CryoEM of Viral Ribonucleoproteins and Nucleocapsids of Single-Stranded RNA Viruses
Abstract
1. Introduction
2. The Single-Stranded RNA Viruses and Ribonucleoproteins
3. Structure of the Nucleocapsid of -ssRNAv
3.1. Nucleocapsids of Non-Segmented, -ssRNAv
3.1.1. Filoviridae: Ebola Virus (EBOV) and Marburg Virus (MARV)
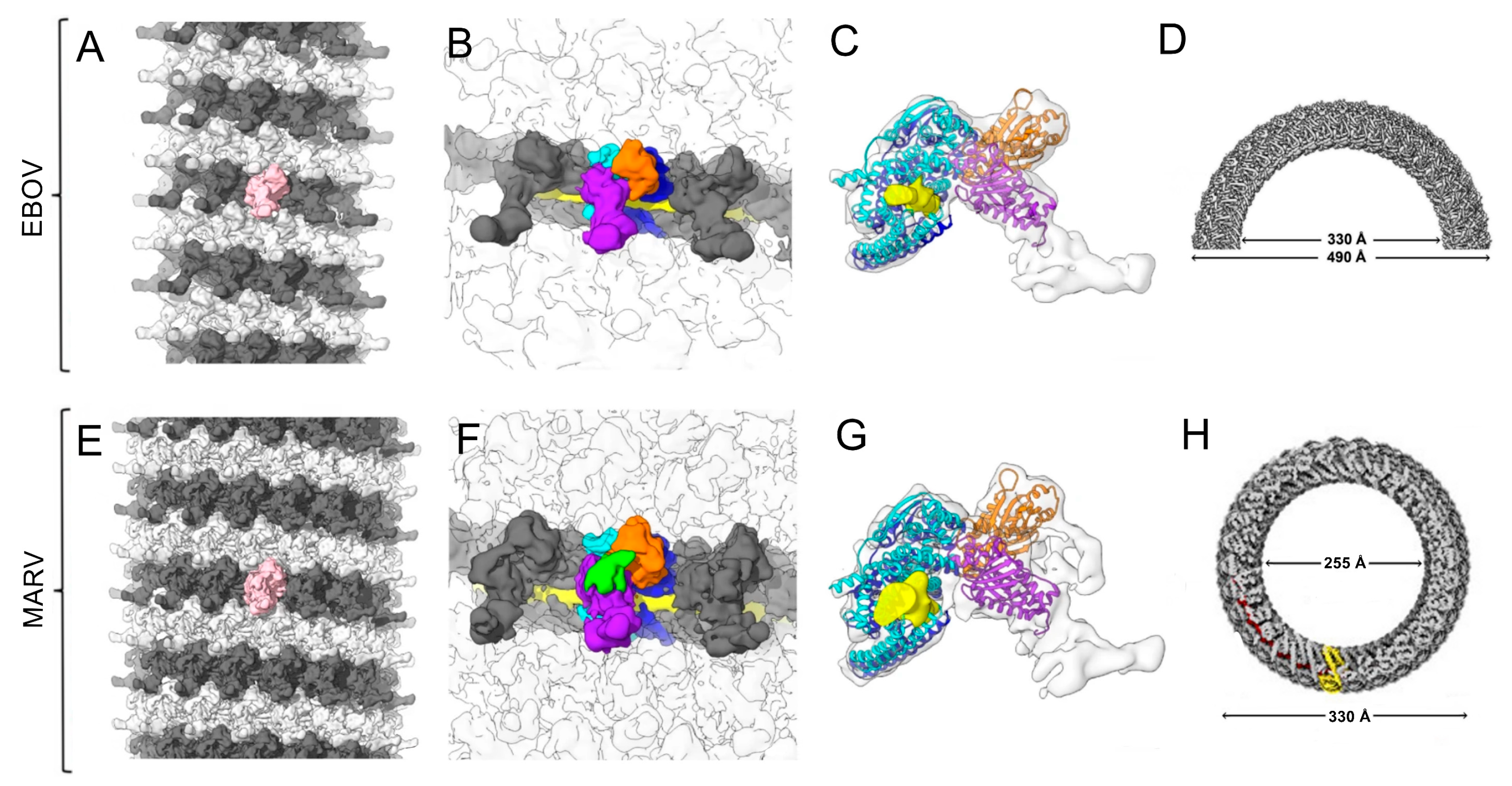
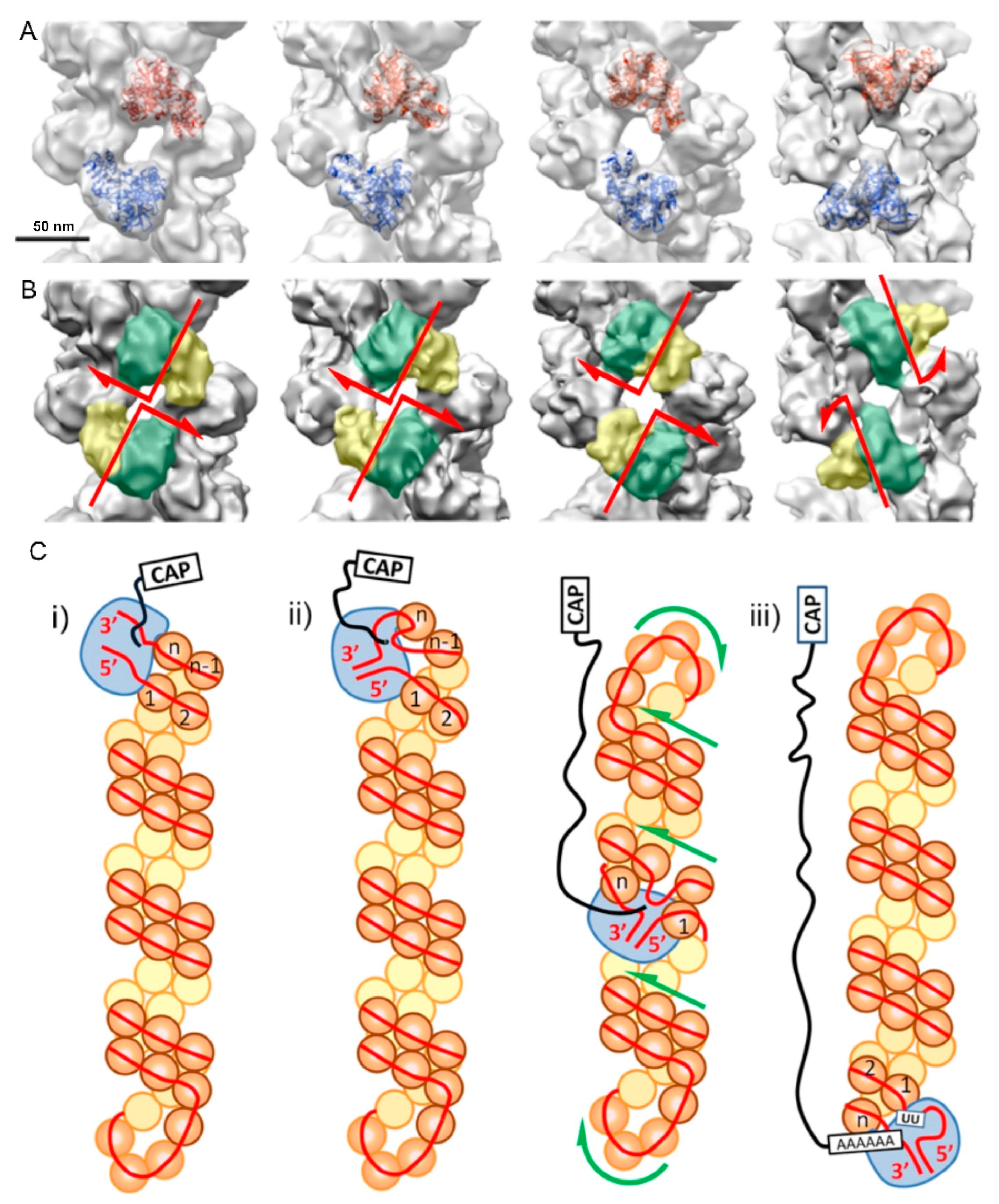
3.1.2. Rhabdoviridae: Rabies Virus (RABV) and Vesicular Stomatitis Virus (VSV)

3.1.3. Paramyxoviridae: Mumps Virus (MuV) and Measles Morbillivirus (MeV)

3.2. Nucleocapsids of Segmented, -ssRNAv
3.2.1. Bunyavirales
Hantaviridae: Hantaan Orthohantavirus (HTNV)
Peribunyaviridae: Bunyamwera Orthobunyavirus (BUNV)
Nairoviridae: Crimean-Congo Hemorrhagic Fever Orthonairovirus (CCHFV)

3.2.2. Articulavirales
Orthomyxoviridae: Influenza A Virus (IAV)

4. Structure of the Nucleocapsid of +ssRNAv
5. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Kühlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, D.; Nutalai, R.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Mentzer, A.J.; Wang, B.; et al. The antibody response to SARS-CoV-2 Beta underscores the antigenic distance to other variants. Cell Host Microbe 2022, 30, 53–68.e12. [Google Scholar] [CrossRef] [PubMed]
- Wandzik, J.M.; Kouba, T.; Karuppasamy, M.; Pflug, A.; Drncova, P.; Provaznik, J.; Azevedo, N.; Cusack, S. A Structure-Based Model for the Complete Transcription Cycle of Influenza Polymerase. Cell 2020, 181, 877–893.e21. [Google Scholar] [CrossRef]
- Wandzik, J.M.; Kouba, T.; Cusack, S. Structure and Function of Influenza Polymerase. Cold Spring Harb. Perspect. Med. 2021, 11, a038372. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Hernandez, R.; Jácome, R.; López Vidal, Y.; Ponce de León, S. Are RNA Viruses Candidate Agents for the Next Global Pandemic? A Review. ILAR J. 2017, 58, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Condit, R.C. Principles of Virology. In Fields Virol; J. B. Lippincott & Co.: Philadelphia, PA, USA, 2007; pp. 21–51. [Google Scholar]
