | 238 [68–433] | 182 [167–242] | 180 [17–330] | 0.7 |
| Neutrophils (×109/L) | 3.4 [1.1–11.6] | 5.5 [1.1–22.8] | 4.1 [2.2–5.7] | 6.8 [6.8–9.5] | 10.6 [8.6–12.7] | 6.8 [1.8–33.4] | 4.3 [3.6–6] | 8.6 [3.1–18.0] | 0.04 |
| Lymphocytes (×109/L) | 0.9 [0.4–3.6] | 0.8 [0.2–2.2] | 1.1 [0.4–1.4] | 0.7 [0.5–1.4] | 1.1 [0.5–1.6] | 0.65 [0.2–0.8] | 0.8 [0.7–1.7] | 1 [0.3–2.0] | 0.3 |
| Monocytes (×109/L) | 0.6 [0–1.2] | 0.6 [0.1–1.5] | 0.4 [0.2–1.2] | 0.5 [0.2–1.1] | 0.8 [0.6–0.9] | 0.5 [0.1–2.1] | 0.6 [0.3–0.9] | 0.8 [0.1–1.4] | >0.9 |
| CD16POS monocytes (×109/L) | 0.07 [0–0.38] | 0.059 [0.003–0.29] | 0.033 [0.022–0.058] | 0.053 [0.005–0.094] | 0.064 | 0.020 [0.005–0.12] | 0.058 [0.044–0.074] | 0.094 [0.004–0.16] | 0.7 |
| CRP (mg/L) | 22 [1–279] | 95 [1–287] | 166 [84–181] | 173 [105–296] | 111 [86–137] | 161 [23–270] | 47 [2–154] | 196 (159–233) | <0.001 |
| D-dimer (mg/L) | 0.79 [0.22–3.80] | 0.66 [0.28–2.21] | 1.07 [0.43–92.86] | 1.59 [0.54–1.81] | 6.961 | 0.95 [0.43–16.25] | 1.29 [0.83–1.74] | NA | 0.02 |
| Lactate (mmol/L) | 0.8 [0.6–1.2] | 1.1 [0.9–5.3] | 1.6 [1.5–1.7] | 2.6 [1.1–2.6] | 4.2 [3.2–5.1] | 1.5 [0.8–2.5] | 1.8 | 1.3 (0.6–8.0) | 0.01 |
| N, lower Damage (<50%) | N, higher Damage (≥50%) | p-Value (t-Test) | AUC | Cut-Off | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| SeptiScore | 53 | 12 | 0.00013 | 0.860 | ≥7 | 100.0 | 61.1 |
| CRP (mg/L) | 49 | 11 | 0.00032 | 0.836 | >154.1 | 81.8 | 78.4 |
| Lymphocytes (109/L) | 52 | 11 | 0.025 | 0.786 | ≤0.7 | 81.8 | 62.3 |
| Monocytes (109/L) | 52 | 11 | 0.016 | 0.758 | ≤0.5 | 81.8 | 56.6 |
| Monocytes CD16POS (109/L) | 43 | 10 | 0.046 | 0.773 | ≤0.037 | 80 | 66.7 |
| Lactate (mmol/L) | 35 | 11 | 0.114 | 0.745 | ˃1.2 | 81.8 | 66.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravrand, V.; Mellot, F.; Ackermann, F.; Ballester, M.-C.; Zuber, B.; Kirk, J.T.; Navalkar, K.; Yager, T.D.; Petit, F.; Pascreau, T.; et al. Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test. Viruses 2023, 15, 419. https://doi.org/10.3390/v15020419
Gravrand V, Mellot F, Ackermann F, Ballester M-C, Zuber B, Kirk JT, Navalkar K, Yager TD, Petit F, Pascreau T, et al. Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test. Viruses. 2023; 15(2):419. https://doi.org/10.3390/v15020419
Chicago/Turabian StyleGravrand, Victor, François Mellot, Felix Ackermann, Marie-Christine Ballester, Benjamin Zuber, James T. Kirk, Krupa Navalkar, Thomas D. Yager, Fabien Petit, Tiffany Pascreau, and et al. 2023. "Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test" Viruses 15, no. 2: 419. https://doi.org/10.3390/v15020419
APA StyleGravrand, V., Mellot, F., Ackermann, F., Ballester, M.-C., Zuber, B., Kirk, J. T., Navalkar, K., Yager, T. D., Petit, F., Pascreau, T., Farfour, E., & Vasse, M. (2023). Stratification of COVID-19 Severity Using SeptiCyte RAPID, a Novel Host Immune Response Test. Viruses, 15(2), 419. https://doi.org/10.3390/v15020419







