Phage Therapy in Germany—Update 2023
Abstract
:1. Introduction
2. Methods
- The regulatory situation or legal framework for phage products in Germany;
- The extent of the existing clinical application of phages;
- Existing activities to produce phages for this purpose;
- The status of phage banks;
- The technical status of sensitivity testing (“phagogram”).
3. Results
3.1. Current Regulatory Situation
3.2. Clinical Application of Phages
3.2.1. Brief Overview of Phage Use in Germany from the 1930s to Modern Times
3.2.2. Phages Use in Germany Today (without Exception under the Umbrella of Article 37 of the Declaration of Helsinki), with a Brief Outlook for the Coming Years
3.3. Production of Phages
3.4. Status of Phage Banks
3.5. Status of Susceptibility Testing (Phagogram)
3.6. Ongoing Translational Research Topics and Projects and Clinical Trials
3.7. One-Health Approach (Food Products, Veterinary Medicine)
3.8. Network Structure and Activities
3.9. Most Urgent Changes Needed in the German Phage Landscape, Most Important Research Topics and the Biggest Hurdles for the Implementation of Phage Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tornimbene, B.; Eremin, S.; Escher, M.; Griskeviciene, J.; Manglani, S.; Pessoa-Silva, C.L. WHO Global Antimicrobial Resistance Surveillance System early implementation 2016–2017. Lancet Infect. Dis. 2018, 18, 241–242. [Google Scholar] [CrossRef]
- Havenga, B.; Ndlovu, T.; Clements, T.; Reyneke, B.; Waso, M.; Khan, W. Exploring the antimicrobial resistance profiles of WHO critical priority list bacterial strains. BMC Microbiol. 2019, 19, 303. [Google Scholar] [CrossRef] [Green Version]
- Babu Rajendran, N.; Mutters, N.T.; Marasca, G.; Conti, M.; Sifakis, F.; Vuong, C.; Voss, A.; Baño, J. Mandatory surveillance and outbreaks reporting of the WHO priority pathogens for research & discovery of new antibiotics in European countries. Clin. Microbiol. Infect. 2020, 26, 943.e1–943.e6. [Google Scholar]
- Zacher, B.; Haller, S.; Willrich, N.; Walter, J.; Abu Sin, M.; Cassini, A.; Plachouras, D.; Suetens, C.; Behnke, M.; Gastmeier, P. Application of a new methodology and R package reveals a high burden of healthcare-associated infections (HAI) in Germany compared to the average in the European Union/European Economic Area, 2011 to 2012. Euro Surveill. 2019, 24, 1900135. [Google Scholar] [CrossRef] [Green Version]
- Behnke, M.; Aghdassi, S.; Hansen, S.; Peña Diaz, L.; Gastmeier, P.; Piening, B. The prevalence of nosocomial infection and antibiotic use in German hospitals. Dtsch. Arztebl. Int. 2017, 114, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Gastmeier, P.; Fätkenheuer, G. Infektiologie: Dilemma mit Begriffen und Zahlen. Dtsch. Arztebl. 2015, 112, A674–A675. (In German) [Google Scholar]
- German National Point Prevalence Study on Nosocomial Infections and Antibiotic Use 2011 Final Report. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/Abschlussbericht_Deutsche_Nationale_Punkt-Praevalenzstudie__zu_nosokomialen_Infektionen_und_Antibiotika-Anwendung_2011.pdf (accessed on 27 December 2022).
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Leaders’ Declaration G7 Summit 7–8 June 2015. Available online: https://www.consilium.europa.eu/media/35254/01_2015-06-08-leaders-statement_final_clean.pdf (accessed on 27 December 2022).
- G20 Leaders’ Declaration Building Consensus for Fair and Sustainable Development. Available online: https://www.consilium.europa.eu/media/37247/buenos_aires_leaders_declaration.pdf (accessed on 27 December 2022).