- Morin, B.; Kranzusch, P.J.; Rahmeh, A.A.; Whelan, S.P. The polymerase of negative-stranded RNA viruses. Curr. Opin. Virol. 2013, 3, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.E. Principles of Virus Structure. In Fields Virol; J. B. Lippincott & Co.: Philadelphia, PA, USA, 2007; pp. 52–86. [Google Scholar]
- York, A.; Hengrung, N.; Vreede, F.T.; Huiskonen, J.T.; Fodor, E. Isolation and characterization of the positive-sense replicative intermediate of a negative-strand RNA virus. Proc. Natl. Acad. Sci. USA 2013, 110, E4238–E4245. [Google Scholar] [CrossRef]
- Coloma, R.; Arranz, R.; de la Rosa-Trevín, J.M.; Sorzano, C.O.S.; Munier, S.; Carlero, D.; Naffakh, N.; Ortín, J.; Martín-Benito, J. Structural insights into influenza A virus ribonucleoproteins reveal a processive helical track as transcription mechanism. Nat. Microbiol. 2020, 5, 727–734. [Google Scholar] [CrossRef]
- Lamb, R.A. Mononegavirales. In Fields Virol; J. B. Lippincott & Co.: Philadelphia, PA, USA, 2007; pp. 880–884. [Google Scholar]
- Ortín, J.; Martín-Benito, J. The RNA synthesis machinery of negative-stranded RNA viruses. Virology 2015, 479–480, 532–544. [Google Scholar] [CrossRef]
- Liang, B. Structures of the Mononegavirales Polymerases. J. Virol. 2020, 94, e00175-20. [Google Scholar] [CrossRef]
- Yu, D.-S.; Weng, T.-H.; Wu, X.-X.; Wang, F.X.C.; Lu, X.-Y.; Wu, H.-B.; Wu, N.-P.; Li, L.-J.; Yao, H.-P. The lifecycle of the Ebola virus in host cells. Oncotarget 2017, 8, 55750–55759. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Hoenen, T.; Canard, B.; Decroly, E. Filovirus proteins for antiviral drug discovery: A structure/function analysis of surface glycoproteins and virus entry. Antiviral Res. 2016, 135, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hoenen, T.; Groseth, A.; Falzarano, D.; Feldmann, H. Ebola virus: Unravelling pathogenesis to combat a deadly disease. Trends Mol. Med. 2006, 12, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Bharat, T.A.M.; Noda, T.; Riches, J.D.; Kraehling, V.; Kolesnikova, L.; Becker, S.; Kawaoka, Y.; Briggs, J.A.G. Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography. Proc. Natl. Acad. Sci. USA 2012, 109, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Kolesnikova, L.; Clarke, M.; Koehler, A.; Noda, T.; Becker, S.; Briggs, J.A.G. Structure and assembly of the Ebola virus nucleocapsid. Nature 2017, 551, 394–397. [Google Scholar] [CrossRef]
- Sugita, Y.; Matsunami, H.; Kawaoka, Y.; Noda, T.; Wolf, M. Cryo-EM structure of the Ebola virus nucleoprotein–RNA complex at 3.6 Å resolution. Nature 2018, 563, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wu, C.; Shi, L.; Luthra, P.; Pintilie, G.D.; Johnson, B.; Porter, J.R.; Ge, P.; Chen, M.; Liu, G.; et al. Electron Cryo-microscopy Structure of Ebola Virus Nucleoprotein Reveals a Mechanism for Nucleocapsid-like Assembly. Cell 2018, 172, 966–978.e12. [Google Scholar] [CrossRef] [PubMed]
- Bharat, T.A.M.; Riches, J.D.; Kolesnikova, L.; Welsch, S.; Krähling, V.; Davey, N.; Parsy, M.-L.; Becker, S.; Briggs, J.A.G. Cryo-Electron Tomography of Marburg Virus Particles and Their Morphogenesis within Infected Cells. PLoS Biol. 2011, 9, e1001196. [Google Scholar] [CrossRef]
- Fujita-Fujiharu, Y.; Sugita, Y.; Takamatsu, Y.; Houri, K.; Igarashi, M.; Muramoto, Y.; Nakano, M.; Tsunoda, Y.; Taniguchi, I.; Becker, S.; et al. Structural insight into Marburg virus nucleoprotein-RNA complex formation. Nat. Commun. 2022, 13, 1191. [Google Scholar] [CrossRef]
- Hu, S.; Noda, T. Filovirus helical nucleocapsid structures. Microscopy 2022. [Google Scholar] [CrossRef]
- Dolnik, O.; Becker, S. Assembly and transport of filovirus nucleocapsids. PLoS Pathog. 2022, 18, e1010616. [Google Scholar] [CrossRef]
- Davis, B.M.; Rall, G.F.; Schnell, M.J. Everything You Always Wanted to Know About Rabies Virus (But Were Afraid to Ask). Annu. Rev. Virol. 2015, 2, 451–471. [Google Scholar] [CrossRef]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Virus Taxonomy. In Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011; p. 727. [Google Scholar]
