Antiviral Peptides in Antimicrobial Surface Coatings—From Current Techniques to Potential Applications
Abstract
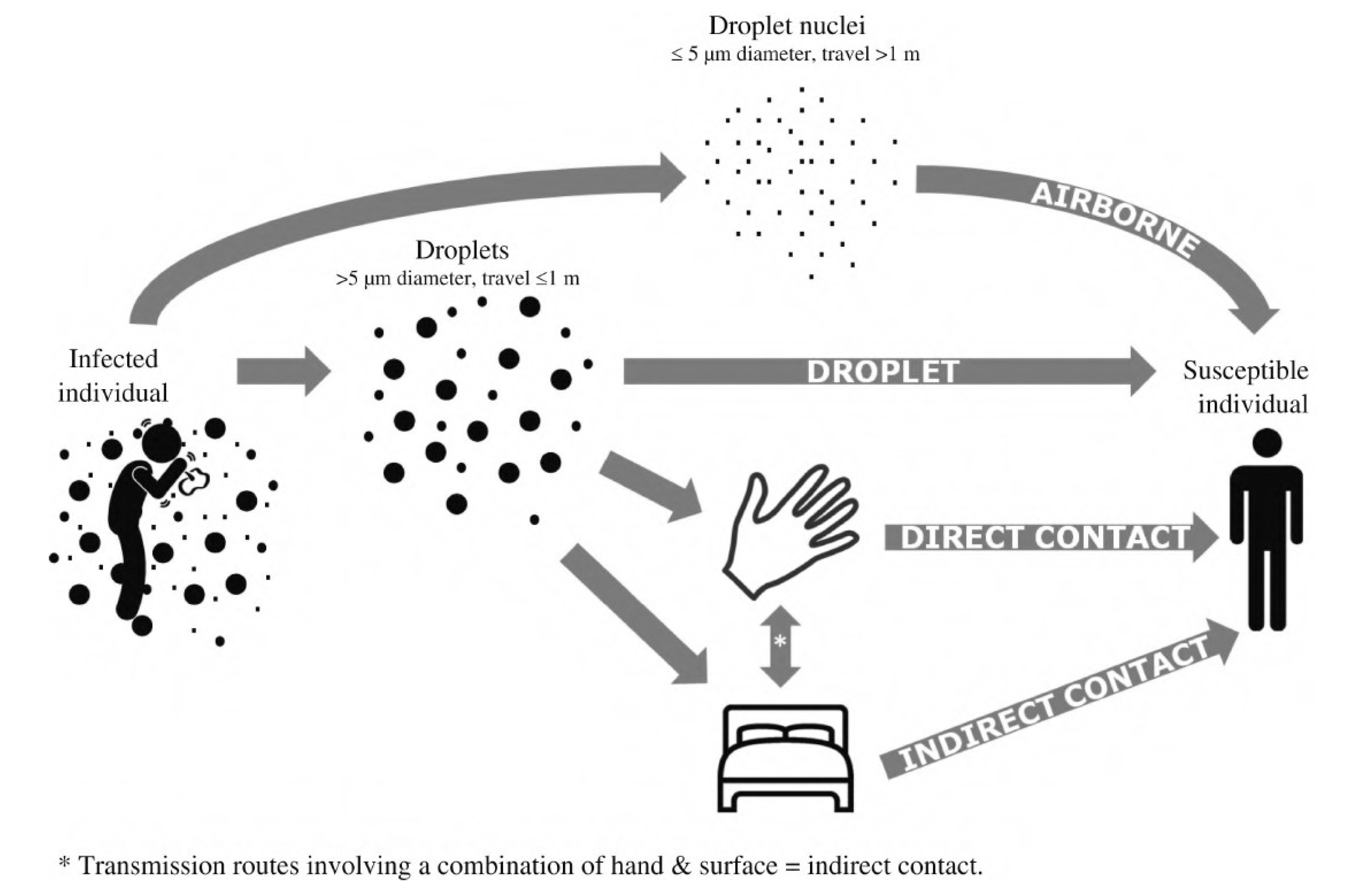
1. Introduction
2. Current Antiviral Surface Coating Techniques
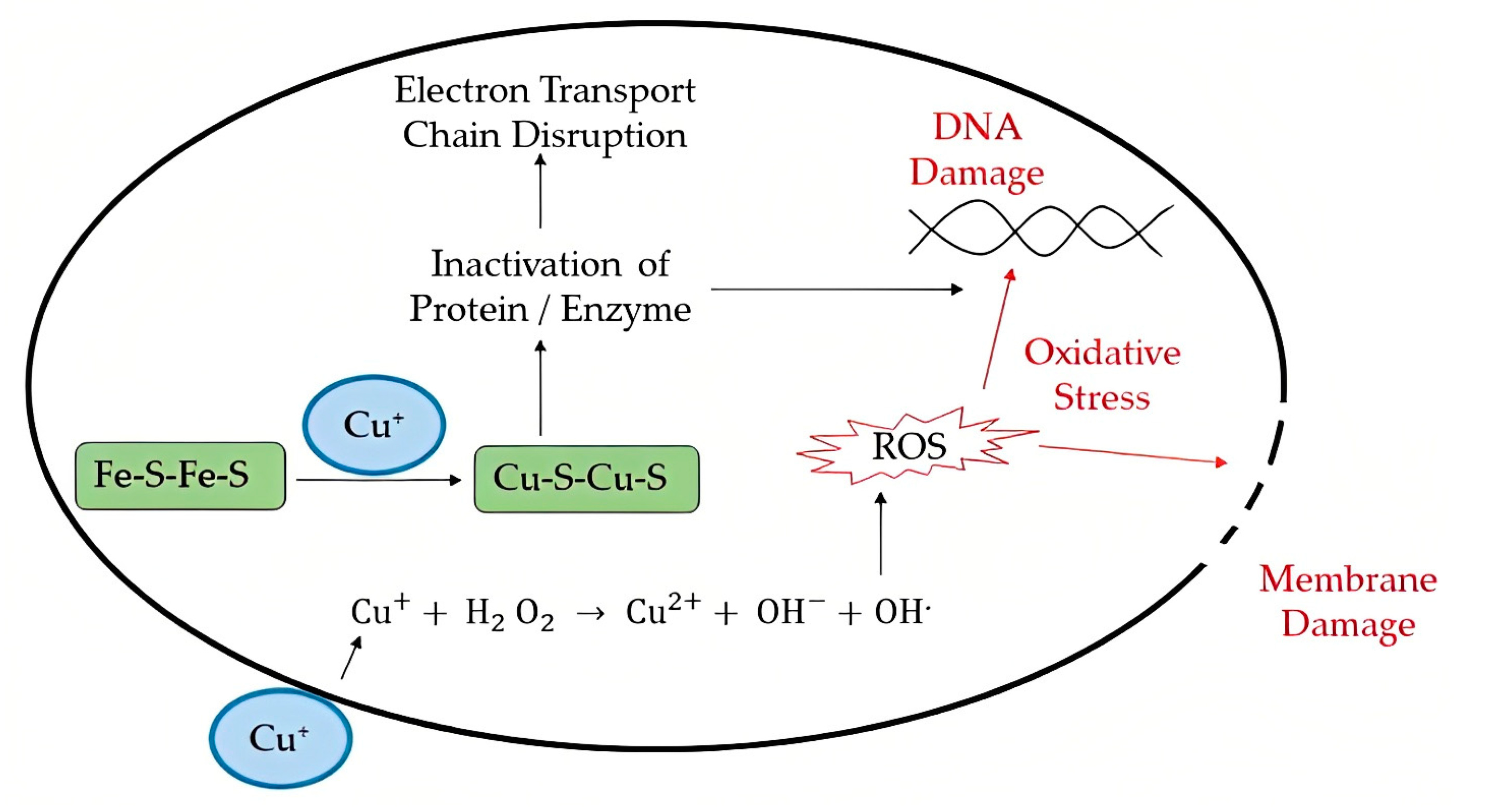
2.1. Metal Ions/Metal Oxide
2.2. Nanoparticle-Based Materials
2.3. Carbon-Based Materials
2.4. Synthetic Polymer-Based Materials
2.5. Natural Polymer-Based Materials
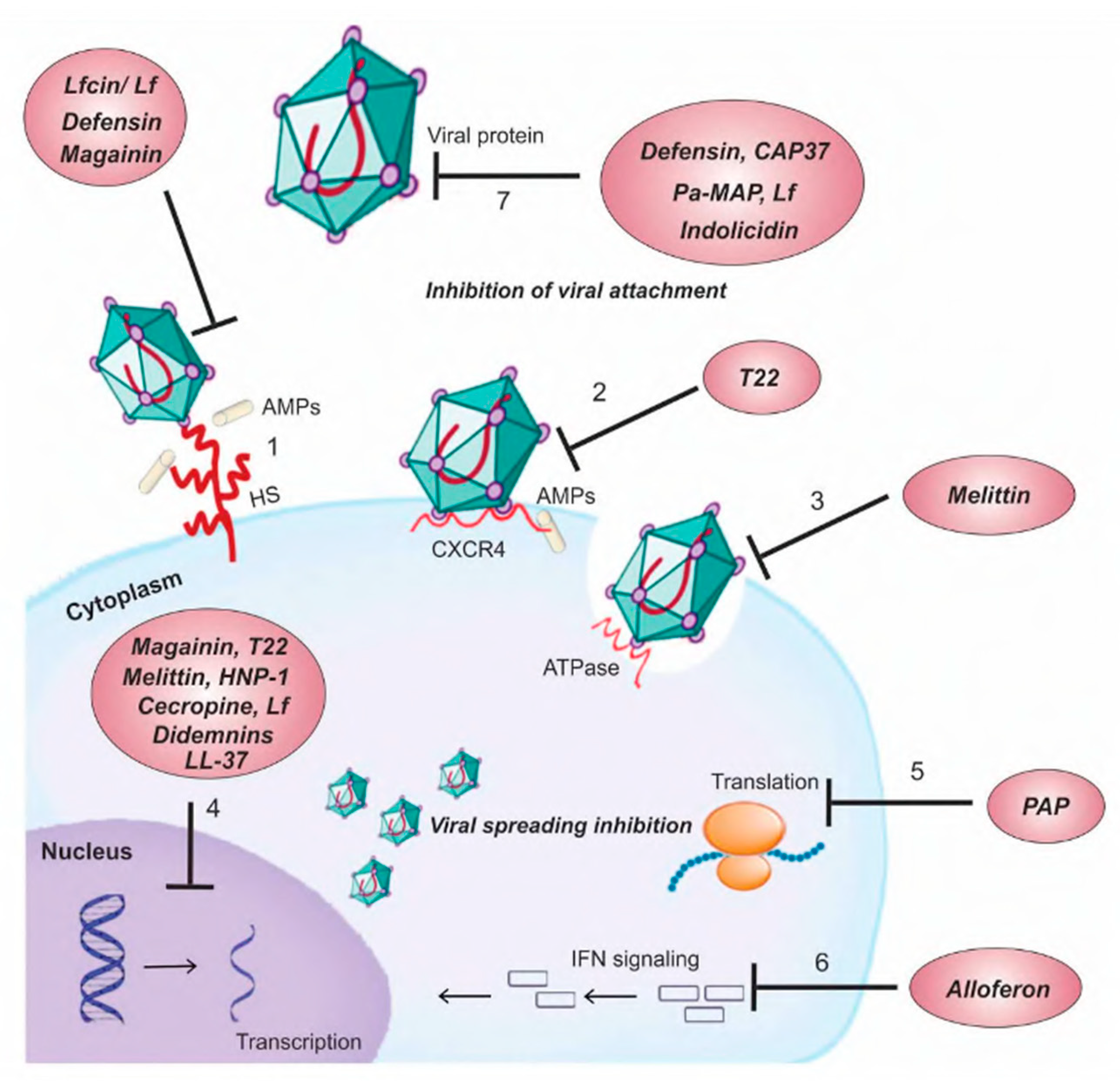
3. Peptides and Antiviral Activity
3.1. Human Cathelicidin and Defensin: Mechanism of Action against Pathogenic Viruses
3.1.1. Human Cathelicidin
3.1.2. Human Defensin
3.1.3. Human Rhinoviruses (HRV)
3.1.4. Influenza Virus (IAV)
3.1.5. Coronavirus, SARS-CoV-2
3.1.6. Human Immunodeficiency Virus (HIV)
3.1.7. Ebola Virus
3.1.8. Zika Virus
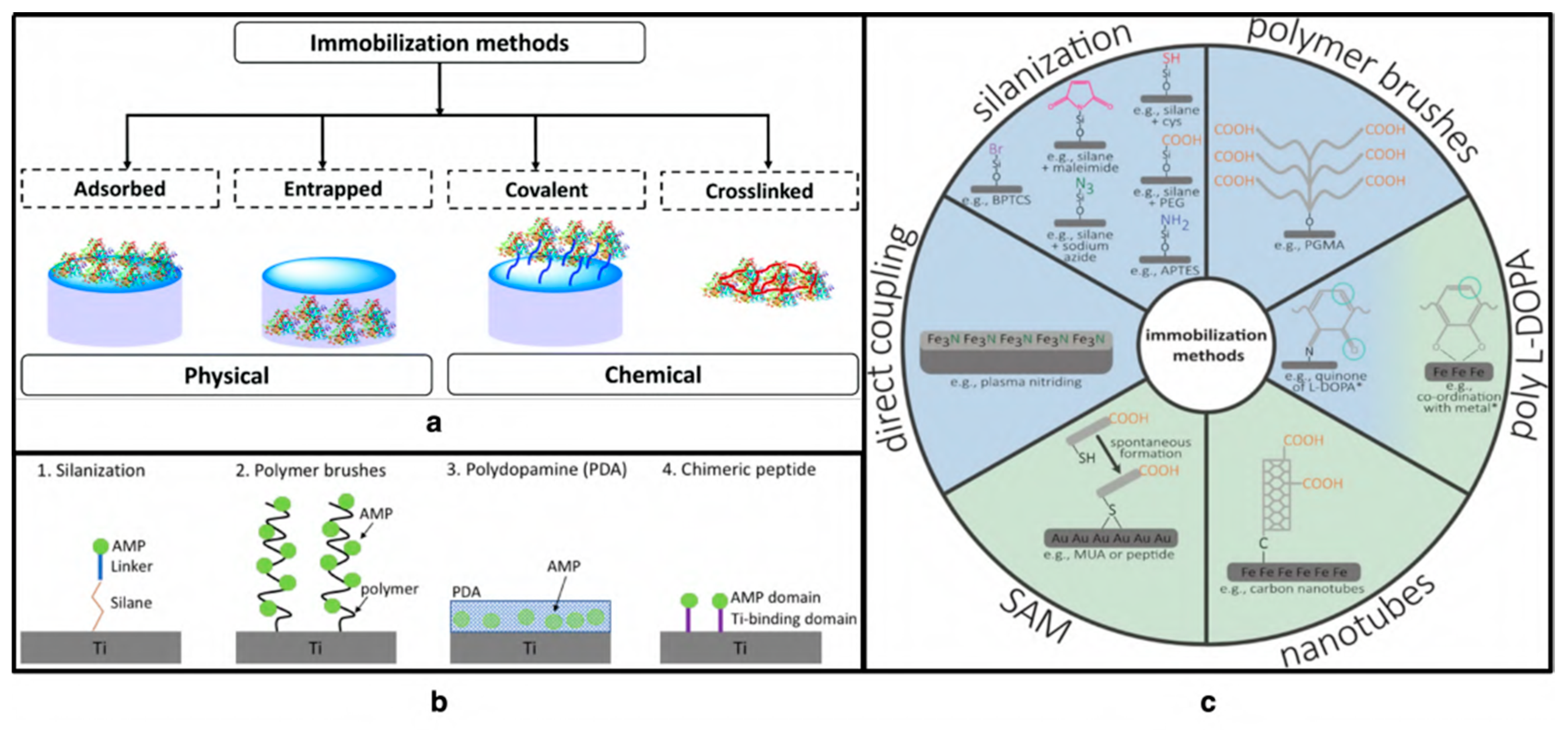
4. Antiviral Peptides as a Surface Coating
4.1. Covalent Immobilization on Modified Surfaces
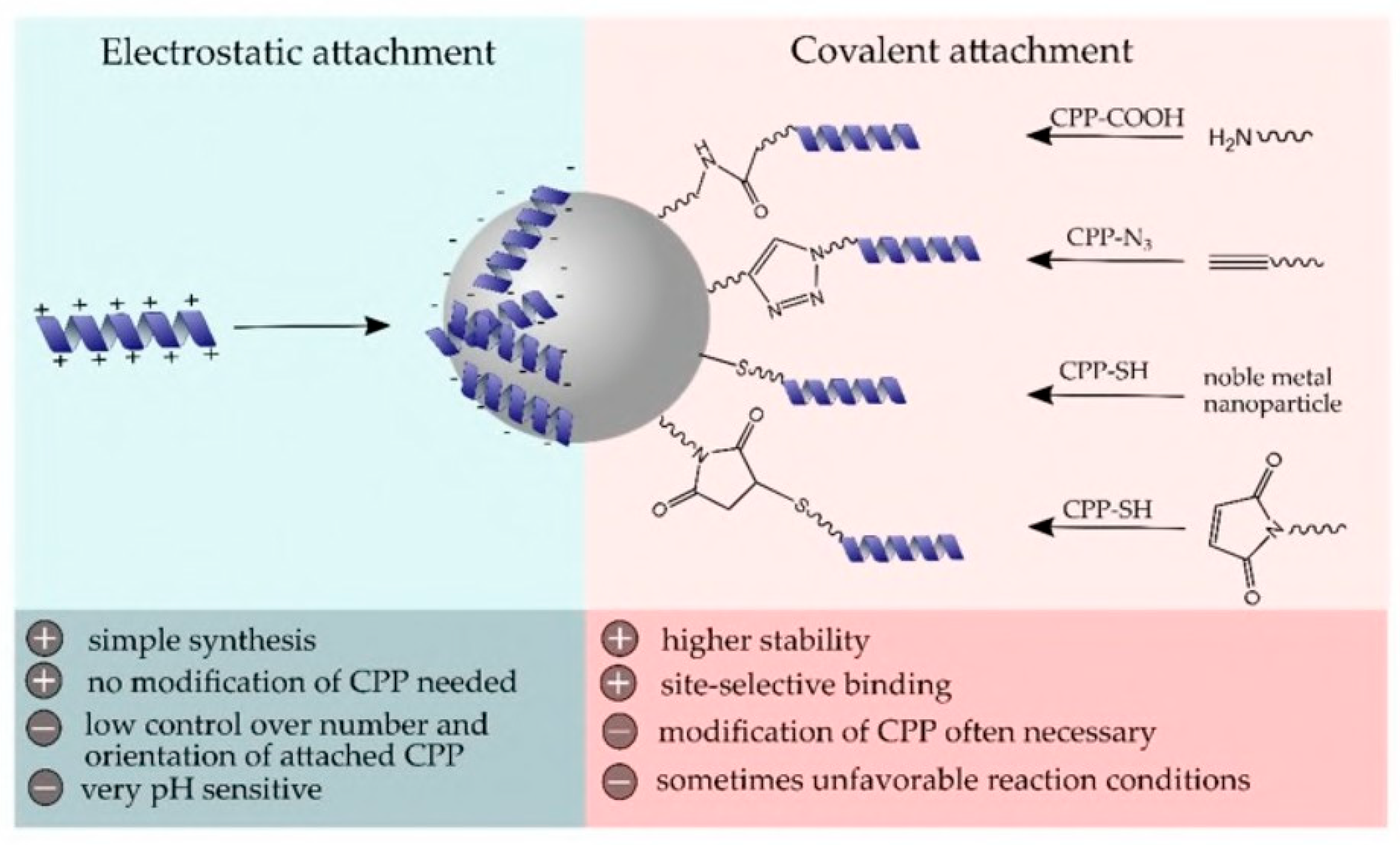
4.2. Electrostatic and Covalent Attachment of AVPs on Surfaces
4.3. Chemical Vapor Deposition
4.4. Physical Adsorption of AVP
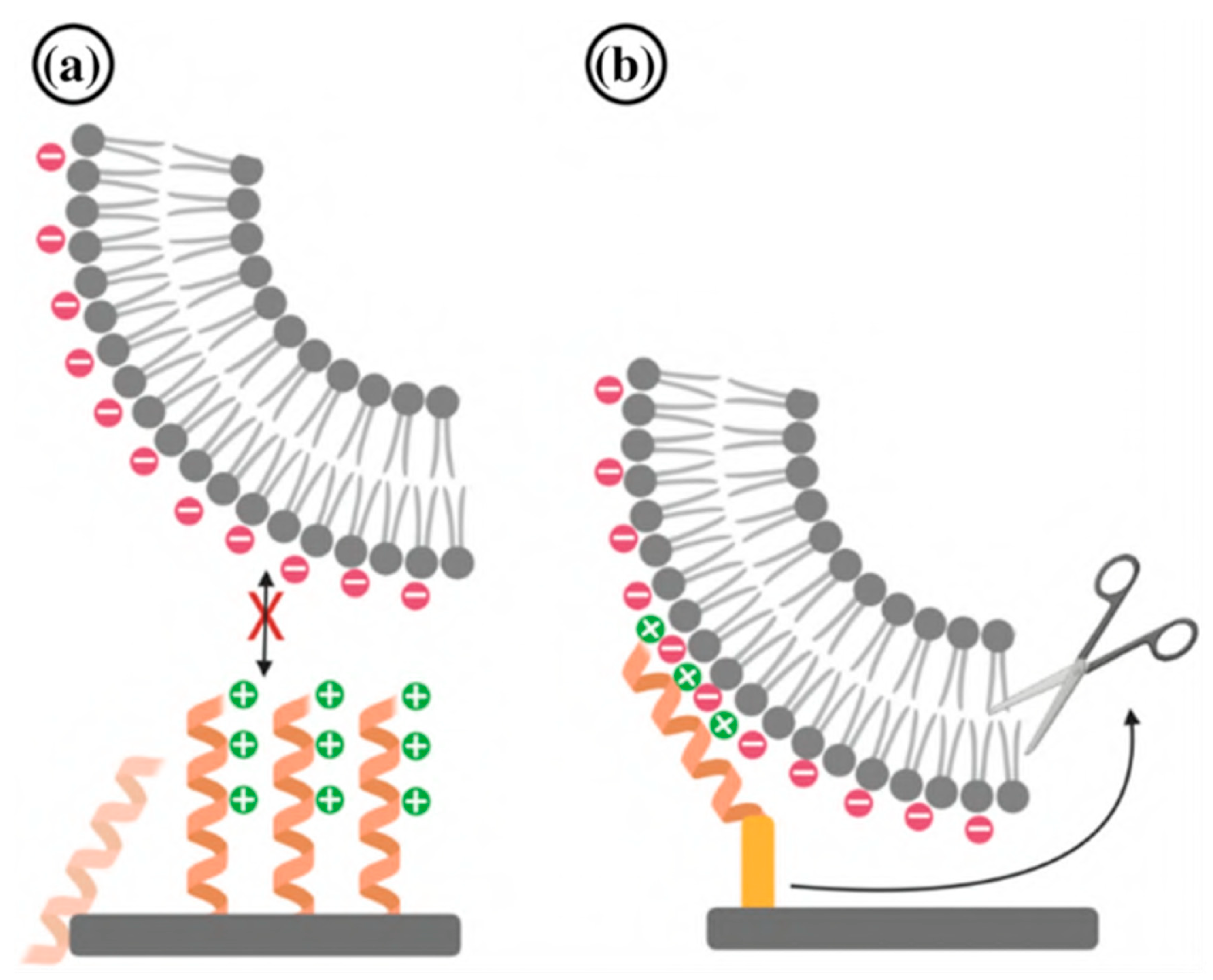
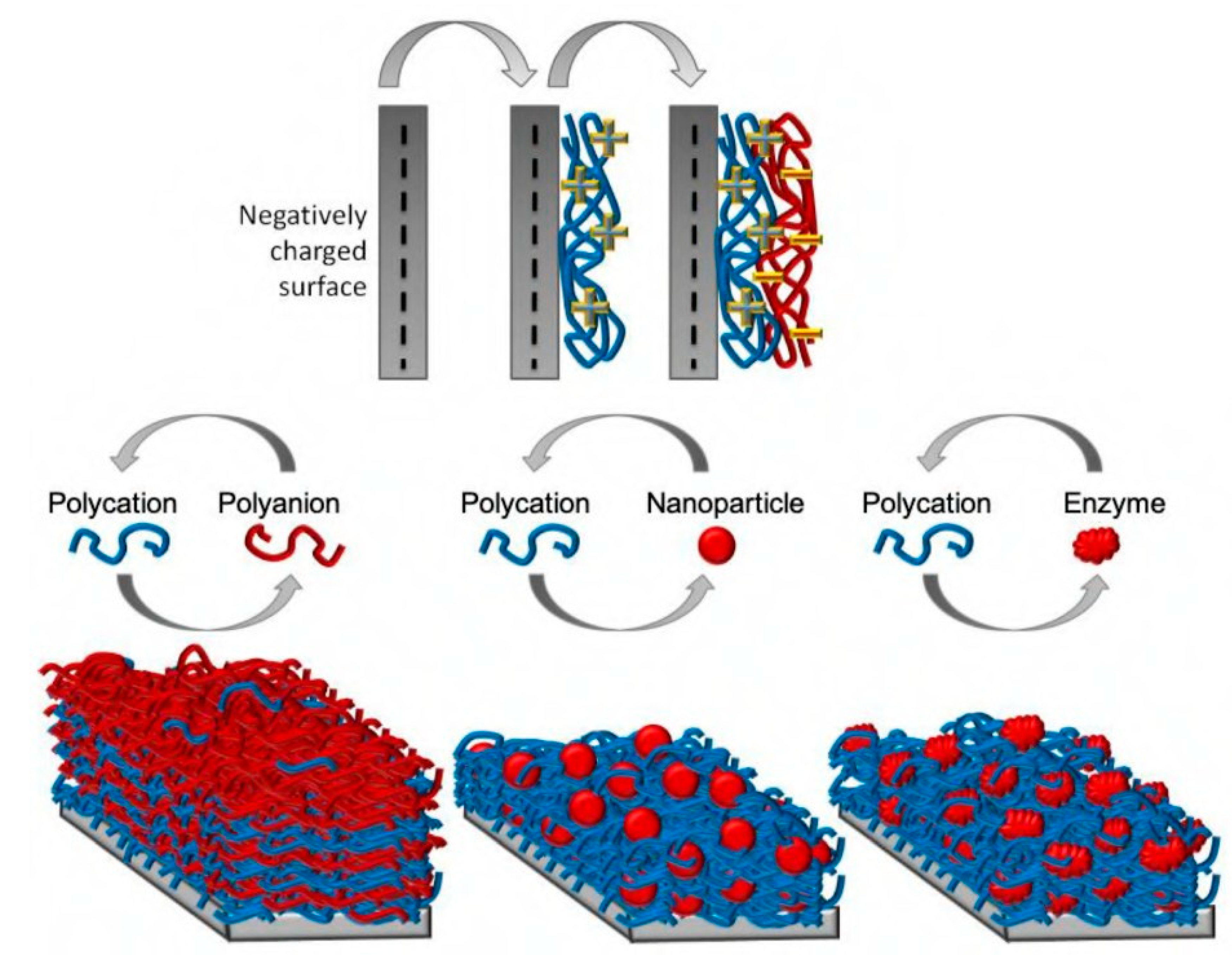
4.5. Layer-by-Layer Adsorption
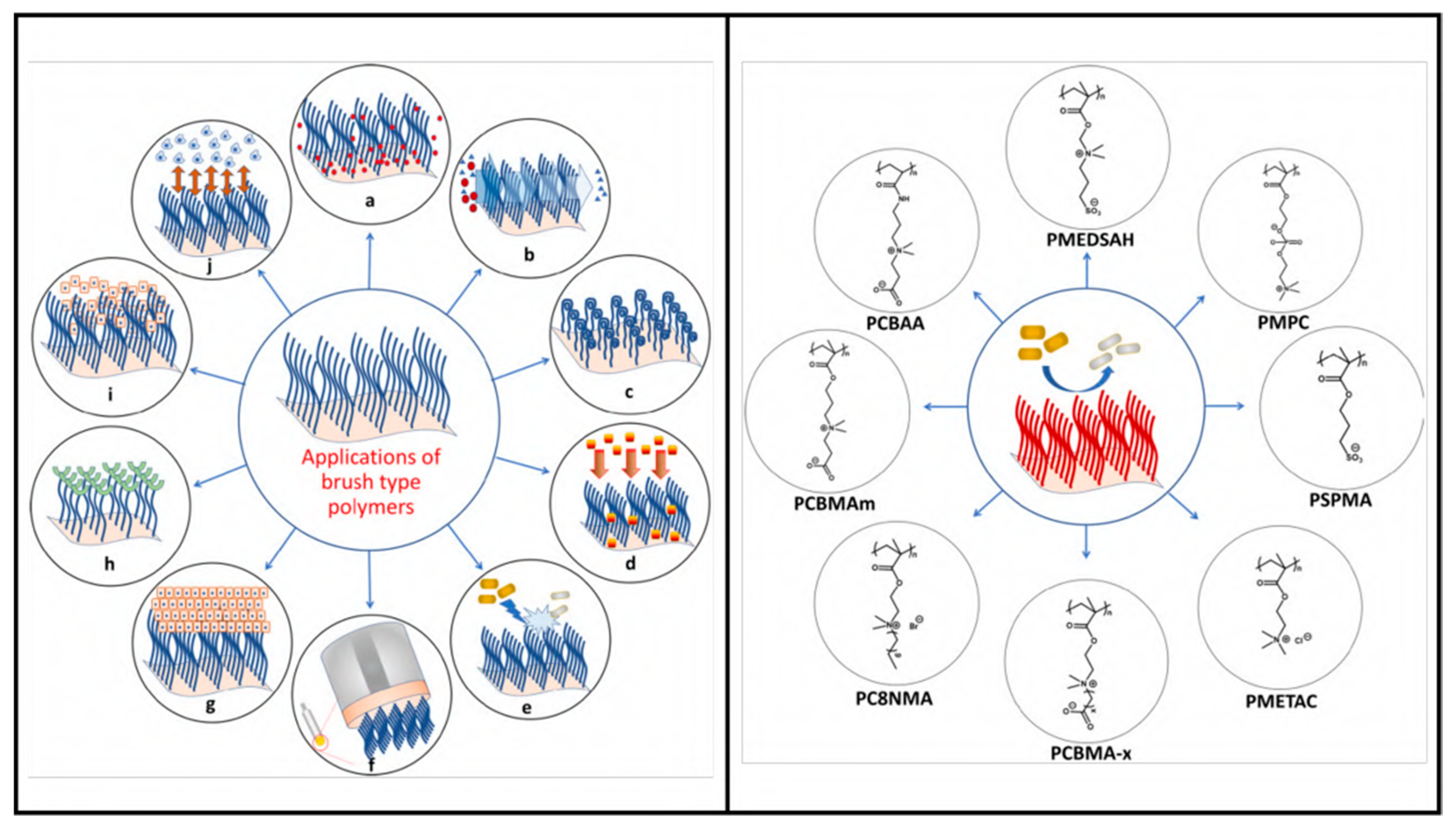
4.6. Polymeric Brush Coating and Peptide Conjugates
4.7. Nanoparticle and Peptide Conjugation
4.8. Recombinant Polymer-Based Coatings
5. Challenges with Peptide Coatings
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gelderblom, H.R. Chapter 41: Structure and classification of viruses. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Anderson, T.F. The reactions of bacterial viruses with their host cells. Bot. Rev. 1949, 15, 464–505. [Google Scholar] [CrossRef]
- Lwoff, A.; Tournier, P. The classification of viruses. Annu. Rev. Microbiol. 1966, 20, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.H. Bacteriophages; Citeseer, Inter-Science Publishers: New York, NY, USA, 1959. [Google Scholar]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Sun, X.; Whittaker, G.R. Role for Influenza Virus Envelope Cholesterol in Virus Entry and Infection. J. Virol. 2003, 77, 12543–12551. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J.M. The Coronavirus Spike Protein Is a Class I Virus Fusion Protein: Structural and Functional Characterization of the Fusion Core Complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef] [PubMed]
- Wisskirchen, K.; Lucifora, J.; Michler, T.; Protzer, U. New pharmacological strategies to fight enveloped viruses. Trends Pharmacol. Sci. 2014, 35, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Altun, E.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Chen, B.; Edirisinghe, M. Surface interactions and viability of coronaviruses. J. R. Soc. Interface 2021, 18, 20200798. [Google Scholar] [CrossRef] [PubMed]
- Brankston, G.; Gitterman, L.; Hirji, Z.; Lemieux, C.; Gardam, M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007, 7, 257–265. [Google Scholar] [CrossRef]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Lexmond, P.; Mulders, A.; Richard, M.; Fouchier, R.A.; Herfst, S. SARS-CoV and SARS-CoV-2 are transmitted through the air between ferrets over more than one meter distance. Nat. Commun. 2021, 12, 1653. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, S.G.; Kim, S.M.; Kim, E.H.; Park, S.J.; Yu, K.M.; Chang, J.H.; Kim, E.J.; Lee, S.; Casel, M.A.B.; et al. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 2020, 27, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Durmus Tekir, S.; Cakir, T.; Ulgen, K. Infection Strategies of Bacterial and Viral Pathogens through Pathogen–Human Protein–Protein Interactions. Front. Microbiol. 2012, 3, 870. [Google Scholar] [CrossRef] [PubMed]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef]