- Häusler, T. Viruses vs. Superbugs: A Solution to the Antibiotics Crisis; Macmillan: New York, NY, USA, 2006; pp. 105–202. [Google Scholar]
- Leupold, F.G. Die Geschichte des VEB Serum-Werk Bernburg von 1954 bis 1990 unter Besonderer Berücksichtigung Biogener Arzneistoffe. PhD Thesis, Philipps-Universität, Marburg, Germany, 21 June 2018. [Google Scholar]
- Mulzer, J.; Trampuz, A.; Potapov, E.V. Treatment of chronic left ventricular assist device infection with local application of bacteriophages. Eur. J. Cardiothorac. Surg. 2020, 57, 1003–1004. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Potapov, E.; Starck, C.; Mulzer, J.; Falk, V.; Trampuz, A.; Schoenrath, F. Bacteriophage therapy as a treatment option for complex cardiovascular implant infection: The German Heart Center Berlin experience. J. Heart Lung Transplant. 2022, 41, 551–555. [Google Scholar] [CrossRef]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef]
- Ferry, T.; Batailler, C.; Petitjean, C.; Chateau, J.; Fevre, C.; Forestier, E.; Brosset, S.; Leboucher, G.; Kolenda, C.; Laurent, F.; et al. The Potential Innovative Use of Bacteriophages Within the DAC((R)) Hydrogel to Treat Patients with Knee Megaprosthesis Infection Requiring “Debridement Antibiotics and Implant Retention” and Soft Tissue Coverage as Salvage Therapy. Front. Med. 2020, 7, 342. [Google Scholar] [CrossRef]
- Falgenhauer, E.; von Schonberg, S.; Meng, C.; Muckl, A.; Vogele, K.; Emslander, Q.; Ludwig, C.; Simmel, F.C. Evaluation of an E. coli Cell Extract Prepared by Lysozyme-Assisted Sonication via Gene Expression, Phage Assembly and Proteomics. Chembiochem 2021, 22, 2805–2813. [Google Scholar] [CrossRef]
- Emslander, Q.; Vogele, K.; Braun, P.; Stender, J.; Willy, C.; Joppich, M.; Hammerl, J.A.; Abele, M.; Meng, C.; Pichlmair, A.; et al. Cell-free production of personalized therapeutic phages targeting multidrug-resistant bacteria. Cell Chem. Biol. 2022, 29, 1434–1445.e7. [Google Scholar] [CrossRef]
- Mitsunaka, S.; Yamazaki, K.; Pramono, A.K.; Ikeuchi, M.; Kitao, T.; Ohara, N.; Kubori, T.; Nagai, H.; Ando, H. Synthetic engineering and biological containment of bacteriophages. Proc. Natl. Acad. Sci. USA 2022, 119, e2206739119. [Google Scholar] [CrossRef]
- Wurstle, S.; Stender, J.; Hammerl, J.A.; Vogele, K.; Rothe, K.; Willy, C.; Bugert, J.J. Practical Assessment of an Interdisciplinary Bacteriophage Delivery Pipeline for Personalized Therapy of Gram-Negative Bacterial Infections. Pharmaceuticals 2022, 15, 186. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Vogele, K.; Malik, D.J. Production of Phage Therapeutics and Formulations: Innovative Approaches. In Phage Therapy: A Practical Approach, 1st ed.; Górski, A., Międzybrodzki, R., Borysowski, J., Eds.; Springer: Cham, Switzerland, 2019; pp. 3–41. [Google Scholar]
- Eckstein, S.; Stender, J.; Mzoughi, S.; Vogele, K.; Wölfel, R.; Ben Moussa, M.; Bugert, J.J. Towards bacteriophage therapy in infections with multidrug resistant Klebsiella pneumoniae. In Proceedings of the 44th ICMM World Congress on Military Medicine, Brussels, Belgium, 5–9 September 2022. [Google Scholar]
- Jakob, N.; Filippov, A.A.; Swierczewski, B.E.; Ellison, D.W.; Wölfel, R.; Bugert, J.J. Modified Appelmans protocol for in vitro Klebsiella pneumoniae phage host range expansion. In Proceedings of the 44th ICMM World Congress on Military Medicine, Brussels, Belgium, 5–9 September 2022. [Google Scholar]
- Braun, P.; Raab, R.; Bugert, J.; Eckstein, S. Recombinant reporter phage rTUN1::nLuc enables rapid detection and real-time antibiotic susceptibility testing of Klebsiella pneumoniae K64 strains. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar]
- Haines, M.E.K.; Hodges, F.E.; Nale, J.Y.; Mahony, J.; van Sinderen, D.; Kaczorowska, J.; Alrashid, B.; Akter, M.; Brown, N.; Sauvageau, D.; et al. Analysis of Selection Methods to Develop Novel Phage Therapy Cocktails Against Antimicrobial Resistant Clinical Isolates of Bacteria. Front. Microbiol. 2021, 12, 613529. [Google Scholar] [CrossRef]
- MAPVAP Project Website. Available online: https://research.pasteur.fr/en/project/mapvap/ (accessed on 8 January 2023).
