4-Phenyl-butyric Acid Inhibits Japanese Encephalitis Virus Replication via Inhibiting Endoplasmic Reticulum Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus, Cell and Animal
2.2. Infection and Treatment
2.3. Plasmid Construction and Transfection
2.4. RNA Extraction and Quantitative Real-Time PCR
2.5. Immunoblots Analysis
2.6. In Vivo Experiment in Mice
2.7. Laser Confocal Microscope
2.8. Statistical Analysis
3. Results
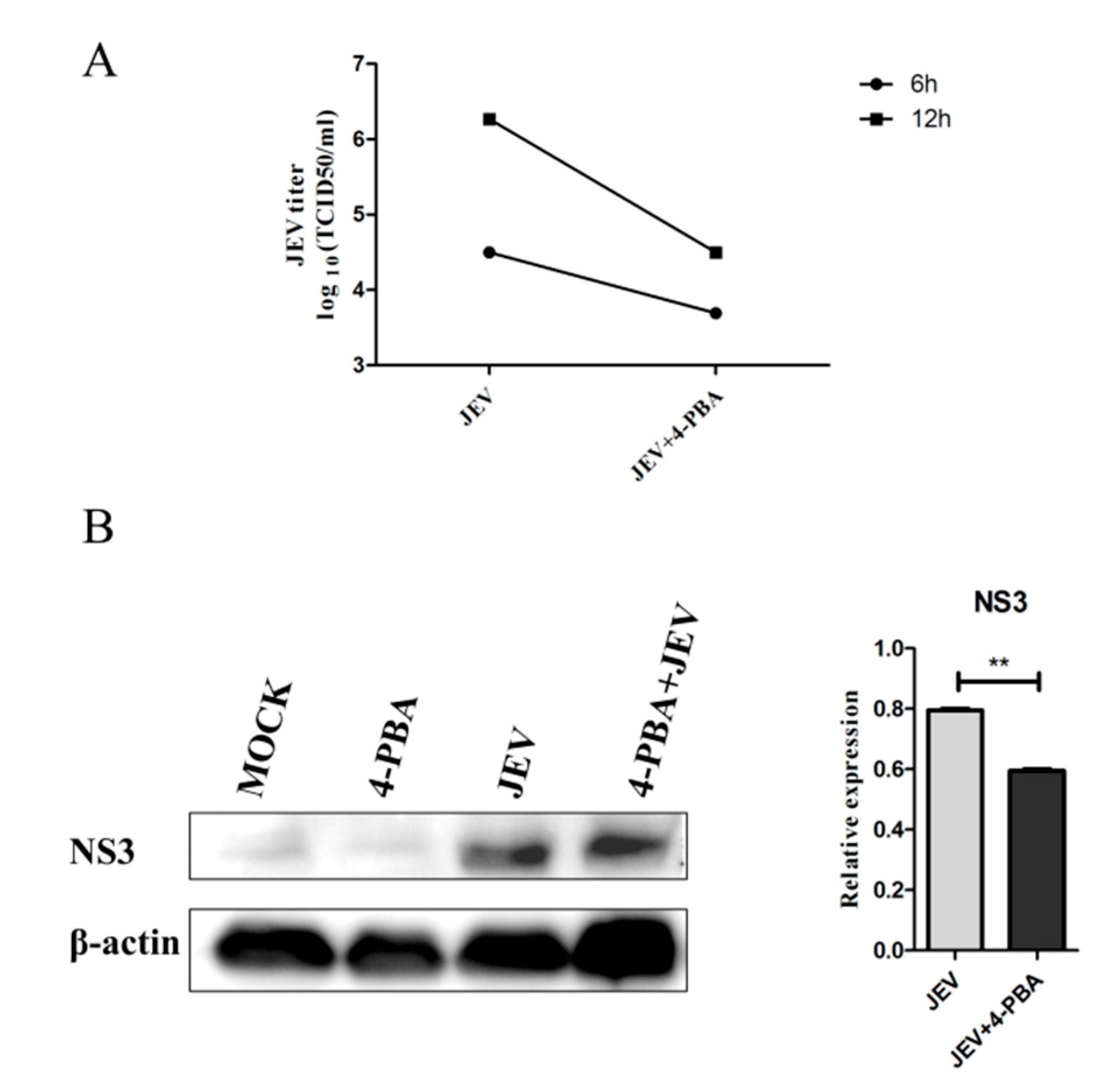
3.1. 4-PBA Inhibits the Replication of JEV In Vitro
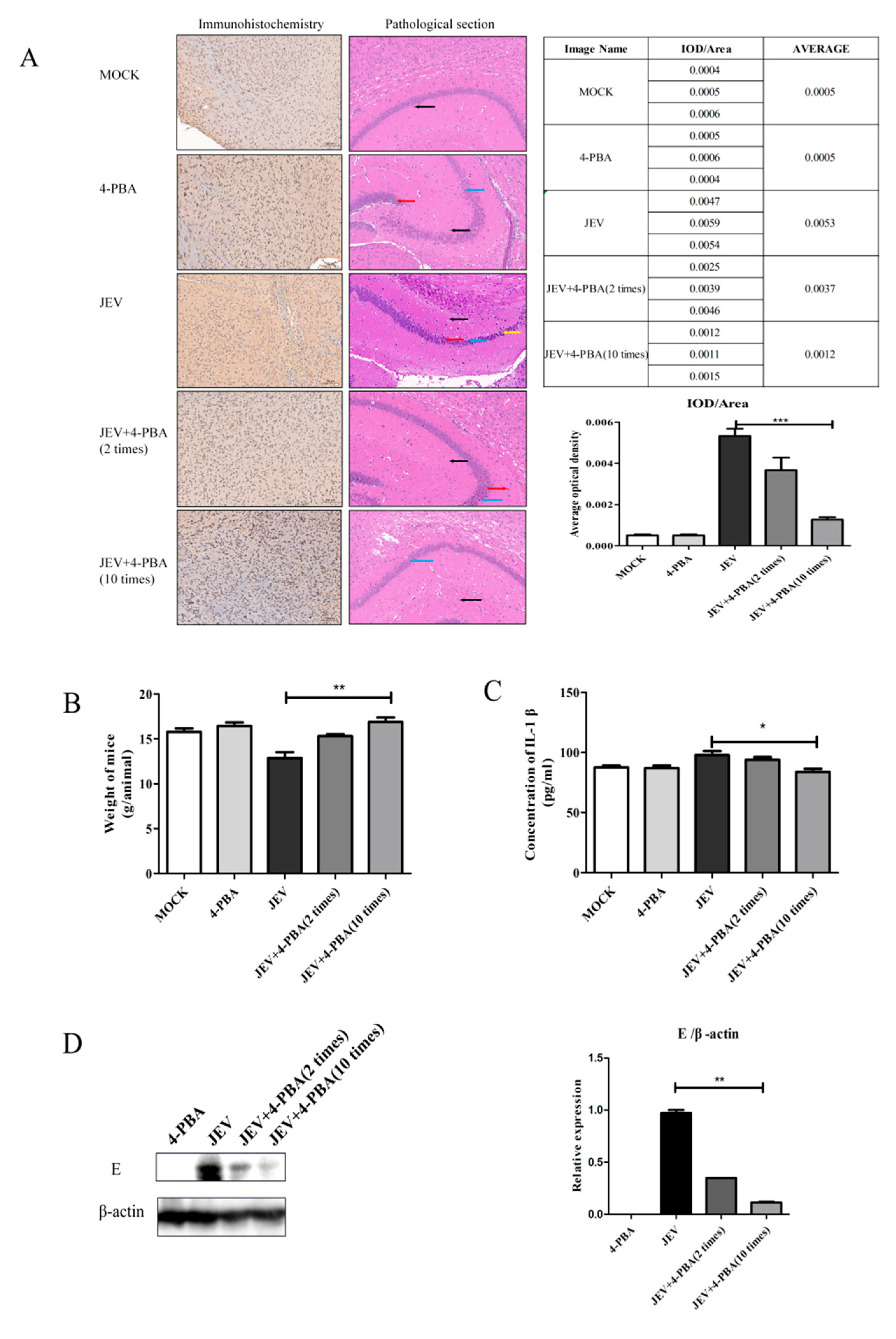
3.2. 4-PBA Inhibits the Replication of JEV In Vivo
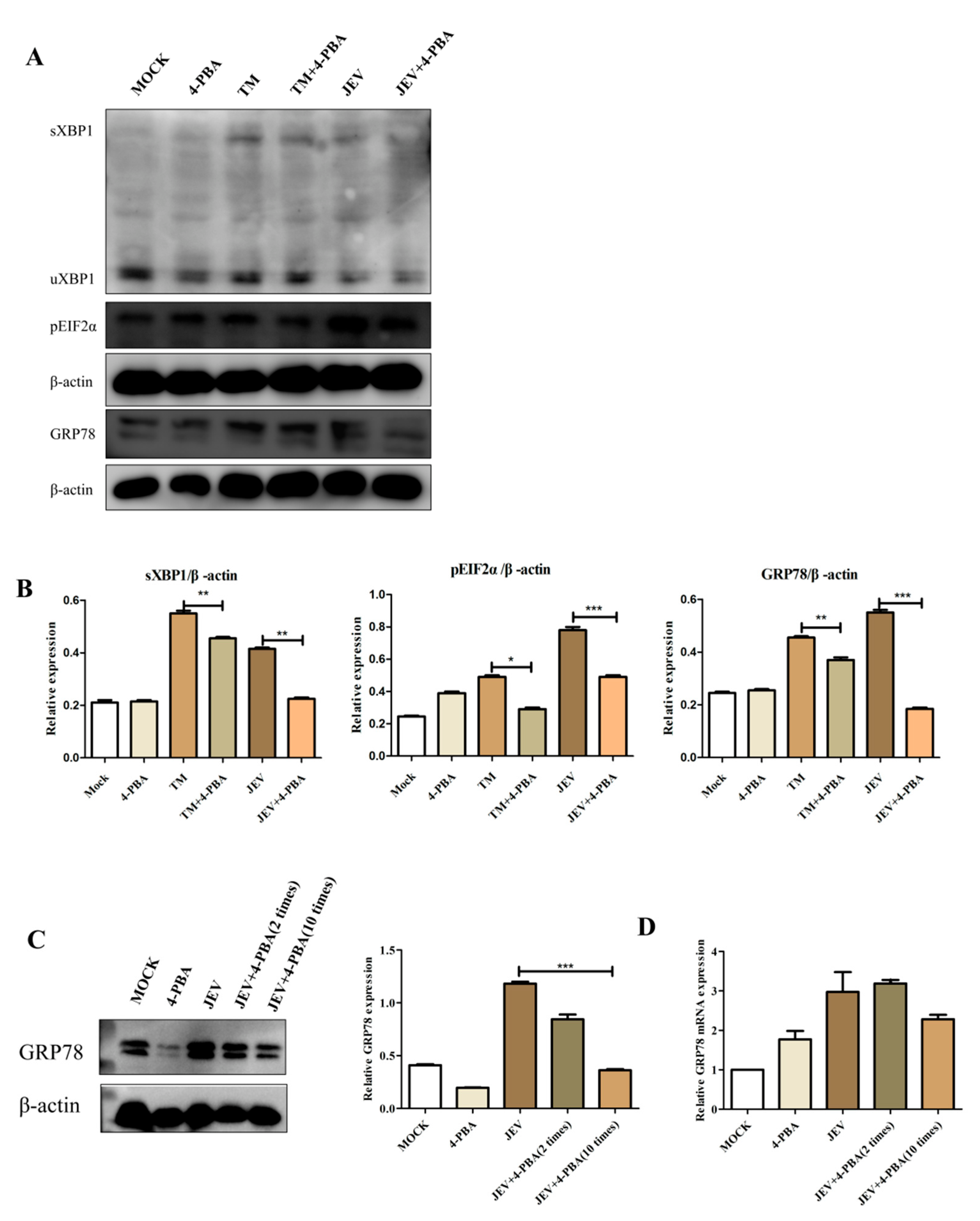
3.3. 4-PBA Inhibits JEV-Induced Activation of the IRE1 and PERK Pathways
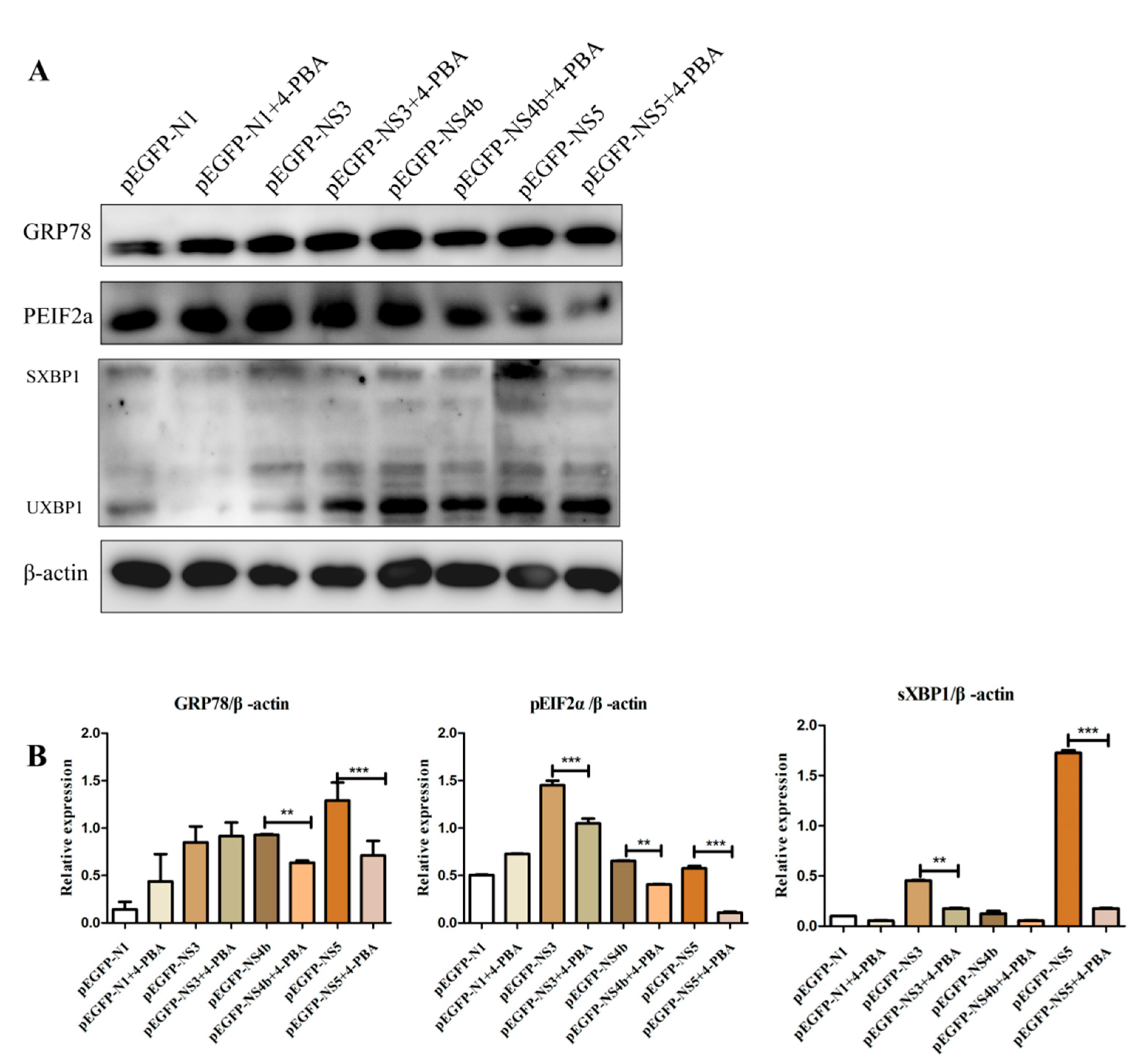
3.4. 4-PBA Inhibits the Activation of ERS Induced by JEV Non-structural Proteins
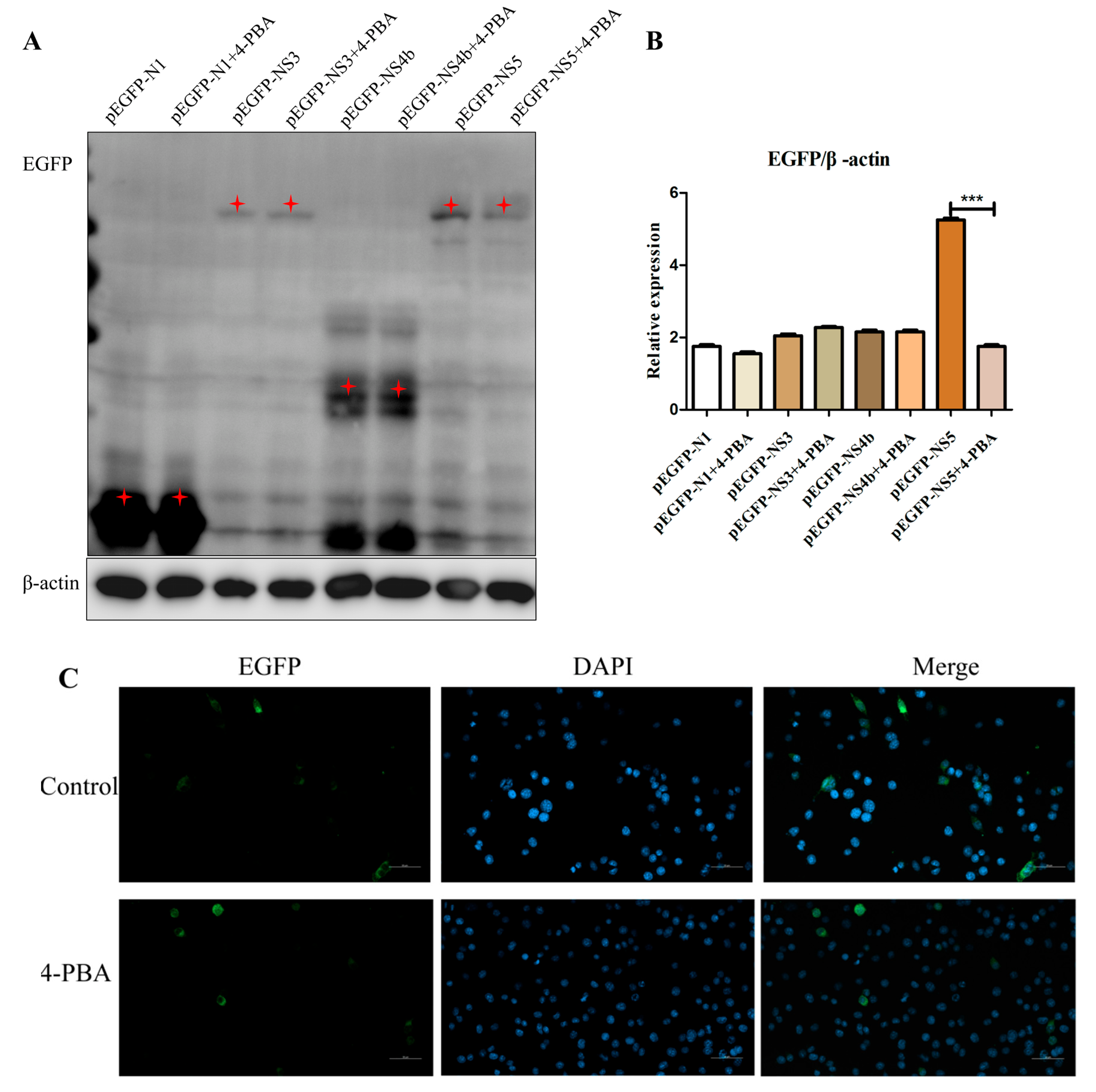
3.5. 4-PBA Inhibits NS5 Expression In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothan, H.A.; Kumar, M. Role of Endoplasmic Reticulum-Associated Proteins in Flavivirus Replication and Assembly Complexes. Pathogens 2019, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Suzuki, T.; Kusakabe, S.; Tokunaga, M.; Hirano, J.; Miyata, Y.; Matsuura, Y. Regulation of Apoptosis during Flavivirus Infection. Viruses 2017, 9, 243. [Google Scholar] [CrossRef] [PubMed]
- Jheng, J.R.; Ho, J.Y.; Horng, J.T. ER stress, autophagy, and RNA viruses. Front. Microbiol. 2014, 5, 388. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Hsu, Y.-W.; Liao, C.-L.; Lin, Y.-L. Flavivirus Infection Activates the XBP1 Pathway of the Unfolded Protein Response to Cope with Endoplasmic Reticulum Stress. J. Virol. 2006, 80, 11868–11880. [Google Scholar] [CrossRef]
