Search of Novel Small Molecule Inhibitors for the Main Protease of SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Preparation and Docking Analysis
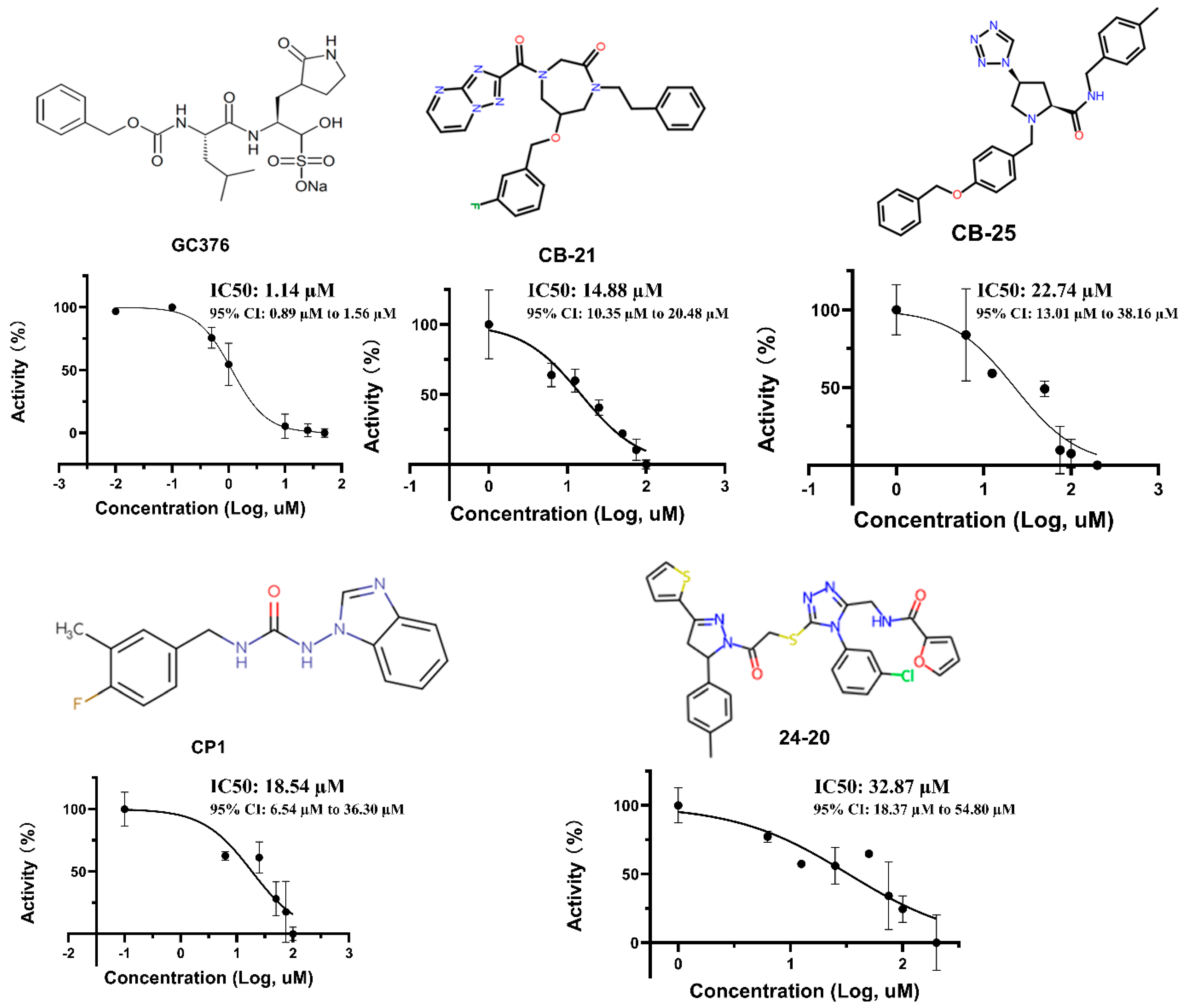
2.2. IC50 Test
2.3. Dissociation Constant (KD) Test
2.4. Compound Pharmacokinetic, Drug Likeness and Toxicity Predictions
2.5. Hydrogen and Hydrophobic Interactions Analysis
3. Results
3.1. Molecular Docking
3.2. IC50 and Inhibitor Binding
3.3. Drug Likeliness, Pharmacokinetic and Oral Toxicity Evaluations of Selected Compounds
3.4. Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Cao, B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Kevadiya, B.D. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Ruggiero, A.; Squeglia, F.; Maga, G.; Berisio, R. A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wei, P.; Feng, Q.; Chen, S.; Huang, C.; Ma, L.; Lai, B.; Pei, J.; Liu, Y.; Chen, J.; et al. Biosynthesis, Purification, and Substrate Specificity of Severe Acute Respiratory Syndrome Coronavirus 3C-like Proteinase. J. Biol. Chem. 2004, 279, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Nitsche, C. The SARS-CoV-2 main protease as drug target. Bioorganic Med. Chem. Lett. 2020, 30, 127377. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.K.; Singh, R.; Das, P.; Purohit, R. Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs. Comput. Biol. Med. 2021, 128, 104117. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Lin, S.X.; Wang, Q.; Wang, Y.L. Interactions between Escherichia coli arginyl-tRNA synthetase and its substrates. Biochemistry 1988, 27, 6348–6353. [Google Scholar] [CrossRef]
- Holler, E.; Bennett, E.L.; Calvin, M. 2-p-Toluidinylnaphthalene-6-sulfonate, a fluorescent reporter group for L-isoleucyl-tRNA synthetase. Biochem. Biophys. Res. Commun. 1971, 45, 409–415. [Google Scholar] [CrossRef]
- Burshteĭn, E.A. Quenching of protein fluorescence. I. Principles of the method. Solutions of tryptophan, tyrosine, and denaturated proteins. Biofizika 1968, 13, 433–442. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Lu, J.; Chen, S.A.; Khan, M.B.; Brassard, R.; Arutyunova, E.; Lamer, T.; Lemieux, M.J. Crystallization of feline coronavirus Mpro with GC376 reveals mechanism of inhibition. Front. Chem. 2022, 10, 852210. [Google Scholar] [CrossRef]
