Real-World Effectiveness of SARS-CoV-2 Vaccine Booster in Hemodialysis Patients with COVID-19 Receiving Molnupiravir
Abstract
:1. Introduction
2. Materials and Methods
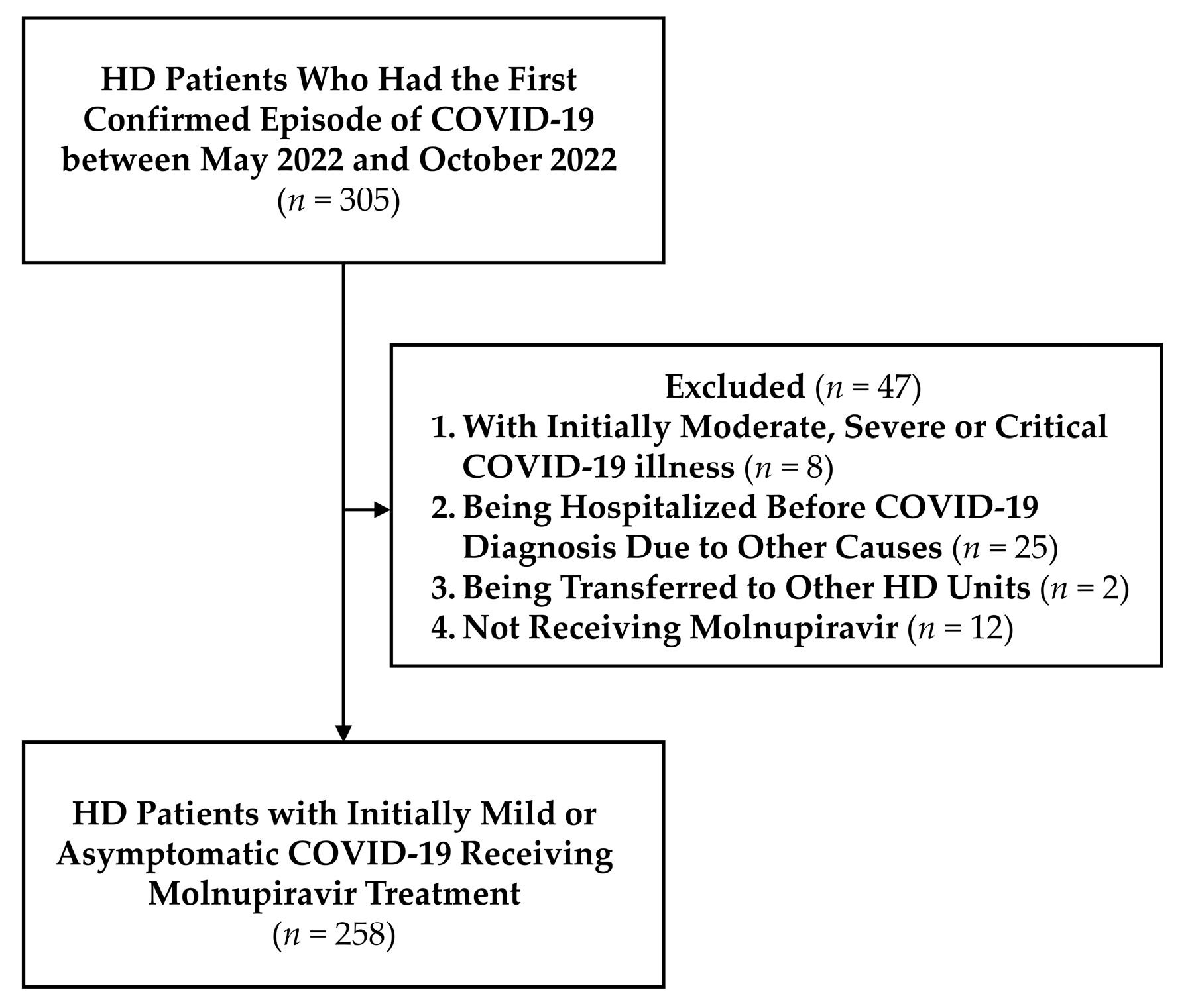
2.1. Enrollment of the Study Cohort
2.2. Vaccination Status of the Study Population
2.3. Clinical Presentations, Molnupiravir Administration, and Composite Events
2.4. Collection of Baseline Demographic and Clinical Profiles
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Enrolled Patients
3.2. Presentations and Composite Events among Patients with Different Vaccination Statuses
3.3. Risk of Composite Events Stratified by Vaccine Doses, Booster Types, and Post-Booster Intervals in the Enrolled HD Patients
3.4. Effects of Vaccine Boosters on Decreasing the Risk of Composite Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19—21 December 2022. Available online: https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update---21-december-2022 (accessed on 24 December 2022).