- Zeng, M.; Sang, W.; Chen, S.; Chen, R.; Zhang, H.; Xue, F.; Li, Z.; Liu, Y.; Gong, Y.; Zhang, H.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, T.; Palmieri, B. Clinical and Experimental Applications of Sodium Phenylbutyrate. Drugs RD 2011, 11, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Bhat, A.; Chakraborty, R.; Adlakha, K.; Sengupta, S.; Roy, S.; Chakraborty, K. Proteomic profile of 4-PBA treated human neuronal cells during ER stress. Mol. Omics 2018, 14, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.-F.; Gaplovska-Kysela, K.; Zuber, C.; Roth, J. Sodium 4-Phenylbutyrate Acts as a Chemical Chaperone on Misfolded Myocilin to Rescue Cells from Endoplasmic Reticulum Stress and Apoptosis. Investig. Opthalmology Vis. Sci. 2007, 48, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Sorrenson, B.; Suetani, R.J.; Williams, M.; Bickley, V.M.; George, P.M.; Jones, G.T.; McCormick, S.P.A. Functional rescue of mutant ABCA1 proteins by sodium 4-phenylbutyrate. J. Lipid Res. 2013, 54, 55–62. [Google Scholar] [CrossRef]
- Pao, H.-P.; Liao, W.-I.; Tang, S.-E.; Wu, S.-Y.; Huang, K.-L.; Chu, S.-J. Suppression of Endoplasmic Reticulum Stress by 4-PBA Protects Against Hyperoxia-Induced Acute Lung Injury via Up-Regulating Claudin-4 Expression. Front. Immunol. 2021, 12, 674316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, W.; Niu, Q.; Sun, Y.; Meng, C.; Tan, L.; Song, C.; Qiu, X.; Liao, Y.; Ding, C. eIF2α-CHOP-BCl-2/JNK and IRE1α-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell Death Dis. 2019, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Liao, Y.-Q.; Li, L.; Sun, L.-Y.; Ding, N.-S.; Wu, Y.-L.; Lu, K.