Targeting Aedes aegypti Metabolism with Next-Generation Insecticides
Abstract
:1. Introduction
1.1. Vector Control Strategies
1.2. Insecticide Resistance
1.3. Novel Insecticide Targets
1.4. Summary
2. Dietary Nutrients and Impact on Mosquito Behavior and Development
2.1. Survival
2.2. Egg Production and Oviposition Site Selection
2.3. Biting Behavior
2.4. Tolerance to Adverse Conditions
3. Dietary Nutrients and Impact on Virus Infection
3.1. Protein
3.2. Sugar
3.3. Lipid
4. Stored Nutrients and Impact on Mosquito Development
4.1. Lipid
4.2. Sugar
5. Stored Nutrients and Impact on Virus Infection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fang, Y.; Shi, W.Q.; Wu, J.T.; Li, Y.Y.; Xue, J.B.; Zhang, Y. Resistance to pyrethroid and organophosphate insecticides, and the geographical distribution and polymorphisms of target-site mutations in voltage-gated sodium channel and acetylcholinesterase 1 genes in Anopheles sinensis populations in Shanghai, China. Parasit. Vectors 2019, 12, 396. [Google Scholar] [CrossRef]
- Amelia-Yap, Z.H.; Chen, C.D.; Sofian-Azirun, M.; Low, V.L. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasit. Vectors 2018, 11, 332. [Google Scholar] [CrossRef]
- Andreazza, F.; Oliveira, E.E.; Martins, G.F. Implications of Sublethal Insecticide Exposure and the Development of Resistance on Mosquito Physiology, Behavior and Pathogen Transmission. Insects 2021, 12, 917. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Sodium Channel Mutations and Pyrethroid Resistance in Aedes aegypti. Insects 2016, 7, 60. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Roberts, D.R.; Andre, R.G. Insecticide resistance issues in vector-borne disease control. Am. J. Trop. Med. Hyg. 1994, 50, 21–34. [Google Scholar] [CrossRef]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, M.S.; Arias-Goeta, C.; Di Genua, C.; Failloux, A.B.; Sinkins, S.P. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl. Trop. Dis. 2013, 7, e2152. [Google Scholar] [CrossRef]
- Dobson, S.L.; Fox, C.W.; Jiggins, F.M. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. Biol. Sci. 2002, 269, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 2017, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.C.; Sommer, U.; Viant, M.R.; Sinkins, S.P. Wolbachia Modulates Lipid Metabolism in Aedes albopictus Mosquito Cells. Appl. Environ. Microbiol. 2016, 82, 3109–3120. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Mousson, L.; Zouache, K.; Arias-Goeta, C.; Raquin, V.; Mavingui, P.; Failloux, A.B. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl. Trop. Dis. 2012, 6, e1989. [Google Scholar] [CrossRef]
- Ross, P.A.; Endersby, N.M.; Hoffmann, A.A. Costs of Three Wolbachia Infections on the Survival of Aedes aegypti Larvae under Starvation Conditions. PLoS Negl. Trop. Dis. 2016, 10, e0004320. [Google Scholar] [CrossRef]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef]
- Soh, L.T.; Ong, Z.; Vasquez, K.; Chen, I.; Li, X.; Niah, W.; Panchapakesan, C.; Sheldenkar, A.; Sim, S.; Ng, L.C.; et al. A Household-Based Survey to Understand Factors Influencing Awareness, Attitudes and Knowledge towards Wolbachia-Aedes Technology. Int. J. Environ. Res. Public. Health 2021, 18, 11997. [Google Scholar] [CrossRef]
- Liew, C.; Soh, L.T.; Chen, I.; Ng, L.C. Public sentiments towards the use of Wolbachia-Aedes technology in Singapore. BMC Public Health 2021, 21, 1417. [Google Scholar] [CrossRef]
- Lwin, M.O.; Ong, Z.; Panchapakesan, C.; Sheldenkar, A.; Soh, L.T.; Chen, I.; Li, X.; Niah, W.; Vasquez, K.; Sim, S.; et al. Influence of public hesitancy and receptivity on reactive behaviours towards releases of male Wolbachia-Aedes mosquitoes for dengue control. PLoS Negl. Trop. Dis. 2022, 16, e0010910. [Google Scholar] [CrossRef]