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease Control. Taiwan National Infectious Disease Statistics System—Severe Pneumonia with Novel Pathogen (COVID-19). Available online: https://nidss.cdc.gov.tw/en/nndss/disease?id=19CoV (accessed on 27 September 2022).
- Velavan, T.P.; Ntoumi, F.; Kremsner, P.G.; Lee, S.S.; Meyer, C.G. Emergence and geographic dominance of Omicron subvariants XBB/XBB.1.5 and BF.7—The public health challenges. Int. J. Infect. Dis. 2023, 128, 307–309. [Google Scholar] [CrossRef]
- Sigal, A. Milder disease with Omicron: Is it the virus or the pre-existing immunity? Nat. Rev. Immunol. 2022, 22, 69–71. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods—United States, December 2020–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 146–152. [Google Scholar] [CrossRef]
- Malahe, S.R.K.; Hoek, R.A.S.; Dalm, V.; Broers, A.E.C.; den Hoed, C.M.; Manintveld, O.C.; Baan, C.C.; van Deuzen, C.M.; Papageorgiou, G.; Bax, H.I.; et al. Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: A prospective observational study. Clin. Infect. Dis. 2022, 76, e172–e178. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Ai, J.; Shen, L.; Lin, K.; Yuan, G.; Sheng, X.; Jin, X.; Deng, Z.; Xu, J.; et al. Identification of CKD, bedridden history and cancer as higher-risk comorbidities and their impact on prognosis of hospitalized Omicron patients: A multi-centre cohort study. Emerg. Microbes Infect. 2022, 11, 2501–2509. [Google Scholar] [CrossRef]
- Aksu, K.; Demir, Ş.; Topel, M.; Yeşilkaya, S.; Ateş, H.; Koca Kalkan, İ.; Öncül, A.; Çuhadar Erçelebi, D.; Türkyılmaz, S. COVID-19 in patients with severe asthma using biological agents. Tuberk. Toraks 2021, 69, 433–436. [Google Scholar] [CrossRef]
- Wang, M.; Xiong, H.; Chen, H.; Li, Q.; Ruan, X.Z. Renal Injury by SARS-CoV-2 Infection: A Systematic Review. Kidney Dis. 2021, 7, 100–110. [Google Scholar] [CrossRef]
- Nopsopon, T.; Kittrakulrat, J.; Takkavatakarn, K.; Eiamsitrakoon, T.; Kanjanabuch, T.; Pongpirul, K. COVID-19 in end-stage renal disease patients with renal replacement therapies: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009156. [Google Scholar] [CrossRef]
- Aydin Bahat, K.; Parmaksiz, E.; Sert, S. The clinical characteristics and course of COVID-19 in hemodialysis patients. Hemodial. Int. 2020, 24, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- D’Marco, L.; Puchades, M.J.; Romero-Parra, M.; Gimenez-Civera, E.; Soler, M.J.; Ortiz, A.; Gorriz, J.L. Coronavirus disease 2019 in chronic kidney disease. Clin. Kidney J. 2020, 13, 297–306. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States—End Stage Renal Disease: Chapter 11. International Comparisons. Available online: https://usrds-adr.niddk.nih.gov/2022/end-stage-renal-disease/11-international-comparisons (accessed on 12 December 2022).