- Peng, X.; Ru, J.; Khan Mirzaei, M.; Deng, L. Replidec—Use naïve Bayes classifier to identify virus lifecycle from metagenomic data. bioRxiv 2022. [Google Scholar] [CrossRef]
- Khan Mirzaei, M.; Deng, L. New technologies for developing phage-based tools to manipulate the human microbiome. Trends Microbiol. 2022, 30, 131–142. [Google Scholar] [CrossRef]
- Smith, S.E.; Huang, W.; Tiamani, K.; Unterer, M.; Khan Mirzaei, M.; Deng, L. Emerging technologies in the study of the virome. Curr. Opin. Virol. 2022, 54, 101231. [Google Scholar] [CrossRef]
- EFSA. Evaluation of the safety and efficacy of ListexTM P100 for the reduction of pathogens on different ready-to-eat (RTE) food products. EFSA 2016, 14, 4565. [Google Scholar]
- Carvalho, C.; Costa, A.R.; Silva, F.; Oliveira, A. Bacteriophages and their derivatives for the treatment and control of food-producing animal infections. Crit. Rev. Microbiol. 2017, 43, 583–601. [Google Scholar] [CrossRef] [Green Version]
- Endersen, L.; Buttimer, C.; Nevin, E.; Coffey, A.; Neve, H.; Oliveira, H.; Lavigne, R.; O’Mahony, J. Investigating the biocontrol and anti-biofilm potential of a three phage cocktail against Cronobacter sakazakii in different brands of infant formula. Int. J. Food Microbiol. 2017, 253, 1–11. [Google Scholar] [CrossRef] [Green Version]
- De Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef]
- Heyer, R.; Schallert, K.; Siewert, C.; Kohrs, F.; Greve, J.; Maus, I.; Klang, J.; Klocke, M.; Heiermann, M.; Hoffmann, M.; et al. Metaproteome analysis reveals that syntrophy, competition, and phage-host interaction shape microbial communities in biogas plants. Microbiome 2019, 7, 69. [Google Scholar] [CrossRef]
- Sáez Moreno, D.; Visram, Z.; Mutti, M.; Restrepo-Córdoba, M.; Hartmann, S.; Kremers, A.I.; Tišáková, L.; Schertler, S.; Wittmann, J.; Kalali, B.; et al. ε2-Phages Are Naturally Bred and Have a Vastly Improved Host Range in Staphylococcus aureus over Wild Type Phages. Pharmaceuticals 2021, 14, 325. [Google Scholar] [CrossRef]