- Arham, A.F.; Amin, L.; Mustapa, M.A.C.; Mahadi, Z.; Yaacob, M.; Ibrahim, M. Determinants of stakeholders’ attitudes and intentions toward supporting the use of Wolbachia-infected Aedes mosquitoes for dengue control. BMC Public Health 2021, 21, 2314. [Google Scholar] [CrossRef]
- Hegazy, M.I.; Hegazy, A.M.; Saad, A.M.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T.; Soliman, S.M.; Taha, A.E.; Alshehri, M.A.; Ezzat Ahmed, A.; et al. ٍSome biologically active microorganisms have the potential to suppress mosquito larvae (Culex pipiens, Diptera: Culicidae). Saudi J. Biol. Sci. 2022, 29, 1998–2006. [Google Scholar] [CrossRef]
- Ye, G.; Wang, Y.; Liu, X.; Dong, Q.; Cai, Q.; Yuan, Z.; Xia, H. Transmission competence of a new mesonivirus, Yichang virus, in mosquitoes and its interference with representative flaviviruses. PLoS Negl. Trop. Dis. 2020, 14, e0008920. [Google Scholar] [CrossRef]
- White, A.V.; Fan, M.; Mazzara, J.M.; Roper, R.L.; Richards, S.L. Mosquito-infecting virus Espirito Santo virus inhibits replication and spread of dengue virus. J. Med. Virol. 2021, 93, 3362–3373. [Google Scholar] [CrossRef]
- Schultz, M.J.; Frydman, H.M.; Connor, J.H. Dual Insect specific virus infection limits Arbovirus replication in Aedes mosquito cells. Virology 2018, 518, 406–413. [Google Scholar] [CrossRef]
- Goenaga, S.; Kenney, J.L.; Duggal, N.K.; Delorey, M.; Ebel, G.D.; Zhang, B.; Levis, S.C.; Enria, D.A.; Brault, A.C. Potential for Co-Infection of a Mosquito-Specific Flavivirus, Nhumirim Virus, to Block West Nile Virus Transmission in Mosquitoes. Viruses 2015, 7, 5801–5812. [Google Scholar] [CrossRef] [Green Version]
- Novak, R.J.; Gubler, D.J.; Underwood, D. Evaluation of slow-release formulations of temephos (Abate) and Bacillus thuringiensis var. israelensis for the control of Aedes aegypti in Puerto Rico. J. Am. Mosq. Control Assoc. 1985, 1, 449–453. [Google Scholar] [PubMed]
- Hare, S.G.; Nasci, R.S. Effects of sublethal exposure to Bacillus thuringiensis var. israelensis on larval development and adult size in Aedes aegypti. J. Am. Mosq. Control Assoc. 1986, 2, 325–328. [Google Scholar] [PubMed]
- Chen, C.; Aldridge, R.L.; Gibson, S.; Kline, J.; Aryaprema, V.; Qualls, W.; Xue, R.D.; Boardman, L.; Linthicum, K.J.; Hahn, D.A. Developing the radiation-based sterile insect technique (SIT) for controlling Aedes aegypti: Identification of a sterilizing dose. Pest Manag. Sci. 2022, 79, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Martín-Park, A.; Che-Mendoza, A.; Contreras-Perera, Y.; Pérez-Carrillo, S.; Puerta-Guardo, H.; Villegas-Chim, J.; Guillermo-May, G.; Medina-Barreiro, A.; Delfín-González, H.; Méndez-Vales, R.; et al. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl. Trop. Dis. 2022, 16, e0010324. [Google Scholar] [CrossRef]
- Silva, E.B.; Mendonça, C.M.; Mendonça, J.A.; Dias, E.S.F.; Florêncio, S.G.L.; Guedes, D.R.D.; Paiva, M.H.S.; Amaral, A.; Netto, A.M.; Melo-Santos, M.A.V. Effects of gamma radiation on the reproductive viability of Aedes aegypti and its descendants (Diptera: Culicidae). Acta Trop. 2022, 228, 106284. [Google Scholar] [CrossRef]
- Hallinan, E.; Rai, K.S. Radiation sterilization of Aedes aegypti in nitrogen and implications for sterile male technique. Nature 1973, 244, 368–369. [Google Scholar] [CrossRef]
- Fu, G.; Lees, R.S.; Nimmo, D.; Aw, D.; Jin, L.; Gray, P.; Berendonk, T.U.; White-Cooper, H.; Scaife, S.; Kim Phuc, H.; et al. Female-specific flightless phenotype for mosquito control. Proc. Natl. Acad. Sci. USA 2010, 107, 4550–4554. [Google Scholar] [CrossRef]