-L. 4-PBA Attenuates Fat Accumulation in Cultured Spotted Seabass Fed High-Fat-Diet via Regulating Endoplasmic Reticulum Stress. Metabolites 2022, 12, 1197. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Bhardwaj, A.; Dhawan, D.K.; Tandon, C.; Kaur, T. 4-PBA rescues hyperoxaluria induced nephrolithiasis by modulating urinary glycoproteins: Cross talk between endoplasmic reticulum, calcium homeostasis and mitochondria. Life Sci. 2022, 305, 120786. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Ta, N.; Liu, L.; Shi, G.; Kang, T.; Zheng, Z. Activation of CaMKII via ER-stress mediates coxsackievirus B3-induced cardiomyocyte apoptosis. Cell Biol. Int. 2019, 44, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, R.; Geng, S.; Shan, Y.; Li, X.; Fang, W. Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium. J. Virol. 2019, 93, e01784-18. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Jones, S.; Daniel, Z.C.T.R.; Brearley, M.C.; Lewis, J.E.; Ebling, F.J.P.; Parr, T.; Brameld, J.M. Effect of sodium 4-phenylbutyrate on Clenbuterol-mediated muscle growth. PLoS ONE 2018, 13, e0201481. [Google Scholar] [CrossRef]
- Bonnemaison, M.L.; Marks-Nelson, E.S.; Boesen, E.I. Sodium 4-phenylbutyrate treatment protects against renal injury in NZBWF1 mice. Clin. Sci. 2019, 133, 167–180. [Google Scholar] [CrossRef]
- Mizukami, T.; Orihashi, K.; Herlambang, B.; Takahashi, S.; Hamaishi, M.; Okada, K.; Sueda, T. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J. Vasc. Surg. 2010, 52, 1580–1586. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.-S.; Jeong, S.; Kim, D.; Chon, S.; Pak, Y.; Kim, S.; Ha, J.; Kang, I.; Choe, W. A Small Molecule, 4-Phenylbutyric Acid, Suppresses HCV Replication via Epigenetically Induced Hepatic Hepcidin. Int. J. Mol. Sci. 2020, 21, 5516. [Google Scholar] [CrossRef]