- Kever, L.; Hardy, A.; Luthe, T.; Hünnefeld, M.; Gätgens, C.; Milke, L.; Wiechert, J.; Wittmann, J.; Moraru, C.; Marienhagen, J.; et al. Aminoglycoside Antibiotics Inhibit Phage Infection by Blocking an Early Step of the Infection Cycle. MBio 2022, 13, e0078322. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef]
- Korf, I.H.E.; Kittler, S.; Bierbrodt, A.; Mengden, R.; Rohde, C.; Rohde, M.; Kroj, A.; Lehnherr, T.; Fruth, A.; Flieger, A.; et al. In Vitro Evaluation of a Phage Cocktail Controlling Infections with Escherichia coli. Viruses 2020, 12, 1470. [Google Scholar] [CrossRef]
- Jahn, M.T.; Lachnit, T.; Markert, S.M.; Stigloher, C.; Pita, L.; Ribes, M.; Dutilh, B.E.; Hentschel, U. Lifestyle of sponge symbiont phages by host prediction and correlative microscopy. ISME J. 2021, 15, 2001–2011. [Google Scholar] [CrossRef]
- Alarcón-Correa, M.; Günther, J.P.; Troll, J.; Kadiri, V.M.; Bill, J.; Fischer, P.; Rothenstein, D. Self-Assembled Phage-Based Colloids for High Localized Enzymatic Activity. ACS Nano 2019, 13, 5810–5815. [Google Scholar] [CrossRef]
- Moreno-Gallego, J.L.; Chou, S.P.; Di Rienzi, S.C.; Goodrich, J.K.; Spector, T.D.; Bell, J.T.; Youngblut, N.D.; Hewson, I.; Reyes, A.; Ley, R.E.; et al. Virome Diversity Correlates with Intestinal Microbiome Diversity in Adult Monozygotic Twins. Cell Host Microbe 2019, 25, 261–272.e5. [Google Scholar] [CrossRef] [Green Version]
- Dherbey, J.R.; Parab, L.; Gallie, J.; Bertels, F.E. E. coli-ΦX174 genotype to phenotype map reveals flexibility and diversity in LPS structures. bioRxiv 2022. [Google Scholar] [CrossRef]
- Simmons, E.L.; Bond, M.C.; Koskella, B.; Drescher, K.; Bucci, V.; Nadell, C.D. Biofilm Structure Promotes Coexistence of Phage-Resistant and Phage-Susceptible Bacteria. mSystems 2020, 5, e00877-e19. [Google Scholar] [CrossRef]
- Irmscher, T.; Roske, Y.; Gayk, I.; Dunsing, V.; Chiantia, S.; Heinemann, U.; Barbirz, S. Pantoea stewartii WceF is a glycan biofilm-modifying enzyme with a bacteriophage tailspike-like fold. J. Biol. Chem. 2021, 296, 100286. [Google Scholar] [CrossRef]