- Bargielowski, I.; Nimmo, D.; Alphey, L.; Koella, J.C. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti. PLoS ONE 2011, 6, e20699. [Google Scholar] [CrossRef]
- Chen, J.; Luo, J.; Wang, Y.; Gurav, A.S.; Li, M.; Akbari, O.S.; Montell, C. Suppression of female fertility in Aedes aegypti with a CRISPR-targeted male-sterile mutation. Proc. Natl. Acad. Sci. USA 2021, 118, e2105075118. [Google Scholar] [CrossRef]
- Phuc, H.K.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Fu, G.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Aryan, A.; Anderson, M.A.; Myles, K.M.; Adelman, Z.N. TALEN-based gene disruption in the dengue vector Aedes aegypti. PLoS ONE 2013, 8, e60082. [Google Scholar] [CrossRef]
- Han, Q.; Calvo, E.; Marinotti, O.; Fang, J.; Rizzi, M.; James, A.A.; Li, J. Analysis of the wild-type and mutant genes encoding the enzyme kynurenine monooxygenase of the yellow fever mosquito, Aedes aegypti. Insect Mol. Biol. 2003, 12, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Kistler, K.E.; Vosshall, L.B.; Matthews, B.J. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015, 11, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bui, M.; Yang, T.; Bowman, C.S.; White, B.J.; Akbari, O.S. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2017, 114, E10540–E10549. [Google Scholar] [CrossRef]
- Li, M.; Yang, T.; Bui, M.; Gamez, S.; Wise, T.; Kandul, N.P.; Liu, J.; Alcantara, L.; Lee, H.; Edula, J.R.; et al. Suppressing mosquito populations with precision guided sterile males. Nat. Commun. 2021, 12, 5374. [Google Scholar] [CrossRef]
- Schairer, C.E.; Triplett, C.; Akbari, O.S.; Bloss, C.S. California Residents’ Perceptions of Gene Drive Systems to Control Mosquito-Borne Disease. Front. Bioeng. Biotechnol. 2022, 10, 848707. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.B.; Mumford, J.D.; Glandorf, D.C.M.; Hartley, S.; Lewis, O.T.; Evans, S.W.; Turner, G.; Beech, C.; Sykes, N.; Coulibaly, M.B.; et al. Recommendations for environmental risk assessment of gene drive applications for malaria vector control. Malar. J. 2022, 21, 152. [Google Scholar] [CrossRef]
- Wise, I.J.; Borry, P. An Ethical Overview of the CRISPR-Based Elimination of Anopheles gambiae to Combat Malaria. J. Bioethical Inq. 2022, 19, 371–380. [Google Scholar] [CrossRef]
- James, S.L.; Marshall, J.M.; Christophides, G.K.; Okumu, F.O.; Nolan, T. Toward the Definition of Efficacy and Safety Criteria for Advancing Gene Drive-Modified Mosquitoes to Field Testing. Vector Borne Zoonotic Dis. 2020, 20, 237–251. [Google Scholar] [CrossRef]
- Famakinde, D.O. Public health concerns over gene-drive mosquitoes: Will future use of gene-drive snails for schistosomiasis control gain increased level of community acceptance? Pathog. Glob. Health 2020, 114, 55–63. [Google Scholar] [CrossRef]
- Fauci, A.S.; Morens, D.M. Zika Virus in the Americas—Yet Another Arbovirus Threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Muller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006845. [Google Scholar] [CrossRef]
- Rose, R.I. Pesticides and public health: Integrated methods of mosquito management. Emerg. Infect. Dis. 2001, 7, 17–23. [Google Scholar] [CrossRef]
- Zulfa, R.; Lo, W.C.; Cheng, P.C.; Martini, M.; Chuang, T.W. Updating the Insecticide Resistance Status of Aedes aegypti and Aedes albopictus in Asia: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2022, 7, 306. [Google Scholar] [CrossRef]
- Kasai, S.; Itokawa, K.; Uemura, N.; Takaoka, A.; Furutani, S.; Maekawa, Y.; Kobayashi, D.; Imanishi-Kobayashi, N.; Amoa-Bosompem, M.; Murota, K.; et al. Discovery of super-insecticide-resistant dengue mosquitoes in Asia: Threats of concomitant knockdown resistance mutations. Sci. Adv. 2022, 8, eabq7345. [Google Scholar] [CrossRef]