- Boone, S.A.; Gerba, C.P. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Hall, C.B.; Suffin, S.C.; Alexson, C.; Harris, P.J.; Manning, J.A. Respiratory Syncytial Viral Infection in Infants with Congenital Heart Disease. N. Engl. J. Med. 1982, 307, 397–400. [Google Scholar] [CrossRef]
- Winther, B.; McCue, K.; Ashe, K.; Rubino, J.R.; Hendley, J.O. Environmental contamination with rhinovirus and transfer to fingers of healthy individuals by daily life activity. J. Med. Virol. 2007, 79, 1606–1610. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Muyembe-Tamfum, J.; Mulangu, S.; Masumu, J.; Kayembe, J.; Kemp, A.; Paweska, J.T. Ebola virus outbreaks in Africa: Past and present. Onderstepoort J. Veter Res. 2012, 79, 8. [Google Scholar] [CrossRef]
- Safari, S.; Baratloo, A.; Rouhipour, A.; Ghelichkhani, P.; Yousefifard, M. Ebola Hemorrhagic Fever as a Public Health Emergency of International Concern: A Review Article. Emergency 2015, 3, 3–7. [Google Scholar] [PubMed]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2017, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-Based Strategies of Antiviral Coatings with Viricidal Properties for the COVID-19 Like Pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Sassi, H.P.; Sifuentes, L.Y.; Koenig, D.W.; Nichols, E.; Clark-Greuel, J.; Wong, L.F.; McGrath, K.; Gerba, C.P.; Reynolds, K.A. Control of the spread of viruses in a long-term care facility using hygiene protocols. Am. J. Infect. Control 2015, 43, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Hess, K.; Gear Hart, J.M.; Geiss, K.T.; Schlarger, J.J. In Vitro Toxicity of Nanoparticles in BRL 3a Rat Liver Cells. Toxicol Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Shirvanimoghaddam, K.; Akbari, M.K.; Yadav, R.; Al-Tamimi, A.K.; Naebe, M. Fight against COVID-19: The case of antivi-ral surfaces. APL Mater. 2021, 9, 031112. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and tox-icity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Med. J. 2019, 120, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Roby, K.D.; Di Nardo, A. Innate immunity and the role of the antimicrobial peptide cathelicidin in inflammatory skin dis-ease. Drug Discov. Today Dis. Mech. 2013, 10, e79–e82. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.E.; Solomon, K.R. Acidity as a factor in leaching of copper, chromium and arsenic from CCA-treated dimension lumber. Environ. Toxicol. Chem. 1990, 9, 1331–1337. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Chakhalian, D.; Shultz, R.B.; Miles, C.E.; Kohn, J. Opportunities for biomaterials to address the challenges ofCOVID-19. J. Biomed. Mater. Res. Part A 2020, 108, 1974–1990. [Google Scholar] [CrossRef]
- Nasri, N.; Rusli, A.; Teramoto, N.; Jaafar, M.; Ku Ishak, K.M.; Shafiq, M.D.; Abdul Hamid, Z.A. Past and Current Progress in the Development of Antiviral/Antimicrobial Polymer Coating towards COVID-19 Prevention: A Review. Polymers 2021, 13, 4234. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Ganesh, M.R.S.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral properties of copper and its alloys to inactivate covid-19 virus: A review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial nanomaterials and coatings: Current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Rifkind, J.M.; Shin, Y.A.; Heim, J.M.; Eichhorn, G.L. Cooperative disordering of single-stranded polynucleotides through copper crosslinking. Biopolym. Orig. Res. Biomol. 1976, 15, 1879–1902. [Google Scholar] [CrossRef]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Silver nanoparticles as antimicrobial agents: Past, present, and future. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.H.; Song, H. Antiviral potential of nanoparti-cles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, M.C.; Koerner, H.; Singh, K.M.; Partlow, B.P.; Kaplan, D.L.; Gazit, E.; Bunning, T.J.; Naik, R.R. Vertically Aligned Peptide Nanostructures Using Plasma-Enhanced Chemical Vapor Deposition. Biomacromolecules 2014, 15, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Pal, U.; Bayan, S.; Mondal, S.; Ghosh, R.; Darbar, S.; Saha-Dasgupta, T.; Ray, S.K.; Pal, S.K. Nanoceutical Fabric Prevents COVID-19 Spread through Expelled Respiratory Droplets: A Combined Computational, Spectroscopic, and Antimicrobial Study. ACS Appl. Bio Mater. 