- Rasmussen, T.S.; Koefoed, A.K.; Jakobsen, R.R.; Deng, L.; Castro-Mejía, J.L.; Brunse, A.; Neve, H.; Vogensen, F.K.; Nielsen, D.S. Bacteriophage-mediated manipulation of the gut microbiome—Promises and presents limitations. FEMS Microbiol. Rev. 2020, 44, 507–521. [Google Scholar] [CrossRef]
- Loessner, H.; Schlattmeier, I.; Anders-Maurer, M.; Bekeredjian-Ding, I.; Rohde, C.; Wittmann, J.; Pokalyuk, C.; Krut, O.; Kamp, C. Kinetic Fingerprinting Links Bacteria-Phage Interactions with Emergent Dynamics: Rapid Depletion of Klebsiella pneumoniae Indicates Phage Synergy. Antibiotics 2020, 9, 408. [Google Scholar] [CrossRef]
- Gogarten, J.F.; Rühlemann, M.; Archie, E.; Tung, J.; Akoua-Koffi, C.; Bang, C.; Deschner, T.; Muyembe-Tamfun, J.-J.; Robbins, M.M.; Schubert, G.; et al. Primate phageomes are structured by superhost phylogeny and environment. Proc. Natl. Acad. Sci. USA 2021, 118, e2013535118. [Google Scholar] [CrossRef]
- Jansen, M.; Wahida, A.; Latz, S.; Krüttgen, A.; Häfner, H.; Buhl, E.M.; Ritter, K.; Horz, H.-P. Enhanced antibacterial effect of the novel T4-like bacteriophage KARL-1 in combination with antibiotics against multi-drug resistant Acinetobacter baumannii. Sci. Rep. 2018, 8, 14140. [Google Scholar] [CrossRef] [Green Version]
- Simon, K.; Pier, W.; Kruttgen, A.; Horz, H.P. Synergy between Phage Sb-1 and Oxacillin against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2021, 10, 849. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [Green Version]
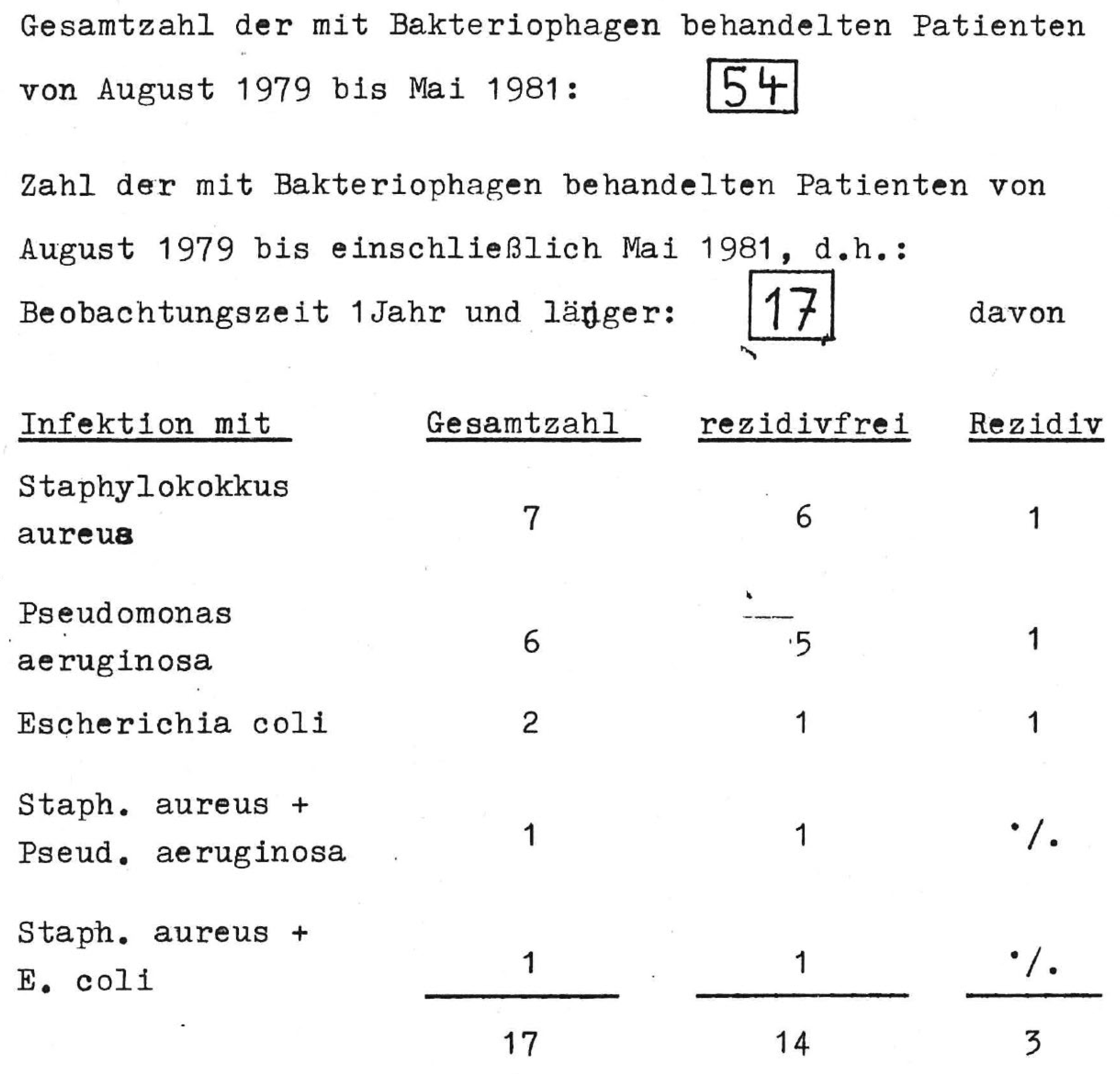



| Compassionate-Use Program (CUP) | “Individual-Treatment Trial” | Magistral Preparation |
|---|---|---|
“Compassionate use” is usually included in the respective national medicinal-products act. In Europe, the European Medicines Agency (EMA) provides recommendations through the Committee for Medicinal Products for Human Use (CHMP), but these do not create a legal framework. CUP are coordinated and implemented by Member States, which set their own rules and procedures (Article 83 of Regulation (EC) No 726/2004).