- Ge, P.; Tsao, J.; Schein, S.; Green, T.J.; Luo, M.; Zhou, Z.H. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 2010, 327, 689–693. [Google Scholar] [CrossRef]
- Jenni, S.; Horwitz, J.A.; Bloyet, L.-M.; Whelan, S.P.J.; Harrison, S.C. Visualizing molecular interactions that determine assembly of a bullet-shaped vesicular stomatitis virus particle. Nat. Commun. 2022, 13, 4802. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, W.F.; Arnheiter, H.; Dubois-Dalcq, M.; Lazzarini, R.A. Stereo images of vesicular stomatitis virus assembly. J. Virol. 1986, 57, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Zhou, K.; Tsao, J.; Luo, M.; Zhou, Z.H. Locations and in situ structure of the polymerase complex inside the virion of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2022, 119, e2111948119. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Si, Z.; Ge, P.; Tsao, J.; Luo, M.; Zhou, Z.H. Atomic model of vesicular stomatitis virus and mechanism of assembly. Nat. Commun. 2022, 13, 5980. [Google Scholar] [CrossRef]
- Guichard, P.; Krell, T.; Chevalier, M.; Vaysse, C.; Adam, O.; Ronzon, F.; Marco, S. Three dimensional morphology of rabies virus studied by cryo-electron tomography. J. Struct. Biol. 2011, 176, 32–40. [Google Scholar] [CrossRef]
- Riedel, C.; Vasishtan, D.; Pražák, V.; Ghanem, A.; Conzelmann, K.-K.; Rümenapf, T. Cryo EM structure of the rabies virus ribonucleoprotein complex. Sci. Rep. 2019, 9, 9639. [Google Scholar] [CrossRef]
- Albertini, A.A.V.; Wernimont, A.K.; Muziol, T.; Ravelli, R.B.G.; Clapier, C.R.; Schoehn, G.; Weissenhorn, W.; Ruigrok, R.W.H. Crystal Structure of the Rabies Virus Nucleoprotein-RNA Complex. Science 2006, 313, 360–363. [Google Scholar] [CrossRef]