2021, 4, 5471–5484. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.J.; Slaine, P.D.; Keltie, E.; Palit, S.; McKinnell, S.L.; Longpre, B.E.; Ko, K.R.; Green, J.; Markle, G.; Kim, J.S.; et al. Non-woven textiles formed from contact drawn poly (ethylene oxide) fibers provide tunable filtration and virucidal proper-ties via entrapment of silver nanoparticles. ACS Appl. Polym. Mater. 2021, 3, 4245–4255. [Google Scholar] [CrossRef]
- Assis, M.; Simoes, L.G.P.; Tremiliosi, G.C.; Coelho, D.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.; Santos, J.R.d.; Ribeiro, L.K.; Rosa, I.L.V.; et al. SiO2-Ag composite as a highly virucidal material: A roadmap that rapidly eliminates SARS-CoV-2. Nanomaterials 2021, 11, 638. [Google Scholar] [CrossRef]
- Chiome, T.J.; Srinivasan, A. Use of antiviral nanocoating in personal protective wear. Int. J. Health Allied Sci. 2020, 9, 62–67. [Google Scholar]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Legge, E.J.; Ahmad, M.; Smith, C.T.; Brennan, B.; Mills, C.A.; Stolojan, V.; Pollard, A.J.; Silva, S.R.P. Physicochemical charac-terisation of reduced graphene oxide for conductive thin films. RSC Adv. 2018, 8, 37540–37549. [Google Scholar] [CrossRef]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef]
- Ziem, B.; Rahn, J.; Donskyi, I.; Silberreis, K.; Cuellar, L.; Dernedde, J.; Keil, G.; Mettenleiter, T.C.; Haag, R. Polyvalent 2D Entry Inhibitors for Pseudorabies and African Swine Fever Virus. Macromol. Biosci. 2017, 17, 1600499. [Google Scholar] [CrossRef] [PubMed]
- Elazzazy, A.M.; Elbeshehy, E.K.; Betiha, M.A. In vitro assessment of activity of graphene silver composite sheets against multidrug-resistant bacteria and Tomato Bushy Stunt Virus. Trop. J. Pharm. Res. 2018, 16, 2705–2711. [Google Scholar] [CrossRef]
- Reina, G.; Iglesias, D.; Samorì, P.; Bianco, A. Graphene: A Disruptive Opportunity for COVID-19 and Future Pandemics? Adv. Mater. 2021, 33, 2007847. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Ivanova, E.P. Mechano-bactericidal mechanism of graphene nanomaterials. Interface Focus 2018, 8, 20170060. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.; Farah, S.; Beyth, N.; Domb, A.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Galos, J.; Linklater, D.P.; Ladani, R.B.; Kandare, E.; Crawford, R.J..; Ivanova, E.P. Towards antiviral polymer composites to combat COVID-19 transmission. Nano Select 2021, 2, 2061. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant role of cationic polymers in drug delivery systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef]
- Kopecˇek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter. 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Thormar, H.; Bergsson, G.; Gunnarsson, E.; Georgsson, G.; Witvrouw, M.; Steingrimsson, O.; De Clercq, E.; Kristmundsdót-tir, T. 976 Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex. Transm. Infect. 1999, 75, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.; Corbitt, T.S.; Parthasarathy, A.; Schanze, K.S.; Whitten, D.G. Light and Dark-Activated Biocidal Activity of Conjugated Polyelectrolytes. ACS Appl. Mater. Interfaces 2011, 3, 2820–2829. [Google Scholar] [CrossRef] [PubMed]
- Chemburu, S.; Corbitt, T.S.; Ista, L.K.; Ji, E.; Fulghum, J.; Lopez, G.P.; Ogawa, K.; Schanze, K.S.; Whitten, D.G. Light-Induced Biocidal Action of Conjugated Polyelectrolytes Supported on Colloids. Langmuir 2008, 24, 11053–11062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Z.; Zhu, J.; Tang, Y.; Canady, T.D.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Dark Antimicrobial Mechanisms of Cationic Phenylene Ethynylene Polymers and Oligomers against Escherichia coli. Polymers 2011, 3, 1199–1214. [Google Scholar] [CrossRef]