- Rants’o, T.A.; Koekemoer, L.L.; Panayides, J.L.; van Zyl, R.L. Potential of Essential Oil-Based Anticholinesterase Insecticides against Anopheles Vectors: A Review. Molecules 2022, 27, 7026. [Google Scholar] [CrossRef]
- Yu, K.X.; Jantan, I.; Ahmad, R.; Wong, C.L. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitol. Res. 2014, 113, 3121–3141. [Google Scholar] [CrossRef]
- Zeni, V.; Baliota, G.V.; Benelli, G.; Canale, A.; Athanassiou, C.G. Diatomaceous Earth for Arthropod Pest Control: Back to the Future. Molecules 2021, 26, 7487. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Marston, A.; Maillard, M.; Hostettmann, K. Search for antifungal, molluscicidal and larvicidal compounds from African medicinal plants. J. Ethnopharmacol. 1993, 38, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, P.M.; Esquivel, C.J.; Denton, J.S. Malpighian Tubules as Novel Targets for Mosquito Control. Int. J. Environ. Res. Public Health 2017, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Swale, D.R.; Engers, D.W.; Bollinger, S.R.; Gross, A.; Inocente, E.A.; Days, E.; Kanga, F.; Johnson, R.M.; Yang, L.; Bloomquist, J.R.; et al. An insecticide resistance-breaking mosquitocide targeting inward rectifier potassium channels in vectors of Zika virus and malaria. Sci. Rep. 2016, 6, 36954. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hopkins, K.; Sabin, L.; Yasunaga, A.; Subramanian, H.; Lamborn, I.; Gordesky-Gold, B.; Cherry, S. ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc. Natl. Acad. Sci. USA 2013, 110, 15025–15030. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Bi, X.; Huang, J.; Zhou, L. The Insulin Receptor: An Important Target for the Development of Novel Medicines and Pesticides. Int. J. Mol. Sci. 2022, 23, 7793. [Google Scholar] [CrossRef] [PubMed]
- Ekoka, E.; Maharaj, S.; Nardini, L.; Dahan-Moss, Y.; Koekemoer, L.L. 20-Hydroxyecdysone (20E) signaling as a promising target for the chemical control of malaria vectors. Parasit. Vectors 2021, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Marten, A.D.; Stothard, A.I.; Kalera, K.; Swarts, B.M.; Conway, M.J. Validamycin A Delays Development and Prevents Flight in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1096–1103. [Google Scholar] [CrossRef]
- Marques, J.; Cardoso, J.C.R.; Felix, R.C.; Santana, R.A.G.; Guerra, M.; Power, D.; Silveira, H. Fresh-blood-free diet for rearing malaria mosquito vectors. Sci. Rep. 2018, 8, 17807. [Google Scholar] [CrossRef]
- da Silva Costa, G.; Rodrigues, M.M.S.; Silva, A.A.E. Toward a blood-free diet for Anopheles darlingi (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 947–951. [Google Scholar] [CrossRef]
- Gonzales, K.K.; Hansen, I.A. Artificial Diets for Mosquitoes. Int. J. Environ. Res. Public Health 2016, 13, 1267. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, K.K.; Rodriguez, S.D.; Chung, H.N.; Kowalski, M.; Vulcan, J.; Moore, E.L.; Li, Y.; Willette, S.M.; Kandel, Y.; Van Voorhies, W.A.; et al. The Effect of SkitoSnack, an Artificial Blood Meal Replacement, on Aedes aegypti Life History Traits and Gut Microbiota. Sci. Rep. 2018, 8, 11023. [Google Scholar] [CrossRef]
- Joy, T.K.; Arik, A.J.; Corby-Harris, V.; Johnson, A.A.; Riehle, M.A. The impact of larval and adult dietary restriction on lifespan, reproduction and growth in the mosquito Aedes aegypti. Exp. Gerontol. 2010, 45, 685–690. [Google Scholar] [CrossRef]