- Taiwan Society of Nephrology. Kidney Disease in Taiwan 2021 Annual Report. Available online: https://www.tsn.org.tw/wowbook.html?id=05b51a9a-fdb2-4183-a9c4-53a85c573acc#book/5 (accessed on 1 October 2022).
- Fu, C.M.; Tsai, K.F.; Kuo, W.H.; Wu, C.H.; Yu, C.I.; You, H.L.; Lee, C.T. The Waxing, Waning, and Predictors of Humoral Responses to Vector-Based SARS-CoV-2 Vaccine in Hemodialysis Patients. Vaccines 2022, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Atluri, K.; Aimlin, I.; Arora, S. Current Effective Therapeutics in Management of COVID-19. J. Clin. Med. 2022, 11, 3838. [Google Scholar] [CrossRef]
- Montez-Rath, M.E.; Garcia, P.; Han, J.; Cadden, L.; Hunsader, P.; Morgan, C.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A.; et al. SARS-CoV-2 Infection during the Omicron Surge among Patients Receiving Dialysis: The Role of Circulating Receptor-Binding Domain Antibodies and Vaccine Doses. J. Am. Soc. Nephrol. 2022, 33, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.G.; Amin, A.B.; Ali, A.R.; Hoots, B.; Cadwell, B.L.; Arora, S.; Avoundjian, T.; Awofeso, A.O.; Barnes, J.; Bayoumi, N.S.; et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4–December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 132–138. [Google Scholar] [CrossRef]
- Shekhar, R.; Garg, I.; Pal, S.; Kottewar, S.; Sheikh, A.B. COVID-19 Vaccine Booster: To Boost or Not to Boost. Infect. Dis. Rep. 2021, 13, 924–929. [Google Scholar] [CrossRef]
- Adams, K.; Rhoads, J.P.; Surie, D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Talbot, H.K.; Casey, J.D.; Zepeski, A.; Shapiro, N.I.; et al. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: Living test negative design study. BMJ 2022, 379, e072065. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Poh, X.Y.; Tan, C.W.; Lee, I.R.; Chavatte, J.M.; Fong, S.W.; Prince, T.; Hartley, C.; Yeoh, A.Y.Y.; Rao, S.; Chia, P.Y.; et al. Antibody Response of Heterologous vs Homologous Messenger RNA Vaccine Boosters Against the Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant: Interim Results from the PRIBIVAC Study, a Randomized Clinical Trial. Clin. Infect. Dis. 2022, 75, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.F.; Boyer, S.; Chalmin, F.; Jeribi, A.; Egasse, C.; Maggi, M.F.; Auvray, P.; Yalaoui, T. Response to three doses of the Pfizer/BioNTech BNT162b2 COVID-19 vaccine: A retrospective study of a cohort of haemodialysis patients in France. BMC Nephrol. 2022, 23, 189. [Google Scholar] [CrossRef]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Humoral and Cellular Immune Response After a 3-Dose Heterologous SARS-CoV-2 Vaccination Using the mRNA-BNT162b2 and Viral Vector Ad26COVS1 Vaccine in Hemodialysis Patients. Front. Immunol. 2022, 13, 907615. [Google Scholar] [CrossRef]
- Boongird, S.; Setthaudom, C.; Kitpermkiat, R.; Prasongtanakij, S.; Srisala, S.; Chuengsaman, P.; Nongnuch, A.; Assanatham, M.; Kiertiburanakul, S.; Malathum, K.; et al. Durability of Humoral and Cellular Immunity after an Extended Primary Series with Heterologous Inactivated SARS-CoV-2 Prime-Boost and ChAdOx1 nCoV-19 in Dialysis Patients (ICON3). Vaccines 2022, 10, 1064. [Google Scholar] [CrossRef]