- Boarino, A.; Wang, H.; Olgiati, F.; Artusio, F.; Özkan, M.; Bertella, S.; Razza, N.; Cagno, V.; Luterbacher, J.S.; Klok, H.-A.; et al. Lignin: A Sustainable Antiviral Coating Material. ACS Sustain. Chem. Eng. 2022, 10, 14001–14010. [Google Scholar] [CrossRef]
- Hosseini, M.; Behzadinasab, S.; Benmamoun, Z.; Ducker, W.A. The viability of SARS-CoV-2 on solid surfaces. Curr. Opin. Colloid Interface Sci. 2021, 55, 101481. [Google Scholar] [CrossRef]
- Patel, S.; Akhtar, N. Antimicrobial peptides (AMPs): The quintessential ‘offense and defense’ molecules are more than antimicrobials. Biomed. Pharmacother. 2017, 95, 1276–1283. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The Broad-Spectrum Antiviral Poten-tial of the Amphibian Peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef]
- Suda, S.; Field, D.; Barron, N. Antimicrobial peptide production and purification. In Protein Chromatography: Methods and Protocols; Springer Nature: Clifton, NJ, USA, 2017; pp. 401–410. [Google Scholar]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.D.; Bataglioli, R.A.; Oshodi, J.; Beppu, M.M. Antimicrobial peptides and their potential application in antiviral coating agents. Colloids Surf. B Biointerfaces 2022, 217, 112693. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Gabrani, R. Antiviral Peptides: Identification and Validation. Int. J. Pept. Res. Ther. 2020, 27, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; da Costa, A.; Dias, S.C.; Casal, M.; Machado, R. Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag. Pharmaceuticals 2021, 14, 956. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current ap-plications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Rothan, H.A.; Mohamed, Z.; Suhaeb, A.M.; Rahman, N.A.; Yusof, R. Antiviral cationic peptides as a strategy for innovation in global health therapeutics for dengue virus: High yield production of the biologically active recombinant plectasin pep-tide. OMICS J. Integr. Biol. 2013, 17, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Ajingi, Y.S.; Rukying, N.; Aroonsri, A.; Jongruja, N. Recombinant active Peptides and their Therapeutic functions. Curr. Pharm. Biotechnol. 2022, 23, 645–663. [Google Scholar] [CrossRef]
- Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Mohanty, A.K.; Kaushik, J.K.; Kumar, S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Vet. Sci. 2020, 7, 206. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Mulder, K.C.L.; Elima, L.A.; Miranda, V.J.; Dias, S.C.; Franco, O.L. Current scenario of peptide-based drugs: The key roles of cationic antitumor and antiviral peptides. Front. Microbiol. 2013, 4, 321. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Follin, P.; Johnsen, A.H.; Calafat, J.; Tjabringa, G.S.; Hiemstra, P.S.; Borregaard, N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood J. Am. Soc. Hematol. 2001, 97, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gennaro, R.; Skerlavaj, B.; Tomasinsig, L.; Circo, R. Cathelicidin Peptides as Candidates for a Novel Class of Antimicrobials. Curr. Pharm. Des. 2002, 8, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Huang, L.C.; Romanowski, E.G.; Yates, K.A.; Proske, R.J.; McDermott, A.M. Human Cathelicidin (LL-37), a Multifunctional Peptide, is Expressed by Ocular Surface Epithelia and has Potent Antibacterial and Antiviral Activity. Curr. Eye Res. 2005, 30, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides in Health and Disease. N. Engl. J. Med. 2002, 347, 1199–1200. [Google Scholar] [CrossRef]
- Fabisiak, A.; Murawska, N.; Fichna, J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol. Rep. 2016, 68, 802–808. [Google Scholar] [CrossRef]
- Nizet, V.; Ohtake, T.; Lauth, X.; Trowbridge, J.; Rudisill, J.; Dorschner, R.A.; Pestonjamasp, V.; Piraino, J.; Huttner, K.; Gallo, R.L. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001, 414, 454–457. [Google Scholar] [CrossRef]