| “Individual treatment trials” are ethically based on Article 37 of the Helsinki Declaration: “In the treatment of an individual patient, where proven interventions do not exist or other known interventions have been ineffective, the physician, after seeking expert advice, with informed consent from the patient or a legally authorized representative, may use an unproven intervention if in the physician’s judgement it offers hope of saving life, re-establishing health or alleviating suffering. This intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available.” *
| In European law, notion of a magistral preparation (compounded-prescription-drug product in the US) is defined as “any medicinal product prepared in a pharmacy in accordance with a medical prescription for an individual patient” (Article 3 of Directive 2001/83 and Article 6 quarter). Today, there are no formal guidelines regarding the clinical use (e.g., medical indications, formulations, and posology) of magistral phage drugs.
|
| Institution or Company | City | Non-Exhaustive Research Topics (Year of First Publication) * |
|---|---|---|
| Anhalt University of Applied Science, Applied Biosciences and Process Engineering (https://www.hs-anhalt.de/hochschule-anhalt/angewandte-biowissenschaften-und-prozesstechnik/uebersicht.html) | Köthen | Bacterium-phage matching, metaproteomics (2019 [35]) |
| BacTrace BioTec AG (https://www.bactrace.de/en/home-english/) | Munich | Development of in-vitro-evolved phages (2021 [36]) |
| Berlin University of Applied Sciences, School of Life Sciences and Technology, Department of Microbiology (https://www.bht-berlin.de/labor/detail/mib) | Berlin | Microbiome dynamics exposed to phages, interplay between phages and antimicrobials applied on biofilms, phage–antibiotic synergy (n.a.) |
| Carl von Ossietzky University Oldenburg, Institute for Chemistry and Biology of the Marine Environment (http://moraru-phage-lab.icbm.de/) | Oldenburg | Phage–antibiotic antagonism (2022 [37]) |
| Charité—Universitätsmedizin Berlin, Center for Musculoskeletal Surgery and Berlin Institute of Health (https://cmsc.charite.de/ and https://www.bihealth.org/) | Berlin | Phage therapy designed for biofilm infections (2018 [38]) |
| Coburg University of Applied Sciences, Institute of Bioanalysis (https://bioanalytik.co/en/home) | Coburg | Application of therapeutic peptides on recombinant phages (n.a.) |
| FINK TEC GmbH (https://www.finktec.com/) | Hamm | Use of phage cocktail in veterinary medicine and food technology (2020 [39]) |
| Forschungszentrum Jülich, Institute of Bio- und Geosciences (https://www.fz-juelich.de/en/ibg) | Jülich | Phage–antibiotic antagonism (2022 [37]) |
| Fraunhofer Institute for Interfacial Engineering and Biotechnology (https://www.igb.fraunhofer.de/en.html) | Stuttgart | Implementation of selected phages in suitable formulations (n.a.) |
| Free University (FU) Berlin, Institute of Chemistry and Biochemistry (https://www.fu-berlin.de/en/einrichtungen/fachbereiche/fb/bio-chem-pharm/chm/index.html) | Berlin | Interaction of phage extracellular glycosidases with bacterial biofilm, biofilm penetration by phages (n.a.) |
| Friedrich-Schiller University, Institute of Physical Chemistry (https://www.ipc.uni-jena.de/en) | Jena | Development of in-vitro-evolved phages (2021 [36]) |
| GEOMAR Helmholtz Centre for Ocean Research (https://www.geomar.de/en/) | Kiel | Imaging approach to studying phage distribution and cellular association (2021 [40]) |