- Sherman, A.C.; Crombie, J.L.; Cheng, C.; Desjardins, M.; Zhou, G.; Ometoruwa, O.; Rooks, R.; Senussi, Y.; McDonough, M.; Guerrero, L.I.; et al. Immunogenicity of a Three-Dose Primary Series of mRNA COVID-19 Vaccines in Patients with Lymphoid Malignancies. Open Forum. Infect. Dis. 2022, 9, ofac417. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Wong, C.K.H.; Au, I.C.H.; Lau, K.T.K.; Lau, E.H.Y.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: A retrospective cohort study. Lancet Infect. Dis. 2022, 22, 1681–1693. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19. Diabetes Metab. Syndr. 2022, 16, 102396. [Google Scholar] [CrossRef]
- Poznański, P.; Augustyniak-Bartosik, H.; Magiera-Żak, A.; Skalec, K.; Jakuszko, K.; Mazanowska, O.; Janczak, D.; Krajewska, M.; Kamińska, D. Molnupiravir When Used Alone Seems to Be Safe and Effective as Outpatient COVID-19 Therapy for Hemodialyzed Patients and Kidney Transplant Recipients. Viruses 2022, 14, 2224. [Google Scholar] [CrossRef]
- Haruta, M.; Otsubo, S.; Otsubo, Y. Characteristics of the 6th Japanese wave of COVID-19 in hemodialysis patients. Ren. Replace. Ther. 2022, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, S.R.; Yau, C.E.; Liew, T.M. Examining the Prevailing Negative Sentiments Related to COVID-19 Vaccination: Unsupervised Deep Learning of Twitter Posts over a 16 Month Period. Vaccines 2022, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Küçükali, H.; Ataç, Ö.; Palteki, A.S.; Tokaç, A.Z.; Hayran, O. Vaccine Hesitancy and Anti-Vaccination Attitudes during the Start of COVID-19 Vaccination Program: A Content Analysis on Twitter Data. Vaccines 2022, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Rungkitwattanakul, D.; Yabusaki, A.; Singh, D.; Lawson, P.; Nwaogwugwu, U.; Iheagwara, O.S.; Mere, C. COVID-19 vaccine hesitancy among African American hemodialysis patients: A single-center experience. Hemodial. Int. 2021, 25, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease Control. Recommendations for COVID-19: Case Definition, Specimen Collection, and Diagnostic Tests. Available online: https://www.cdc.gov.tw/File/Get/Dp3P8Z-Sporp5a1qSi3haA (accessed on 22 September 2022).
- World Health Organization. Living Guidance for Clinical Management of COVID-19: Living Guidance. 23 November 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 22 September 2022).
- Taiwan Ministry of Health and Welfare. The Domestic COVID-19 IVD Granted Emergency Use Authorization by Ministry of Health and Welfare (MOHW), Taiwan. Available online: https://covid19.mohw.gov.tw/en/cp-5281-63752-206.html (accessed on 22 September 2022).
- Hsieh, S.M.; Liu, M.C.; Chen, Y.H.; Lee, W.S.; Hwang, S.J.; Cheng, S.H.; Ko, W.C.; Hwang, K.P.; Wang, N.C.; Lee, Y.L.; et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: Interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; Cuenca-Torres, O.; Barrios, L.; Armoa-Garcia, L.; Estigarribia, G.; Sanabria, G.; Lin, M.Y.; Antonio Estrada, J.; Estephan, L.; Cheng, H.Y.; et al. An evaluation of the safety and immunogenicity of MVC-COV1901: Results of an interim analysis of a phase III, parallel group, randomized, double-blind, active-controlled immunobridging study in Paraguay. Vaccine 2023, 41, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease Control. Practical Principles for Immunization of the COVID-19 Vaccines. Available online: https://www.cdc.gov.tw/File/Get/-niFMIOgUA8x4m-kfOoW2A (accessed on 12 December 2022).
- World Health Organization. Therapeutics and COVID-19: Living Guideline. 14 July 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4 (accessed on 22 September 2022).