- Bandurska, K.; Berdowska, A.; Barczyn’ska-Felusiak, R.; Krupa, P. Unique features of human cathelicidin LL-37. Biofactors 2015, 41, 289–300. [Google Scholar] [CrossRef]
- Bals, R.; Wilson, J.M. Cathelicidins—A family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. CMLS 2003, 60, 711–720. [Google Scholar] [CrossRef]
- Sousa, F.H.; Casanova, V.; Findlay, F.; Stevens, C.; Svoboda, P.; Pohl, J.; Proudfoot, L.; Barlow, P.G. Cathelicidins display con-served direct antiviral activity towards rhinovirus. Peptides 2017, 95, 76–83. [Google Scholar] [CrossRef]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Söderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral Mechanisms of Human Defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef] [PubMed]
- Casanova, V.; Sousa, F.H.; Shakamuri, P.; Svoboda, P.; Buch, C.; D’Acremont, M.; Christophorou, M.A.; Pohl, J.; Stevens, C.; Barlow, P.G. Citrullination Alters the Antiviral and Immunomodulatory Activities of the Human Cathelicidin LL-37 During Rhinovirus Infection. Front. Immunol. 2020, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Kalenik, B.M.; Góra-Sochacka, A.; Sirko, A. Β-defensins—Underestimated peptides in influenza combat. Virus Res. 2018, 247, 10–14. [Google Scholar] [CrossRef]
- Tripathi, S.; Tecle, T.; Verma, A.; Crouch, E.; White, M.; Hartshorn, K. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J. Gen. Virol. 2013, 94, 40–49. [Google Scholar] [CrossRef]
- Aloul, K.M.; Nielsen, J.E.; Defensor, E.B.; Lin, J.S.; Fortkort, J.A.; Shamloo, M.; Cirillo, J.D.; Gombart, A.F.; Barron, A.E. Upregulating Human Cathelicidin Antimicrobial Peptide LL-37 Expression May Prevent Severe COVID-19 Inflammatory Responses and Reduce Microthrombosis. Front. Immunol. 2022, 13, 880961. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, D.; Wei, D.Q.; Zhao, J.; Wang, J. Human intestinal defensin 5 inhibits SARS-CoV-2 invasion by cloaking ACE2. Gastroenterology 2020, 159, 1145–1147. [Google Scholar] [CrossRef]
- Kudryashova, E.; Zani, A.; Vilmen, G.; Sharma, A.; Lu, W.; Yount, J.S.; Kudryashov, D.S. Inhibition of SARS-CoV-2 infection by human defensin HNP1 and retrocyclin RC-101. J. Mol. Biol. 2022, 434, 167225. [Google Scholar] [CrossRef]
- Tonk, M.; Ržek, D.; Vilcinskas, A. Compelling evidence for the activity of antiviral peptides against SARS-CoV-2. Viruses 2021, 13, 912. [Google Scholar] [CrossRef]
- Tangpricha, V.; Judd, S.E.; Ziegler, T.R.; Hao, L.; Alvarez, J.A.; Fitzpatrick, A.M.; McComsey, G.A.; Eckard, A.R. LL-37 Concentrations and the Relationship to Vitamin D, Immune Status, and Inflammation in HIV-Infected Children and Young Adults. AIDS Res. Hum. Retrovir. 2014, 30, 670–676. [Google Scholar] [CrossRef]
- Wang, G.; Watson, K.M.; Buckheit, R.W., Jr. Anti-Human Immunodeficiency Virus Type 1 Activities of Antimicrobial Peptides Derived from Human and Bovine Cathelicidins. Antimicrob. Agents Chemother. 2008, 52, 3438–3440. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Hong, T.; Boo, L.M.; Nguyen, T.; Zhao, C.; Bristol, G.; Zack, J.A.; Waring, A.J.; Yang, O.O.; Lehrer, R.I. Retrocy-clin: A primate peptide that protects cells from infection by T-and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 2002, 99, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury—Nucleoside Analogues. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 1 May 2020).
- Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; et al. BCX4430–a broad-spectrum antiviral adenosine nucleoside analog under development for the treatment of Ebola virus disease. J. Infect. Public Health 2016, 9, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Yu, Y.; Cooper, C.L.; Wang, G.; Morwitzer, M.J.; Kota, K.; Tran, J.P.; Bradfute, S.B.; Liu, Y.; Shao, J.; Zhang, A.K.; et al. Engi-neered human cathelicidin antimicrobial peptides inhibit Ebola virus infection. iScience 2020, 23, 100999. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Li, Y.; Wang, G.; Tang, B.; Zhao, J.; Huang, Y.; Zheng, J. Cathelicidin-Derived Antimicrobial Peptides Inhibit Zika Virus Through Direct Inactivation and Interferon Pathway. Front. Immunol. 2018, 9, 722. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.H.; O’Neal, J.T.; Dale, G.; Holthausen, D.J.; Bowen, J.R.; Quicke, K.M.; Skountzou, I.; Gopal, S.; George, S.; et al. The amphibian peptide Yodha is virucidal for Zika and dengue viruses. Sci. Rep. 2021, 11, 602. [Google Scholar] [CrossRef]
- Krepstakies, M.; Lucifora, J.; Nagel, C.-H.; Zeisel, M.B.; Holstermann, B.; Hohenberg, H.; Kowalski, I.; Gutsmann, T.; Baumert, T.F.; Brandenburg, K.; et al. A New Class of Synthetic Peptide Inhibitors Blocks Attachment and Entry of Human Pathogenic Viruses. J. Infect. Dis. 2012, 205, 1654–1664. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Goddard, J.M. Antimicrobial Coatings with Dual Cationic and N-Halamine Character: Characterization and Biocidal Efficacy. J. Agric. Food Chem. 2015, 63, 4243–4251. [Google Scholar] [CrossRef]
- Bagheri, M.; Beyermann, M.; Dathe, M. Immobilization Reduces the Activity of Surface-Bound Cationic Antimicrobial Peptides with No Influence upon the Activity Spectrum. Antimicrob. Agents Chemother. 2009, 53, 1132–1141. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme entrapment, biocatalyst immobilization without covalent attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Andrea, A.; Molchanova, N.; Jenssen, H. Antibiofilm Peptides and Peptidomimetics with Focus on Surface Immobilization. Biomolecules 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Stillger, L.; Müller, D. Peptide-coating combating antimicrobial contaminations: A review of covalent immobilization strategies for industrial applications. J. Mater. Sci. 2022, 57, 10863–10885. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.Y.; Mei, Y.; Haney, E.F.; Siren, E.; Kalathottukaren, M.T.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Toward Infection-Resistant Surfaces: Achieving High Antimicrobial Peptide Potency by Modulating the Functionality of Polymer Brush and Peptide. ACS Appl. Mater. Interfaces 2015, 7, 28591–28605. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, K.; Elliott, M.; Jenssen, H.; Kindrachuk, J.; Fjell, C.D.; Körner, J.; Winkler, D.F.; Weaver, L.L.; Henklein, P.; Ulrich, A.S.; et al. Screening and Characterization of Surface-Tethered Cationic Peptides for Antimicrobial Activity. Chem. Biol. 2009, 16, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pinto, S.; Evangelista, M.B.; Gil, H.; Kallip, S.; Ferreira, M.G.; Ferreira, L. High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 2016, 33, 64–77. [Google Scholar] [CrossRef]
- Jeong, G.M.; Seong, H.; Im, S.G.; Sung, B.H.; Kim, S.C.; Jeong, K.J. Coating of an antimicrobial peptide on solid substrate via initiated chemical vapor deposition. J. Ind. Eng. Chem. 2018, 58, 51–56. [Google Scholar] [CrossRef]
- Steven, M.D.; Hotchkiss, J.H. Covalent immobilization of an antimicrobial peptide on poly(ethylene) film. J. Appl. Polym. Sci. 2008, 110, 2665–2670. [Google Scholar] [CrossRef]
- Rapsch, K.; Bier, F.F.; Tadros, M.; Von Nickisch-Rosenegk, M. Identification of Antimicrobial Peptides and Immobilization Strategy Suitable for a Covalent Surface Coating with Biocompatible Properties. Bioconjugate Chem. 2014, 25, 308–319. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, M.; Chen, S.; Lv, G.; Yao, J. Nanolayer biofilm coated on magnetic nanoparticles by using a dielectric barrier discharge glow plasma fluidized bed for immobilizing an antimicrobial peptide. Nanotechnology 2009, 20, 465706. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, C.W.; Kim, H.J.; Jung, H.-H.; Kim, J.I.; Shin, S.Y.; Shin, S.