- Hung, H.C.; Ke, Y.Y.; Huang, S.Y.; Huang, P.N.; Kung, Y.A.; Chang, T.Y.; Hsu, J.T.A. Discovery of M protease inhibitors encoded by SARS-CoV-2. Antimicrob. Agents Chemother. 2020, 64, e00872-e20. [Google Scholar] [CrossRef] [PubMed]
- Vuong, W.; Fischer, C.; Khan, M.B.; van Belkum, M.J.; Lamer, T.; Willoughby, K.D.; Vederas, J.C. Improved SARS-CoV-2 Mpro inhibitors based on feline antiviral drug GC376: Structural enhancements, increased solubility, and micellar studies. Eur. J. Med. Chem. 2021, 222, 113584. [Google Scholar] [CrossRef]
- Fu, L.; Ye, F.; Feng, Y.; Yu, F.; Wang, Q.; Wu, Y.; Gao, G.F. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Commun. 2020, 11, 4417. [Google Scholar] [CrossRef]
- Dampalla, C.S.; Zheng, J.; Perera, K.D.; Wong, L.Y.R.; Meyerholz, D.K.; Nguyen, H.N.; Chang, K.O. Postinfection treatment with a protease inhibitor increases survival of mice with a fatal SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101555118. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C. Progress in developing inhibitors of SARS-CoV-2 3C-like protease. Microorganisms 2020, 8, 1250. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Perera, L.; Tillekeratne, L.V. Potential SARS-CoV-2 main protease inhibitors. Drug Discov. Today 2021, 26, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Park, K.D.; Lee, D.-W. Editorial: Interactions Between Small Molecule Ligands and Target Enzymes. Front. Mol. Biosci. 2021, 8, 649450. [Google Scholar] [CrossRef] [PubMed]
- Breznik, M.; Ge, Y.; Bluck, J.P.; Briem, H.; Hahn, D.F.; Christ, C.D.; Mortier, J.; Mobley, D.L.; Meier, K. Prioritizing Small Sets of Molecules for Synthesis through in-silico Tools: A Comparison of Common Ranking Methods. Chemmedchem 2022, 18, 202200425. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed]

| Name | Company | ID | Molecular Mass (Da) | ChemPLP Score |
|---|---|---|---|---|
| CB-21 | Chembridge | 25917626 | 489 | 98.08 |
| CB-25 | Chembridge | 55464888 | 483 | 92.36 |
| CP-1 | ChemSpace | CSCS00026316954 | 298 | 81.19 |