- Rocha-Santos, C.; Dutra, A.; Fróes Santos, R.; Cupolillo, C.; de Melo Rodovalho, C.; Bellinato, D.F.; Dos Santos Dias, L.; Jablonka, W.; Lima, J.B.P.; Silva Neto, M.A.C.; et al. Effect of Larval Food Availability on Adult Aedes Aegypti (Diptera: Culicidae) Fitness and Susceptibility to Zika Infection. J. Med. Entomol. 2021, 58, 535–547. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Janairo, M.S. Late-instar Behavior of Aedes aegypti (Diptera: Culicidae) Larvae in Different Thermal and Nutritive Environments. J. Med. Entomol. 2015, 52, 789–796. [Google Scholar] [CrossRef]
- Marten, A.D.; Tift, C.T.; Tree, M.O.; Bakke, J.; Conway, M.J. Chronic depletion of vertebrate lipids in Aedes aegypti. cells dysregulates lipid metabolism and inhibits innate immunity without altering dengue infectivity. PLoS Negl. Trop. Dis. 2022, 16, e0010890. [Google Scholar] [CrossRef]
- Talyuli, O.A.; Bottino-Rojas, V.; Taracena, M.L.; Soares, A.L.; Oliveira, J.H.; Oliveira, P.L. The use of a chemically defined artificial diet as a tool to study Aedes aegypti physiology. J. Insect Physiol. 2015, 83, 1–7. [Google Scholar] [CrossRef]
- Naksathit, A.T.; Scott, T.W. Effect of female size on fecundity and survivorship of Aedes aegypti fed only human blood versus human blood plus sugar. J. Am. Mosq. Control Assoc. 1998, 14, 148–152. [Google Scholar]
- Mostowy, W.M.; Foster, W.A. Antagonistic effects of energy status on meal size and egg-batch size of Aedes aegypti (Diptera: Culicidae). J. Vector Ecol. 2004, 29, 84–93. [Google Scholar]
- Canyon, D.V.; Hii, J.L.; Muller, R. Effect of diet on biting, oviposition, and survival of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1999, 36, 301–308. [Google Scholar] [CrossRef]
- Khan, A.A.; Maibach, H.I. A study of the probing response of Aedes aegypti. 1. Effect of nutrition on probing. J. Econ. Entomol. 1970, 63, 974–976. [Google Scholar] [CrossRef]
- Kessler, S.; Vlimant, M.; Guerin, P.M. Sugar-sensitive neurone responses and sugar feeding preferences influence lifespan and biting behaviours of the Afrotropical malaria mosquito, Anopheles gambiae. J. Comp. Physiol. Neuroethol. Sens. Neural Behav. Physiol. 2015, 201, 317–329. [Google Scholar] [CrossRef]
- Duvall, L.B.; Ramos-Espiritu, L.; Barsoum, K.E.; Glickman, J.F.; Vosshall, L.B. Small-Molecule Agonists of Ae. aegypti Neuropeptide Y Receptor Block Mosquito Biting. Cell 2019, 176, 687–701.e685. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.J.; Brown, E.S.; Sharma, D.; Nguyen, Q.; Spangler, A.A.; Pathak, A.; Payton, B.; Warden, M.; Shah, A.J.; Shaw, S.; et al. Bloodmeal regulation in mosquitoes curtails dehydration-induced mortality, altering vectorial capacity. J. Insect Physiol. 2022, 137, 104363. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Kongmee, M.; Bangs, M.J.; Sathantriphop, S.; Meunworn, V.; Parbaripai, A.; Suwonkerd, W.; Akratanakul, P. Influence of nutritional and physiological status on behavioral responses of Aedes aegypti (Diptera: Culicidae) to deltamethrin and cypermethrin. J. Vector Ecol. 2006, 31, 89–101. [Google Scholar] [CrossRef]
- Norris, E.J.; Bloomquist, J.R. Nutritional status significantly affects toxicological endpoints in the CDC bottle bioassay. Pest Manag. Sci. 2022, 78, 743–748. [Google Scholar] [CrossRef]
- Huang, Y.S.; Lyons, A.C.; Hsu, W.W.; Park, S.L.; Higgs, S.; Vanlandingham, D.L. Differential outcomes of Zika virus infection in Aedes aegypti orally challenged with infectious blood meals and infectious protein meals. PLoS ONE 2017, 12, e0182386. [Google Scholar] [CrossRef]
- Almire, F.; Terhzaz, S.; Terry, S.; McFarlane, M.; Gestuveo, R.J.; Szemiel, A.M.; Varjak, M.; McDonald, A.; Kohl, A.; Pondeville, E. Sugar feeding protects against arboviral infection by enhancing gut immunity in the mosquito vector Aedes aegypti. PLoS Pathog. 2021, 17, e1009870. [Google Scholar] [CrossRef]