-H. Structural analysis and mode of action of BMAP-27, a cathelicidin-derived antimicrobial peptide. Peptides 2019, 118, 170106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mulvenon, A.; Makarov, E.; Wagoner, J.; Knibbe, J.; Kim, J.O.; Osna, N.; Bronich, T.K.; Poluektova, L.Y. Antiviral peptide nanocomplexes as a potential therapeutic modality for HIV/HCV co-infection. Biomaterials 2013, 34, 3846–3857. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Willcox, M.; Cole, N.; Ho, K.K.; Rasul, R.; Denman, J.; Kumar, N. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012, 8, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.; Maia, S.R.; Gomes, P.A.; Martins, M.C.L. Dhvar5 antimicrobial peptide (AMP) chemoselective covalent im-mobilization results on higher antiadherence effect than simple physical adsorption. Biomaterials 2015, 52, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Mishra, A. Exploring potential of glass surface immobilized short antimicrobial peptide (AMP) as antibacterial coatings. Mater. Today Proc. 2021, 49, 1367–1377. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Surface modification of poly(styrene) by the attachment of an antimicrobial peptide. J. Appl. Polym. Sci. 2001, 81, 609–616. [Google Scholar] [CrossRef]
- Héquet, A.; Humblot, V.; Berjeaud, J.-M.; Pradier, C.-M. Optimized grafting of antimicrobial peptides on stainless steel surface and biofilm resistance tests. Colloids Surfaces B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef]
- Stepulane, A.; Rajasekharan, A.K.; Andersson, M. Multifunctional Surface Modification of PDMS for Antibacterial Contact Killing and Drug-Delivery of Polar, Nonpolar, and Amphiphilic Drugs. ACS Appl. Bio Mater. 2022, 5, 5289–5301. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Hossain, K.R.; Chen, R.; Ho, K.K.K.; Kuppusamy, R.; Clarke, R.J.; Kumar, N.; Willcox, M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas aeruginosa. Front. Microbiol. 2020, 10, 3053. [Google Scholar] [CrossRef]
- Atefyekta, S. Antibacterial Surface Coatings for Biomedical Applications. Ph.D. Thesis, Chalmers Tekniska Hogskola, Göteborg, Sweden, 2016. [Google Scholar]
- Townsend, L.; Williams, R.L.; Anuforom, O.; Berwick, M.R.; Halstead, F.; Hughes, E.; Stamboulis, A.; Oppenheim, B.; Gough, J.; Grover, L.; et al. Antimicrobial peptide coatings for hydroxyapatite: Electrostatic and covalent attachment of antimicrobial peptides to surfaces. J. R. Soc. Interface 2017, 14, 20160657. [Google Scholar] [CrossRef]
- Xiao, M.; Jasensky, J.; Gerszberg, J.; Chen, J.; Tian, J.; Lin, T.; Lu, T.; Lahann, J.; Chen, Z. Chemically immobilized anti-microbial peptide on polymer and self-assembled monolayer substrates. Langmuir 2018, 34, 12889–12896. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; Kooi, S.; Chang, S.; Sedransk, K.; Gleason, K. Initiated chemical vapor deposition of antimicrobial polymer coatings. Biomaterials 2006, 28, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.; Bhattacharjee, S.; Kaur, K. Adsorption of an Antimicrobial Peptide on Self-Assembled Monolayers by Molecular Dynamics Simulation. J. Phys. Chem. B 2010, 114, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Kobe, A.C.; Sang, T.; Aparicio, C. Unraveling dominant surface physicochemistry to build antimicrobial peptide coatings with supramolecular amphiphiles. Nanoscale 2020, 12, 20767–20775. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, W.; Wang, L.; Li, R.; Gong, H.; Zhang, Y.; Xu, H.; Lu, J. CAS: 528: DC% 2BC1cXht1eksb7L: Graphene ox-ide-assisted accumulation and layer-by-layer assembly of antibacterial peptide for sustained release applications. ACS Appl. Mater. Interfaces 2018, 10, 24937–24946. [Google Scholar] [CrossRef]
- Otto, D.P.; de Villiers, M.M. Layer-by-layer nanocoating of antiviral polysaccharides on surfaces to prevent coronavirus infections. Molecules 2020, 25, 3415. [Google Scholar] [CrossRef]
- Açarı, İ.K.; Sel, E.; Özcan, İ.; Ateş, B.; Köytepe, S.; Thakur, V.K. Chemistry and engineering of brush type polymers: Perspective towards tissue engineering. Adv. Colloid Interface Sci. 2022, 305, 102694. [Google Scholar] [CrossRef]