- Taiwan Centers for Disease Control. Interim Guidelines for Clinical Management of SARS-CoV-2 Infection (20th Edition). Available online: https://www.cdc.gov.tw/File/Get/xPGgnorFhF_7Xx-PuKDRDg (accessed on 22 September 2022).
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.L.; Housset, P. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e181. [Google Scholar] [CrossRef]
- Matrajt, L.; Brown, E.R.; Cohen, M.S.; Dimitrov, D.; Janes, H. Could widespread use of antiviral treatment curb the COVID-19 pandemic? A modeling study. BMC Infect. Dis. 2022, 22, 683. [Google Scholar] [CrossRef]
- Butler, C.C.; Hobbs, F.D.R.; Gbinigie, O.A.; Rahman, N.M.; Hayward, G.; Richards, D.B.; Dorward, J.; Lowe, D.M.; Standing, J.F.; Breuer, J.; et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): An open-label, platform-adaptive randomised controlled trial. Lancet 2022, 401, 281–293. [Google Scholar] [CrossRef]
- Blanchi, S.; Torreggiani, M.; Chatrenet, A.; Fois, A.; Mazé, B.; Njandjo, L.; Bianco, G.; Lepori, N.; Pili, A.; Michel, P.A.; et al. COVID-19 Vaccine Hesitancy in Patients on Dialysis in Italy and France. Kidney Int. Rep. 2021, 6, 2763–2774. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, M.; Fuentes, L.R.; Thwin, O.; Grobe, N.; Wang, K.; Wang, Y.; Kotanko, P. SARS-CoV-2 neutralizing antibody response after three doses of mRNA1273 vaccine and COVID-19 in hemodialysis patients. Front. Nephrol. 2022, 2, 926635. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Ben-Dor, N.; Agur, T.; Guetta, T.; Raiter, A.; Meisel, E.; Alkeesh, W.; Ori, Y.; Rozen-Zvi, B.; Zingerman, B. BNT162b2 Booster Vaccination Induced Immunity against SARS-CoV-2 Variants among Hemodialysis Patients. Vaccines 2022, 10, 967. [Google Scholar] [CrossRef]
- Housset, P.; Kubab, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Caudwell, V.; Faucon, A.L. Waning but persistent humoral response 6 months after the third dose of the mRNA BNT162b2 vaccine in hemodialysis and peritoneal dialysis patients. J. Nephrol. 2022, 35, 783–785. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Bao, W.; Fu, S.; Jin, H. Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2070. [Google Scholar] [CrossRef]
- Mallory, R.M.; Formica, N.; Pfeiffer, S.; Wilkinson, B.; Marcheschi, A.; Albert, G.; McFall, H.; Robinson, M.; Plested, J.S.; Zhu, M.; et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): A secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2022, 22, 1565–1576. [Google Scholar] [CrossRef]
- Hsieh, S.M.; Chang, S.C.; Cheng, H.Y.; Shih, S.R.; Lien, C.E. Durability and Immunogenicity of Neutralizing Antibodies Response Against Omicron Variants After Three Doses of Subunit SARS-CoV-2 Vaccine MVC-COV1901: An Extension to an Open-Label, Dose-Escalation Phase 1 Study. Infect. Dis. Ther. 2022, 11, 1493–1504. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against COVID-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef] [PubMed]