- Jung, K.I.; Ko, D.-H.; Shin, N.; Pyo, C.W.; Choi, S.-Y. Endoplasmic reticulum-associated degradation potentiates the infectivity of influenza A virus by regulating the host redox state. Free. Radic. Biol. Med. 2019, 135, 293–305. [Google Scholar] [CrossRef]
- Wang, X.; Hou, L.; Du, J.; Ge, X.; Guo, X.; Yang, H. Capsid, membrane and NS3 are the major viral proteins involved in autophagy induced by Japanese encephalitis virus. Vet. Microbiol. 2015, 178, 217–229. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Picazo, E.; Giordanetto, F. Small molecule inhibitors of ebola virus infection. Drug Discov. Today 2015, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Rothan, H.A.; Zhong, Y.; Yan, W.; Henderson, M.J.; Chen, F.; Fang, S. A small molecule inhibitor of ER-to-cytosol protein dislocation exhibits anti-dengue and anti-Zika virus activity. Sci. Rep. 2019, 9, 10901. [Google Scholar] [CrossRef]
- Rothan, H.A.; Zhong, Y.; Sanborn, M.A.; Teoh, T.C.; Ruan, J.; Yusof, R.; Hang, J.; Henderson, M.J.; Fang, S. Small molecule grp94 inhibitors block dengue and Zika virus replication. Antivir. Res. 2019, 171, 104590. [Google Scholar] [CrossRef] [PubMed]
- Frouco, G.; Freitas, F.B.; Martins, C.; Ferreira, F. Sodium phenylbutyrate abrogates African swine fever virus replication by disrupting the virus-induced hypoacetylation status of histone H3K9/K14. Virus Res. 2017, 242, 24–29. [Google Scholar] [CrossRef]