- Muszanska, A.K.; Rochford, E.T.J.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; Van Der Mei, H.C.; Herrmann, A. Antiadhesive Polymer Brush Coating Functionalized with Antimicrobial and RGD Peptides to Reduce Biofilm Formation and Enhance Tissue Integration. Biomacromolecules 2014, 15, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Song, L.; Luan, S.; Xin, Z.; Du, S.; Shi, H.; Yuan, S.; Yang, Y.; Yin, J. A hierarchical polymer brush coating with du-al-function antibacterial capability. Colloids Surf. B Biointerfaces 2017, 150, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Xue, Y.; Ding, T.; Zhu, S.; Mao, M.; Zhang, L.; Han, Y. Polymer brush grafted antimicrobial peptide on hydroxyapatite nanorods for highly effective antibacterial performance. Chem. Eng. J. 2021, 423, 130133. [Google Scholar] [CrossRef]
- Alghrair, Z.K.; Fernig, D.G.; Ebrahimi, B. Enhanced inhibition of influenza virus infection by peptide–noble-metal nano-particle conjugates. Beilstein J. Nanotechnol. 2019, 10, 1038–1047. [Google Scholar] [CrossRef]
- Baram-Pinto, D.; Shukla, S.; Richman, M.; Gedanken, A.; Rahimipour, S.; Sarid, R. Surface-modified protein nanospheres as potential antiviral agents. Chem. Commun. 2012, 48, 8359–8361. [Google Scholar] [CrossRef]
- Gessner, I.; Neundorf, I. Nanoparticles Modified with Cell-Penetrating Peptides: Conjugation Mechanisms, Physicochemical Properties, and Application in Cancer Diagnosis and Therapy. Int. J. Mol. Sci. 2020, 21, 2536. [Google Scholar] [CrossRef]
- da Costa, A.; Pereira, A.M.; Sampaio, P.; Rodríguez-Cabello, J.C.; Gomes, A.C.; Casal, M.; Machado, R. Protein-Based Films Functionalized with a Truncated Antimicrobial Peptide Sequence Display Broad Antimicrobial Activity. ACS Biomater. Sci. Eng. 2021, 7, 451–461. [Google Scholar] [CrossRef]
- Lima, L.F.; Sousa, M.G.D.C.; Rodrigues, G.R.; de Oliveira, K.B.S.; Pereira, A.M.; da Costa, A.; Machado, R.; Franco, O.L.; Dias, S.C. Elastin-like Polypeptides in Development of Nanomaterials for Application in the Medical Field. Front. Nanotechnol. 2022, 1169, 31. [Google Scholar] [CrossRef]
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings: Functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256. [Google Scholar] [CrossRef]
- Dominy, B.N.; Perl, D.; Schmid, F.X.; Brooks III, C.L. The effects of ionic strength on protein stability: The cold shock protein family. J. Mol. Biol. 2002, 319, 541–554. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Evaluation of Strategies for Improving Proteolytic Resistance of Antimicrobial Peptides by Using Variants of EFK17, an Internal Segment of LL-37. Antimicrob. Agents Chemother. 2009, 53, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Knappe, D.; Henklein, P.; Hoffmann, R.; Hilpert, K. Easy Strategy To Protect Antimicrobial Peptides from Fast Degradation in Serum. Antimicrob. Agents Chemother. 2010, 54, 4003–4005. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, M.; Biswas, P.; Islam, M.T.; Paul, R. Antiviral Peptides in Antimicrobial Surface Coatings—From Current Techniques to Potential Applications. Viruses 2023, 15, 640. https://doi.org/10.3390/v15030640
Jabeen M, Biswas P, Islam MT, Paul R. Antiviral Peptides in Antimicrobial Surface Coatings—From Current Techniques to Potential Applications. Viruses. 2023; 15(3):640. https://doi.org/10.3390/v15030640
Chicago/Turabian StyleJabeen, Mahe, Payel Biswas, Md Touhidul Islam, and Rajesh Paul. 2023. "Antiviral Peptides in Antimicrobial Surface Coatings—From Current Techniques to Potential Applications" Viruses 15, no. 3: 640. https://doi.org/10.3390/v15030640
APA StyleJabeen, M., Biswas, P., Islam, M. T., & Paul, R. (2023). Antiviral Peptides in Antimicrobial Surface Coatings—From Current Techniques to Potential Applications. Viruses, 15(3), 640. https://doi.org/10.3390/v15030640