| Baseline Demographic Profiles, n (%) or median (IQR) | |
| Age (year) | 66 (59–71) |
| Female | 144 (55.81) |
| BMI (kg/m2) | 22.98 (20.51–25.81) |
| HD Vintage (year) | 6.25 (2.25–14.17) |
| Diabetes | 113 (43.80) |
| Hypertension | 237 (91.86) |
| Dyslipidemia | 207 (80.23) |
| Vascular Disease | 106 (41.09) |
| Heart Failure | 78 (30.23) |
| Liver Cirrhosis | 23 (8.91) |
| Lung Disease | 27 (10.47) |
| Smoking | 28 (10.85) |
| Malignancy | 62 (24.03) |
| Autoimmune Disease | 10 (3.88) |
| Immunosuppressant | 15 (5.81) |
| Transplantation History | 9 (3.49) |
| Baseline Clinical Profiles, median (IQR) | |
| Hemoglobin (g/L) | 105.00 (97.00–112.00) |
| Leukocyte (109/L) | 5.80 (4.80–7.13) |
| Platelet (109/L) | 174.50 (141.00–218.25) |
| Blood Urea Nitrogen (mmol/L) | 24.28 (20.71–28.92) |
| Serum Creatinine (μmol/L) | 889.30 (750.96–1051.30) |
| Kt/V | 1.56 (1.37–1.75) |
| Blood Total Calcium (mmol/L) | 2.40 (2.24–2.55) |
| Blood Phosphorus (mmol/L) | 1.65 (1.36–1.94) |
| Blood Potassium (mmol/L) | 4.50 (4.10–4.90) |
| Blood Bicarbonate (mmol/L) | 21.70 (20.08–23.05) |
| ALT (U/L) | 13.00 (9.00–19.00) |
| Alkaline Phosphatase (U/L) | 75.00 (55.75–105.00) |
| Intact-PTH (ng/L) | 204.00 (72.90–427.00) |
| Transferrin Saturation (%) | 29.54 (23.97–37.70) |
| Serum Albumin (g/L) | 39.90 (37.60–41.93) |
| Total Cholesterol (mmol/L) | 3.99 (3.36–4.64) |
| Onset to Molnupiravir (day) | 1 (0–1) |
| ≤Two Vaccine Doses (n = 66) | Three Vaccine Doses (n = 123) | Four Vaccine Doses (n = 69) | p-Value | |
|---|---|---|---|---|
| Demographic and Clinical Profiles, n (%) or median (IQR) | ||||
| Age (year) | 66.50 (57.75–72.25) | 66.00 (59.00–71.00) | 65.00 (58.00–70.00) | 0.566 |
| Female | 43 (65.15) | 68 (55.28) | 33 (47.83) | 0.129 |
| BMI (kg/m2) | 21.74 (19.26–25.15) | 23.06 (21.01–26.71) | 23.44 (20.70–25.40) | 0.121 |
| HD Vintage (year) | 4.71 (1.29–10.56) | 7.67 (2.67–17.25) b | 5.58 (2.75–10.42) | 0.050 # |
| Diabetes | 28 (42.42) | 56 (45.53) | 29 (42.03) | 0.884 |
| Hypertension | 65 (98.48) | 108 (87.80) a | 64 (92.75) | 0.025 * |
| Dyslipidemia | 50 (75.76) | 99 (80.49) | 58 (84.06) | 0.485 |
| Vascular Disease | 33 (50.00) | 45 (36.59) | 28 (40.58) | 0.202 |
| Heart Failure | 25 (37.88) | 33 (26.83) | 20 (28.99) | 0.272 |
| Liver Cirrhosis | 6 (9.09) | 11 (8.94) | 6 (8.70) | 1.000 |
| Lung Disease | 7 (10.61) | 12 (9.76) | 8 (11.59) | 0932 |
| Smoking | 9 (13.64) | 10 (8.13) | 9 (13.04) | 0.390 |
| Malignancy | 21 (31.82) | 32 (26.02) | 9 (13.04) a | 0.026 * |