| Heidelberg University, Institute for Molecular Systems Engineering and Advanced Materials (https://www.imseam.uni-heidelberg.de/) | Heidelberg | Platform technology based on genetically modified phages (2019 [41]) |
| Helmholtz-Centre for Infection Research (HZI), Central Facility for Microscopy (https://www.helmholtz-hzi.de/en/research/technology-platforms/overview/zeim/our-expertise/) | Braunschweig | Phage cocktails for veterinary medicine and food technology (2020 [39]) |
| InfectoGnostics Research Campus (https://www.infectognostics.de/) | Jena | Development of in-vitro-evolved phages (2021 [36]) |
| Justus-Liebig University Giessen, Clinic for Urology, Pediatric Urology and Andrology (https://www.ukgm.de/ugm_2/deu/ugi_uro/index.html) | Giessen | Development of in-vitro-evolved phages (2021 [36]) |
| Leibniz Institute for Photonic Technologies Jena e.V. (https://www.leibniz-ipht.de/en/homepage/) | Jena | On-site identification of phage-mediated bacterial lysis (n.a.); development of in-vitro-evolved phages (2021 [36]) |
| Leibniz Research Institute for Molecular Pharmacology (https://leibniz-fmp.de/) | Berlin | Development of biofilm-penetrating recombinant phages (n.a.) |
| Max Planck Institute for Developmental Biology, Department of Microbiome Science (https://www.bio.mpg.de/48843/microbiome-science-ruth-ley) | Tübingen | Computational tools for the analysis of uncultivated phage genomes (2019 [42]) |
| Max Planck Institute for Evolutionary Biology (https://www.evolbio.mpg.de/2169/en) | Plön | Phage resistance affected by antibiotics (2022 [43]) |
| Max Planck Institute for Medical Research, Department for Cellular Biophysics (https://www.mr.mpg.de/13943505/cellular_biophysics) | Heidelberg | Synthetic phages for personalized treatment on demand (n.a.) |
| Max Planck Institute for Intelligent Systems | Stuttgart | Genetically engineered phages (2019 [41]) |
| Max Planck Institute for Molecular Genetics, Department Computational Molecular Biology (https://www.molgen.mpg.de/en/bioinformatics) | Berlin | Machine-learning framework for translatable phage research (n.a.) |
| Max Planck Institute for Terrestrial Microbiology (https://www.mpi-marburg.mpg.de/) | Marburg | Coexistence of phage-resistant and phage-susceptible bacteria (2020 [44]) |
| Max Planck Institute of Colloids and Interfaces, Department Theory and Bio-Systems (https://www.mpikg.mpg.de/theory) | Potsdam | Interaction of extracellular glycosidases of phages with bacterial biofilm (2021 [45]) |
| Max Rubner Institute, Institute of Microbiology and Biotechnology (https://www.mri.bund.de/en/institutes/microbiology-and-biotechnology/) | Kiel | Developing of a broad-spectrum phage collection and phage-mediated manipulation of the gut microbiome (2020 [46]) |
| Max Delbruck Center for Molecular Medicine, Crystallography (https://www.mdc-berlin.de/heinemann) | Berlin | Interaction of extracellular glycosidases of phages with bacterial biofilm (2021 [45]) |
| Medea Biopharma (https://www.medea-bio.com/) | Munich | Development of a scalable phage-therapy pipeline (n.a.) |
| Paul Ehrlich Institute (https://www.pei.de/EN/home/home-node.html) | Langen | Kinetic fingerprinting to infer phage–host interactions (2020 [47]) |
| Philipps-University Marburg, Department of Physics (https://www.uni-marburg.de/en/fb13) | Marburg | Coexistence of phage-resistant and phage-susceptible bacteria (2020 [44]) |
| PTC Phage Technology Center GmbH (https://www.finktec.com/applied-phage) | Bönen | Phages in veterinary medicine and food technology, phages against uropathogenic bacteria (n.a.) |
| Robert Koch Institute (https://www.rki.de/EN/Home/homepage_node.html) | Berlin | Phage communities over evolutionary history (2021 [48]) |