- Weng, S.C.; Tsao, P.N.; Shiao, S.H. Blood glucose promotes dengue virus infection in the mosquito Aedes aegypti. Parasit. Vectors 2021, 14, 376. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
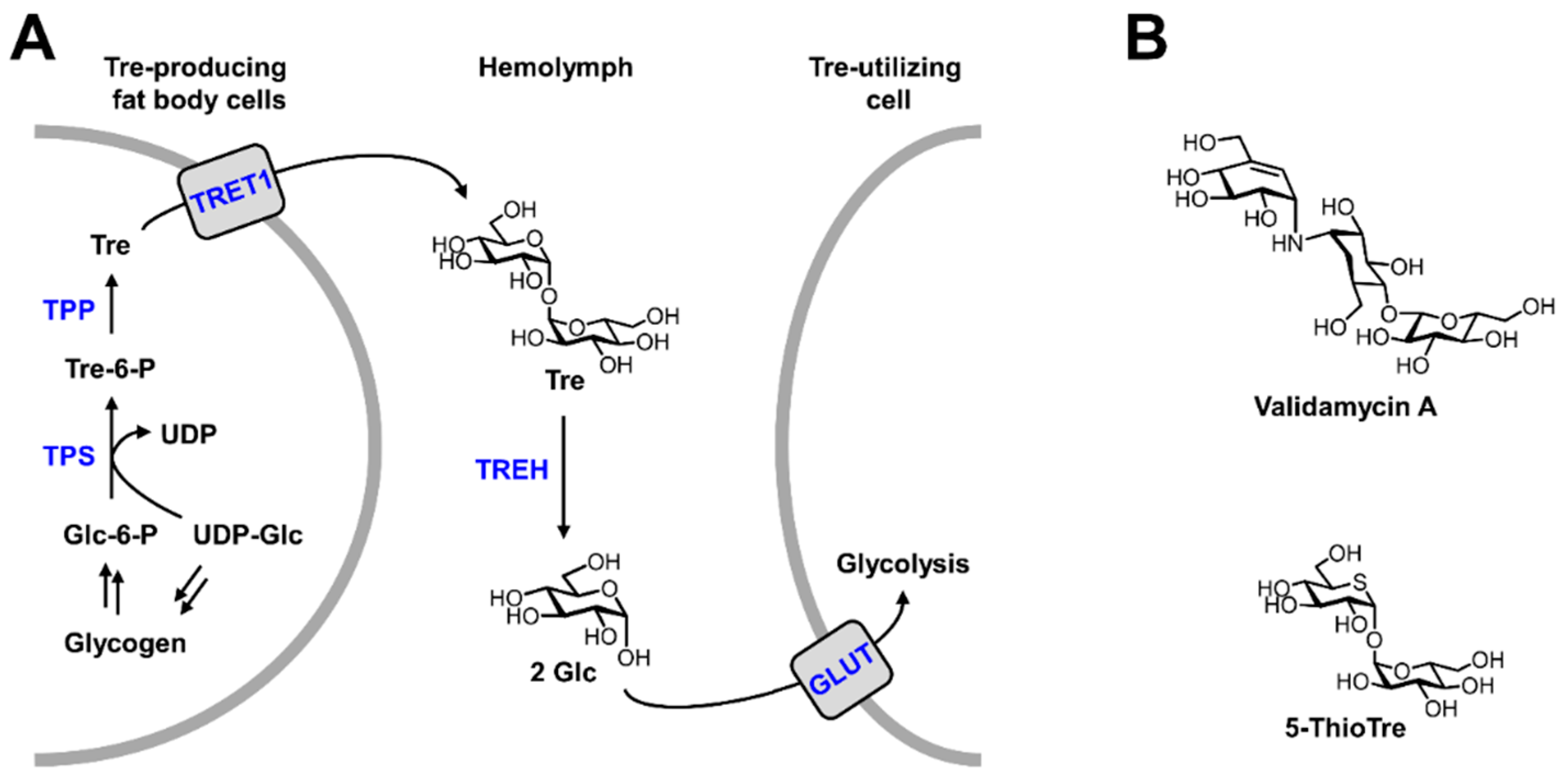
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef]
- Alabaster, A.; Isoe, J.; Zhou, G.; Lee, A.; Murphy, A.; Day, W.A.; Miesfeld, R.L. Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochem. Mol. Biol. 2011, 41, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Santos, L.V.; Caiado, M.S.; Hastenreiter, L.S.N.; Fonseca, S.R.R.; Carbajal-de-la-Fuente, A.L.; Carvalho, M.G.; Pontes, E.G. The influence of larval density on triacylglycerol content in Aedes aegypti (Linnaeus) (Diptera: Culicidae). Arch. Insect Biochem. Physiol. 2021, 106, e21757. [Google Scholar] [CrossRef] [PubMed]
- Tose, L.V.; Weisbrod, C.R.; Michalkova, V.; Nouzova, M.; Noriega, F.G.; Fernandez-Lima, F. Following de novo triglyceride dynamics in ovaries of Aedes aegypti during the previtellogenic stage. Sci. Rep. 2021, 11, 9636. [Google Scholar] [CrossRef]
- Mensch, J.; Di Battista, C.; De Majo, M.S.; Campos, R.E.; Fischer, S. Increased size and energy reserves in diapausing eggs of temperate Aedes aegypti populations. J. Insect Physiol. 2021, 131, 104232. [Google Scholar] [CrossRef]
- Dou, X.; Chen, K.; Brown, M.R.; Strand, M.R. Multiple endocrine factors regulate nutrient mobilization and storage in Aedes aegypti during a gonadotrophic cycle. Insect Sci. 2022. Online Version of Record. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Saha, T.T.; Pei, G.; Raikhel, A.S.; Zou, Z. Hormone and receptor interplay in the regulation of mosquito lipid metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, E2709–E2718. [Google Scholar] [CrossRef]
- Morou, E.; Lirakis, M.; Pavlidi, N.; Zotti, M.; Nakagawa, Y.; Smagghe, G.; Vontas, J.; Swevers, L. A new dibenzoylhydrazine with insecticidal activity against Anopheles mosquito larvae. Pest Manag. Sci. 2013, 69, 827–833. [Google Scholar] [CrossRef]
- Childs, L.M.; Cai, F.Y.; Kakani, E.G.; Mitchell, S.N.; Paton, D.; Gabrieli, P.; Buckee, C.O.; Catteruccia, F. Disrupting Mosquito Reproduction and Parasite Development for Malaria Control. PLoS Pathog. 2016, 12, e1006060. [Google Scholar] [CrossRef]
- Price, D.P.; Schilkey, F.D.; Ulanov, A.; Hansen, I.A. Small mosquitoes, large implications: Crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasit. Vectors 2015, 8, 252. [Google Scholar] [CrossRef]
- Lyu, X.Y.; Wang, X.L.; Geng, D.Q.; Jiang, H.; Zou, Z. Juvenile hormone acts on male accessory gland function via regulating l-asparaginase expression and triacylglycerol mobilization in Aedes aegypti. Insect Sci. 2022. Online Version of Record. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.L.; Saha, T.T.; Roy, S.; Zhao, B.; Raikhel, A.S.; Zou, Z. Temporal Coordination of Carbohydrate Metabolism during Mosquito Reproduction. PLoS Genet. 2015, 11, e1005309. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.R.; Kale, G.F. The chemistry of insect hemolymph. II. Trehalose and other carbohydrates. J. Gen. Physiol. 1957, 40, 833–847. [Google Scholar] [CrossRef]
- Van Handel, E. The obese mosquito. J. Physiol. 1965, 181, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.A.; Wyatt, G.R. Enzymatic regulation of trehalose and glycogen synthesis in the fat body of an insect. Nature 1964, 202, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- da Rocha Fernandes, M.; Martins, R.; Pessoa Costa, E.; Pacidônio, E.C.; Araujo de Abreu, L.; da Silva Vaz, I., Jr.; Moreira, L.A.; da Fonseca, R.N.; Logullo, C. The modulation of the symbiont/host interaction between Wolbachia pipientis and Aedes fluviatilis embryos by glycogen metabolism. PLoS ONE 2014, 9, e98966. [Google Scholar] [CrossRef]