| Autoimmune Disease | 6 (9.09) | 2 (1.63) a | 2 (2.90) | 0.037 * |
| Immunosuppressant | 7 (10.61) | 6 (4.88) | 2 (2.90) | 0.157 |
| Transplantation History | 3 (4.55) | 3 (2.44) | 3 (4.35) | 0.684 |
| Hemoglobin (g/L) | 101.00 (91.50–109.25) | 106.00 (97.00–114.00) a | 106.00 (99.50–113.00) a | 0.007 * |
| Leukocyte (109/L) | 5.60 (4.58–7.03) | 5.70 (4.90–7.20) | 6.00 (4.70–7.30) | 0.666 |
| Platelet (109/L) | 178.00 (128.50–223.75) | 175.00 (141.00–215.00) | 173.00 (143.50–215.00) | 0.957 |
| Blood Urea Nitrogen (mmol/L) | 23.21 (20.35–27.94) | 24.99 (20.71–28.92) | 24.99 (21.06–29.99) | 0.529 |
| Serum Creatinine (μmol/L) | 807.98 (701.45–920.47) | 893.72 (771.73–1098.81) a | 948.53 (820.35–1091.30) a | <0.001 * |
| Kt/V | 1.54 (1.32–1.71) | 1.59 (1.41–1.78) | 1.54 (1.37–1.72) | 0.234 |
| Blood Total Calcium (mmol/L) | 2.39 (2.23–2.50) | 2.40 (2.23–2.55) | 2.43 (2.26–2.58) | 0.292 |
| Blood Phosphorus (mmol/L) | 1.65 (1.41–2.04) | 1.68 (1.32–1.91) | 1.58 (1.29–1.91) | 0.799 |
| Blood Potassium (mmol/L) | 4.60 (4.20–4.93) | 4.50 (4.10–4.90) | 4.50 (4.10–4.80) | 0.423 |
| Blood Bicarbonate (mmol/L) | 21.85 (20.18–23.05) | 21.40 (19.80–23.00) | 21.90 (20.65–23.35) | 0.309 |
| ALT (U/L) | 13.50 (8.75–18.00) | 13.00 (10.00–20.00) | 12.00 (9.00–18.00) | 0.299 |
| Alkaline Phosphatase (U/L) | 80.00 (62.00–126.00) | 75.00 (58.00–103.00) | 63.00 (49.00–97.00) a | 0.003 * |
| Intact-PTH (ng/L) | 220.50 (84.58–629.00) | 160.00 (64.40–498.50) | 215.00 (89.45–352.25) | 0.565 |
| Transferrin Saturation (%) | 29.78 (24.01–39.92) | 29.44 (23.33–37.70) | 29.60 (24.95–36.35) | 0.863 |
| Serum Albumin (g/L) | 38.50 (35.65–41.05) | 40.00 (37.80–42.30) a | 40.40 (38.45–42.40) a | 0.002 * |
| Total Cholesterol (mmol/L) | 3.74 (3.30–4.46) | 4.14 (3.42–4.74) | 3.99 (3.46–4.64) | 0.312 |
| Onset to Molnupiravir (day) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Huang, C.-C.; Fu, C.-M.; Chang, Y.-C.; Wu, P.-J.; Lee, W.-C.; Lee, C.-T.; Tsai, K.-F. Real-World Effectiveness of SARS-CoV-2 Vaccine Booster in Hemodialysis Patients with COVID-19 Receiving Molnupiravir. Viruses 2023, 15, 543. https://doi.org/10.3390/v15020543
Chen P-C, Huang C-C, Fu C-M, Chang Y-C, Wu P-J, Lee W-C, Lee C-T, Tsai K-F. Real-World Effectiveness of SARS-CoV-2 Vaccine Booster in Hemodialysis Patients with COVID-19 Receiving Molnupiravir. Viruses. 2023; 15(2):543. https://doi.org/10.3390/v15020543
Chicago/Turabian StyleChen, Po-Chun, Chiang-Chi Huang, Chung-Ming Fu, Yi-Chin Chang, Po-Jung Wu, Wen-Chin Lee, Chien-Te Lee, and Kai-Fan Tsai. 2023. "Real-World Effectiveness of SARS-CoV-2 Vaccine Booster in Hemodialysis Patients with COVID-19 Receiving Molnupiravir" Viruses 15, no. 2: 543. https://doi.org/10.3390/v15020543