| Robert Koch Institute (https://www.rki.de/EN/Home/homepage_node.html) | Wernigerode | Use of phage cocktail in veterinary medicine and food technology (2020 [39]) |
| RWTH Aachen University, Institute of Biotechnology (https://www.biotec.rwth-aachen.de/go/id/imne/?lidx=1) | Aachen | Phage–antibiotic antagonism (2022 [37]) |
| RWTH Aachen University, Institute of Medical Microbiology (https://www.medizin.rwth-aachen.de/cms/Medizin/Die-Fakultaet/Institute-und-Kliniken/Die-Institute/Klinisch-theoretische-Institute/~ezkx/Institut-fuer-Medizinische-Mikrobiologie/) | Aachen | Phage–antibiotic synergy (2018 [49]) |
| Technical University Dresden, Institute of Medical Microbiology and Hygiene (https://tu-dresden.de/med/mf/mib) | Dresden | Development of in-vitro-evolved phages (2021 [36]) |
| University Hospital Münster, Institute of Hygiene (https://www.ukm.de/institute/hygiene) | Münster | Phages against uropathogenic bacteria (n.a.) |
| University of Hamburg, Institute of Biochemistry and Molecular Biology (https://www.chemie.uni-hamburg.de/en/institute/bc.html) | Hamburg | Synergistic/antagonistic interactions of bacterium, phage, and antibiotic (n.a.) |
| University of Potsdam, Physical Biochemistry Group (https://www.uni-potsdam.de/en/ibb-physbiochem/index) | Potsdam | Interaction of phage extracellular glycosidases with bacterial biofilm (2021 [45]) |
| University of Stuttgart, Institute for Materials Science (https://www.imw.uni-stuttgart.de/en/) | Stuttgart | Genetically engineered phages (n.a.) |
| University of Tubingen, Interfaculty Institute for Microbiology and Infection Medicine (https://uni-tuebingen.de/en/faculties/faculty-of-science/departments/interfaculty-facilities/imit/) | Tübingen | Predicting the host range of phages, based on computational clustering (n.a.) |
| University of Veterinary Medicine Hannover, Institute for Food Quality and Food Safety (www.tiho-hannover.de) | Hannover | Phage cocktails for veterinary medicine and food technology (2020 [39]) |
| University of Wurzburg, Imaging Core Facility (https://www.biozentrum.uni-wuerzburg.de/em/startseite/) | Würzburg | Imaging approach to studying phage distribution and cellular association (2021 [40]) |
| Zuse Institute Berlin (https://www.zib.de/) | Berlin | Mathematical methods for predicting bacteria–phage interactions and possible side effects (n.a.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willy, C.; Bugert, J.J.; Classen, A.Y.; Deng, L.; Düchting, A.; Gross, J.; Hammerl, J.A.; Korf, I.H.E.; Kühn, C.; Lieberknecht-Jouy, S.; et al. Phage Therapy in Germany—Update 2023. Viruses 2023, 15, 588. https://doi.org/10.3390/v15020588
Willy C, Bugert JJ, Classen AY, Deng L, Düchting A, Gross J, Hammerl JA, Korf IHE, Kühn C, Lieberknecht-Jouy S, et al. Phage Therapy in Germany—Update 2023. Viruses. 2023; 15(2):588. https://doi.org/10.3390/v15020588
Chicago/Turabian StyleWilly, Christian, Joachim J. Bugert, Annika Y. Classen, Li Deng, Anja Düchting, Justus Gross, Jens A. Hammerl, Imke H. E. Korf, Christian Kühn, Simone Lieberknecht-Jouy, and et al. 2023. "Phage Therapy in Germany—Update 2023" Viruses 15, no. 2: 588. https://doi.org/10.3390/v15020588
APA StyleWilly, C., Bugert, J. J., Classen, A. Y., Deng, L., Düchting, A., Gross, J., Hammerl, J. A., Korf, I. H. E., Kühn, C., Lieberknecht-Jouy, S., Rohde, C., Rupp, M., Vehreschild, M. J. G. T., Vogele, K., Wienecke, S., Witzenrath, M., Würstle, S., Ziehr, H., Moelling, K., & Broecker, F. (2023). Phage Therapy in Germany—Update 2023. Viruses, 15(2), 588. https://doi.org/10.3390/v15020588