- Vital, W.; Rezende, G.L.; Abreu, L.; Moraes, J.; Lemos, F.J.; Vaz Ida, S., Jr.; Logullo, C. Germ band retraction as a landmark in glucose metabolism during Aedes aegypti embryogenesis. BMC Dev. Biol. 2010, 10, 25. [Google Scholar] [CrossRef]
- Briegel, H.; Gut, T.; Lea, A.O. Sequential deposition of yolk components during oogenesis in an insect, Aedes aegypti (Diptera: Culicidae). J. Insect Physiol. 2003, 49, 249–260. [Google Scholar] [CrossRef]
- Naksathit, A.T.; Edman, J.D.; Scott, T.W. Partitioning of glycogen, lipid, and sugar in ovaries and body remnants of female Aedes aegypti (Diptera: Culicidae) fed human blood. J. Med. Entomol. 1999, 36, 18–22. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Becker, A.; Schloder, P.; Steele, J.E.; Wegener, G. The regulation of trehalose metabolism in insects. Experientia 1996, 52, 433–439. [Google Scholar] [CrossRef]
- Katagiri, N.; Ando, O.; Yamashita, O. Reduction of glycogen in eggs of the silkworm, Bombyx mori, by use of a trehalase inhibitor, trehazolin, and diapause induction in glycogen-reduced eggs. J. Insect Physiol. 1998, 44, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Liebl, M.; Nelius, V.; Kamp, G.; Ando, O.; Wegener, G. Fate and effects of the trehalase inhibitor trehazolin in the migratory locust (Locusta migratoria). J. Insect Physiol. 2010, 56, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Tatun, N.; Wangsantitham, O.; Tungjitwitayakul, J.; Sakurai, S. Trehalase activity in fungus-growing termite, Odontotermes feae (Isoptera: Termitideae) and inhibitory effect of validamycin. J. Econ. Entomol. 2014, 107, 1224–1232. [Google Scholar] [CrossRef]
- Thorat, L.J.; Gaikwad, S.M.; Nath, B.B. Trehalose as an indicator of desiccation stress in Drosophila melanogaster larvae: A potential marker of anhydrobiosis. Biochem. Biophys. Res. Commun. 2012, 419, 638–642. [Google Scholar] [CrossRef]
- Wegener, G.; Macho, C.; Schloder, P.; Kamp, G.; Ando, O. Long-term effects of the trehalase inhibitor trehazolin on trehalase activity in locust flight muscle. J. Exp. Biol. 2010, 213, 3852–3857. [Google Scholar] [CrossRef]
- Wegener, G.; Tschiedel, V.; Schloder, P.; Ando, O. The toxic and lethal effects of the trehalase inhibitor trehazolin in locusts are caused by hypoglycaemia. J. Exp. Biol. 2003, 206, 1233–1240. [Google Scholar] [CrossRef]
- Wolber, J.M.; Urbanek, B.L.; Meints, L.M.; Piligian, B.F.; Lopez-Casillas, I.C.; Zochowski, K.M.; Woodruff, P.J.; Swarts, B.M. The trehalose-specific transporter LpqY-SugABC is required for antimicrobial and anti-biofilm activity of trehalose analogues in Mycobacterium smegmatis. Carbohydr. Res. 2017, 450, 60–66. [Google Scholar] [CrossRef]
- Xia, Y.; Clarkson, J.M.; Charnley, A.K. Trehalose-hydrolysing enzymes of Metarhizium anisopliae and their role in pathogenesis of the tobacco hornworm, Manduca sexta. J. Invertebr. Pathol. 2002, 80, 139–147. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, L.Y.; Yang, H.L.; Wang, H.J.; Zhou, M.; Wang, S.G.; Tang, B. Study on the Effect of Wing Bud Chitin Metabolism and Its Developmental Network Genes in the Brown Planthopper, Nilaparvata lugens, by Knockdown of TRE Gene. Front. Physiol. 2017, 8, 750. [Google Scholar] [CrossRef]
- García, M.D.; Argüelles, J.C. Trehalase inhibition by validamycin A may be a promising target to design new fungicides and insecticides. Pest Manag. Sci. 2021, 77, 3832–3835. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Yao, Q.; Zhang, J.; Dong, X.; Tian, H.; Zhang, W. Feeding-based RNA interference of a trehalose phosphate synthase gene in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2010, 19, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, B.; Chen, H.; Yao, Q.; Huang, X.; Zhang, D.; Zhang, W. Different functions of the insect soluble and membrane-bound trehalase genes in chitin biosynthesis revealed by RNA interference. PLoS ONE 2010, 5, e10133. [Google Scholar] [CrossRef]