- Mufrrih, M.; Chen, B.; Chan, S.W. Zika Virus Induces an Atypical Tripartite Unfolded Protein Response with Sustained Sensor and Transient Effector Activation and a Blunted BiP Response. mSphere 2021, 6, e0036121. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; He, J.J. HIV-1 Tat Induces Unfolded Protein Response and Endoplasmic Reticulum Stress in Astrocytes and Causes Neurotoxicity through Glial Fibrillary Acidic Protein (GFAP) Activation and Aggregation. J. Biol. Chem. 2016, 291, 22819–22829. [Google Scholar] [CrossRef]
- Gething, M.-J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Dash, S.; Chava, S.; Aydin, Y.; Chandra, P.K.; Ferraris, P.; Chen, W.; Balart, L.A.; Wu, T.; Garry, R.F. Hepatitis C Virus Infection Induces Autophagy as a Prosurvival Mechanism to Alleviate Hepatic ER-Stress Response. Viruses 2016, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Kuo, S.-H.; Lin, C.-Y.; Fu, P.-J.; Lin, Y.-S.; Yeh, T.-M.; Liu, H.-S. Dengue virus-induced ER stress is required for autophagy activation, viral replication, and pathogenesis both in vitro and in vivo. Sci. Rep. 2018, 8, 489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Bhattacharyya, S.; Sharma, K.B.; Chauhan, S.; Asthana, S.; Abdin, M.Z.; Vrati, S.; Kalia, M. Japanese encephalitis virus activates autophagy through XBP1 and ATF6 ER stress sensors in neuronal cells. J. Gen. Virol. 2017, 98, 1027–1039. [Google Scholar] [CrossRef]
- Nain, M.; Mukherjee, S.; Karmakar, S.P.; Paton, A.W.; Paton, J.C.; Abdin, M.Z.; Basu, A.; Kalia, M.; Vrati, S. GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells. J. Virol. 2017, 91, e02274-16. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Wu, J.-L.; Hong, J.