| LC24-20 | Life Chemicals | F0514-4479 | 617 | 90.29 |
| ID | Lipophilicity Consensus Log Po/w | Water Solubility (mol/L) | GI Absorption | BBB Permeability | P-gp Substrate | Bioavailability Score | LD50 (mg/kg) |
|---|---|---|---|---|---|---|---|
| GC376 | −2.18 | Soluble (8.47 × 10−4) | Low | No | Yes | 0.17 | 300 |
| CB-21 | 2.45 | Poorly Soluble (3.00 × 10−5) | High | No | Yes | 0.55 | 1600 |
| CB-25 | 3.25 | Poorly Soluble (4.00 × 10−6) | High | No | Yes | 0.55 | 576 |
| CP-1 | 2.75 | Moderatery soluble (1.36 × 10−4) | High | Yes | No | 0.55 | 1000 |
| LC24-20 | 5.02 | Insoluble (3.59 × 10−8) | Low | No | No | 0.55 | 3000 |
| Compound | Hydrogen Bond Interaction | Hydrophobic Interaction |
|---|---|---|
| Z369936976 | Gly143, His163 | Thr25, Thr26, His41, Phe140, Leu141, Asn142, Cys145, His164, Glu166 |
| GC376 | Phe140, Gly143, Cys145, His163, Glu166 | His41, Leu141, Asn142, Ser144, His164, Met165, Pro168, His172, Gln189, Gln192 |
| CB-21 | Thr25, Thr26, Leu27, His41, Phe140, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, Gln189 | |
| CB-25 | His41 | Thr24, Thr26, Phe140, Leu141, Asn142, Gly143, Cys145, His163, His164, Met165, Glu166, Pro168, Arg188, Gln189, Thr190 |
| CP-1 | His41, His163 | Met49, Phe140, Leu141, Asn142, His164, Met165, Glu166, Arg188, Gln189, Thr190 |
| LC24-20 | Thr24, Thr25, His41, Cys44, Thr45, Ser46, Met49, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, Pro168, Arg188, Gln189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Lin, S.-X. Search of Novel Small Molecule Inhibitors for the Main Protease of SARS-CoV-2. Viruses 2023, 15, 580. https://doi.org/10.3390/v15020580
Zhang W, Lin S-X. Search of Novel Small Molecule Inhibitors for the Main Protease of SARS-CoV-2. Viruses. 2023; 15(2):580. https://doi.org/10.3390/v15020580
Chicago/Turabian StyleZhang, Wenfa, and Sheng-Xiang Lin. 2023. "Search of Novel Small Molecule Inhibitors for the Main Protease of SARS-CoV-2" Viruses 15, no. 2: 580. https://doi.org/10.3390/v15020580
APA StyleZhang, W., & Lin, S.-X. (2023). Search of Novel Small Molecule Inhibitors for the Main Protease of SARS-CoV-2. Viruses, 15(2), 580. https://doi.org/10.3390/v15020580