- Kalera, K.; Stothard, A.I.; Woodruff, P.J.; Swarts, B.M. The role of chemoenzymatic synthesis in advancing trehalose analogues as tools for combatting bacterial pathogens. Chem. Commun. 2020, 56, 11528–11547. [Google Scholar] [CrossRef]
- Vaidyanathan, R.; Fleisher, A.E.; Minnick, S.L.; Simmons, K.A.; Scott, T.W. Nutritional stress affects mosquito survival and vector competence for West Nile virus. Vector Borne Zoonotic Dis. 2008, 8, 727–732. [Google Scholar] [CrossRef]
- Oringanje, C.; Delacruz, L.R.; Han, Y.; Luckhart, S.; Riehle, M.A. Overexpression of Activated AMPK in the Anopheles stephensi Midgut Impacts Mosquito Metabolism, Reproduction and Plasmodium Resistance. Genes 2021, 12, 119. [Google Scholar] [CrossRef]
- Liu, K.; Dong, Y.; Huang, Y.; Rasgon, J.L.; Agre, P. Impact of trehalose transporter knockdown on Anopheles gambiae stress adaptation and susceptibility to Plasmodium falciparum infection. Proc. Natl. Acad. Sci. USA 2013, 110, 17504–17509. [Google Scholar] [CrossRef]
- Somerville, A.G.T.; Gleave, K.; Jones, C.M.; Reimer, L.J. The consequences of Brugia malayi infection on the flight and energy resources of Aedes aegypti mosquitoes. Sci. Rep. 2019, 9, 18449. [Google Scholar] [CrossRef]
- Li, M.J.; Lan, C.J.; Gao, H.T.; Xing, D.; Gu, Z.Y.; Su, D.; Zhao, T.Y.; Yang, H.Y.; Li, C.X. Transcriptome analysis of Aedes aegypti Aag2 cells in response to dengue virus-2 infection. Parasit. Vectors 2020, 13, 421. [Google Scholar] [CrossRef]
- Lan, Q.; Massey, R.J. Subcellular localization of the mosquito sterol carrier protein-2 and sterol carrier protein-x. J. Lipid Res. 2004, 45, 1468–1474. [Google Scholar] [CrossRef]
- Fu, Q.; Inankur, B.; Yin, J.; Striker, R.; Lan, Q. Sterol Carrier Protein 2, a Critical Host Factor for Dengue Virus Infection, Alters the Cholesterol Distribution in Mosquito Aag2 Cells. J. Med. Entomol. 2015, 52, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.B.; Navarro, D.M.; da Silva, A.G.; Santos, G.K.; Dutra, K.A.; Moreira, D.R.; Ramos, M.N.; Espíndola, J.W.; de Oliveira, A.D.; Brondani, D.J.; et al. Thiosemicarbazones as Aedes aegypti larvicidal. Eur. J. Med. Chem. 2015, 100, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Poirier, E.Z.; Passoni, G.; Simon-Loriere, E.; Cesaro, T.; Prot, M.; Stapleford, K.A.; Moratorio, G.; Sakuntabhai, A.; Levraud, J.P.; et al. Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016, 20, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Danielson, N.D.; Collins, J.; Stothard, A.I.; Dong, Q.Q.; Kalera, K.; Woodruff, P.J.; DeBosch, B.J.; Britton, R.A.; Swarts, B.M. Degradation-resistant trehalose analogues block utilization of trehalose by hypervirulent Clostridioides difficile. Chem. Commun. 2019, 55, 5009–5012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conway, M.J.; Haslitt, D.P.; Swarts, B.M. Targeting Aedes aegypti Metabolism with Next-Generation Insecticides. Viruses 2023, 15, 469. https://doi.org/10.3390/v15020469
Conway MJ, Haslitt DP, Swarts BM. Targeting Aedes aegypti Metabolism with Next-Generation Insecticides. Viruses. 2023; 15(2):469. https://doi.org/10.3390/v15020469
Chicago/Turabian StyleConway, Michael J., Douglas P. Haslitt, and Benjamin M. Swarts. 2023. "Targeting Aedes aegypti Metabolism with Next-Generation Insecticides" Viruses 15, no. 2: 469. https://doi.org/10.3390/v15020469