-R. Betanodavirus up-regulates chaperone GRP78 via ER stress: Roles of GRP78 in viral replication and host mitochondria-mediated cell death. Apoptosis 2010, 16, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Viktorovskaya, O.V.; Greco, T.M.; Cristea, I.M.; Thompson, S.R. Identification of RNA Binding Proteins Associated with Dengue Virus RNA in Infected Cells Reveals Temporally Distinct Host Factor Requirements. PLOS Negl. Trop. Dis. 2016, 10, e0004921. [Google Scholar] [CrossRef]
- Mairiang, D.; Zhang, H.; Sodja, A.; Murali, T.; Suriyaphol, P.; Malasit, P.; Limjindaporn, T.; Finley, L.R., Jr. Identification of New Protein Interactions between Dengue Fever Virus and Its Hosts, Human and Mosquito. PLoS ONE 2013, 8, e53535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xin, X.; Wang, T.; Wan, J.; Ou, Y.; Yang, Z.; Yu, Q.; Zhu, L.; Guo, Y.; Wu, Y.; et al. Japanese Encephalitis Virus Induces Apoptosis and Encephalitis by Activating the PERK Pathway. J. Virol. 2019, 93, e00887-19. [Google Scholar] [CrossRef]
- Zou, D.; Xu, J.; Duan, X.; Xu, X.; Li, P.; Cheng, L.; Zheng, L.; Li, X.; Zhang, Y.; Wang, X.; et al. Porcine epidemic diarrhea virus ORF3 protein causes endoplasmic reticulum stress to facilitate autophagy. Vet. Microbiol. 2019, 235, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Whitby, K.; Pierson, T.C.; Geiss, B.; Lane, K.; Engle, M.; Zhou, Y.; Doms, R.W.; Diamond, M.S. Castanospermine, a Potent Inhibitor of Dengue Virus Infection In Vitro and In Vivo. J. Virol. 2005, 79, 8698–8706. [Google Scholar] [CrossRef] [Green Version]
- Courageot, M.P.; Frenkiel, M.P.; Dos Santos, C.D.; Deubel, V.; Desprès, P. Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J. Virol. 2000, 74, 564–572. [Google Scholar] [CrossRef]
- Takhampunya, R.; Padmanabhan, R.; Ubol, S. Antiviral action of nitric oxide on dengue virus type 2 replication. J. Gen. Virol. 2006, 87, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, R.C.; Steel, J.J.; Moon, S.; Soltani, E.; Geiss, B.J. Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology 2015, 475, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Wang, C.; Chan, K.; Vasudevan, S.; Jans, D. Novel dengue virus inhibitor 4-HPR activates ATF4 independent of protein kinase R–like Endoplasmic Reticulum Kinase and elevates levels of eIF2α phosphorylation in virus infected cells. Antivir. Res. 2016, 130, 1–6. [Google Scholar] [CrossRef]
- Oraby, A.K.; Gardner, C.L.; Needle, R.F.; Kofahi, H.M.; Everard, K.R.; Taylor, N.G.A.; Rutihinda, S.G.; Barry, J.P.; Hirasawa, K.; Georghiou, P.E.; et al. A Novel Small Molecule Inhibits Hepatitis C Virus Propagation in Cell Culture. Microbiol. Spectr. 2021, 9, e0043921. [Google Scholar] [CrossRef]
- Almasy, K.M.; Davies, J.P.; Lisy, S.M.; Tirgar, R.; Tran, S.C.; Plate, L. Small-molecule endoplasmic reticulum proteostasis regulator acts as a broad-spectrum inhibitor of dengue and Zika virus infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2012209118. [Google Scholar] [CrossRef]
- Lam, K.H.K.; Ellis, T.M.; Williams, D.T.; Lunt, R.A.; Daniels, P.W.; Watkins, K.L.; Riggs, C.M. Japanese encephalitis in a racing thoroughbred gelding in Hong Kong. Vet. Rec. 2005, 157, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Park, S.L.; Huang, Y.-J.S.; Vanlandingham, D.L. Re-Examining the Importance of Pigs in the Transmission of Japanese Encephalitis Virus. Pathogens 2022, 11, 575. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Production |
|---|---|---|
| NS3 | F:CGGCTAGCGCCACCATGGGGGGCGTGTTTTGGGACAC | 1857 bp |
| R: CCCTCGAGTCTCTTCCCTGCTGCAAAGTCTTTG | ||
| NS4b | F:CGGCTAGCGCCACCATGAACGAGTACGGGATGCTAGA | 765 bp |
| R: CCCTCGAGCCTTTTCAAGGAGGGCTTGTC | ||
| NS5 rdrp | F: CGGCTAGCGCCACCATGGAGGAAGATGTCAACCTAG | 1920 bp |
| R: CCCTCGAGGTATCTCCTGAGTGAAGTC |
| Gene | Sequence | Product Length/bp | Accession |
|---|---|---|---|
| GRP78 | F:TCGGACGCACTTGGAATGA | 181 bp | NM_001163434.1 |
| R:GCCTCAGCAGTCTCCTTCA | |||
| β-actin | F:GTGCTTCTAGGCGGACTGT | 239 bp | NM_007393.5 |
| R:GCTCCAACCAACTGCTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yang, K.; Li, C.; Liu, W.; Gao, T.; Yuan, F.; Guo, R.; Liu, Z.; Tan, Y.; Hu, X.; et al. 4-Phenyl-butyric Acid Inhibits Japanese Encephalitis Virus Replication via Inhibiting Endoplasmic Reticulum Stress Response. Viruses 2023, 15, 534. https://doi.org/10.3390/v15020534
Wang S, Yang K, Li C, Liu W, Gao T, Yuan F, Guo R, Liu Z, Tan Y, Hu X, et al. 4-Phenyl-butyric Acid Inhibits Japanese Encephalitis Virus Replication via Inhibiting Endoplasmic Reticulum Stress Response. Viruses. 2023; 15(2):534. https://doi.org/10.3390/v15020534
Chicago/Turabian StyleWang, Shuangshuang, Keli Yang, Chang Li, Wei Liu, Ting Gao, Fangyan Yuan, Rui Guo, Zewen Liu, Yiqing Tan, Xianwang Hu, and et al. 2023. "4-Phenyl-butyric Acid Inhibits Japanese Encephalitis Virus Replication via Inhibiting Endoplasmic Reticulum Stress Response" Viruses 15, no. 2: 534. https://doi.org/10.3390/v15020534
APA StyleWang, S., Yang, K., Li, C., Liu, W., Gao, T., Yuan, F., Guo, R., Liu, Z., Tan, Y., Hu, X., Tian, Y., & Zhou, D. (2023). 4-Phenyl-butyric Acid Inhibits Japanese Encephalitis Virus Replication via Inhibiting Endoplasmic Reticulum Stress Response. Viruses, 15(2), 534. https://doi.org/10.3390/v15020534