- Riedel, C.; Hennrich, A.A.; Conzelmann, K.-K. Components and Architecture of the Rhabdovirus Ribonucleoprotein Complex. Viruses 2020, 12, 959. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Green, T.J.; Zhang, X.; Tsao, J.; Qiu, S. Conserved characteristics of the rhabdovirus nucleoprotein. Virus Res. 2007, 129, 246–251. [Google Scholar] [CrossRef]
- Cox, R.M.; Plemper, R.K. Structure and organization of paramyxovirus particles. Curr. Opin. Virol. 2017, 24, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Liljeroos, L.; Huiskonen, J.T.; Ora, A.; Susi, P.; Butcher, S.J. Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions. Proc. Natl. Acad. Sci. USA 2011, 108, 18085–18090. [Google Scholar] [CrossRef]
- Desfosses, A.; Goret, G.; Farias Estrozi, L.; Ruigrok, R.W.H.; Gutsche, I. Nucleoprotein-RNA Orientation in the Measles Virus Nucleocapsid by Three-Dimensional Electron Microscopy. J. Virol. 2011, 85, 1391–1395. [Google Scholar] [CrossRef]
- Gutsche, I.; Desfosses, A.; Effantin, G.; Ling, W.L.; Haupt, M.; Ruigrok, R.W.H.; Sachse, C.; Schoehn, G. Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science 2015, 348, 704–707. [Google Scholar] [CrossRef]
- Desfosses, A.; Milles, S.; Jensen, M.R.; Guseva, S.; Colletier, J.-P.; Maurin, D.; Schoehn, G.; Gutsche, I.; Ruigrok, R.W.H.; Blackledge, M. Assembly and cryo-EM structures of RNA-specific measles virus nucleocapsids provide mechanistic insight into paramyxoviral replication. Proc. Natl. Acad. Sci. USA 2019, 116, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Pickar, A.; Qiu, S.; Tsao, J.; Rodenburg, C.; Dokland, T.; Elson, A.; He, B.; Luo, M. Structural studies on the authentic mumps virus nucleocapsid showing uncoiling by the phosphoprotein. Proc. Natl. Acad. Sci. USA 2014, 111, 15208–15213. [Google Scholar] [CrossRef]
- Shan, H.; Su, X.; Li, T.; Qin, Y.; Zhang, N.; Yang, L.; Ma, L.; Bai, Y.; Qi, L.; Liu, Y.; et al. Structural plasticity of mumps virus nucleocapsids with cryo-EM structures. Commun. Biol. 2021, 4, 833. [Google Scholar] [CrossRef] [PubMed]
- Zinzula, L.; Beck, F.; Klumpe, S.; Bohn, S.; Pfeifer, G.; Bollschweiler, D.; Nagy, I.; Plitzko, J.M.; Baumeister, W. Cryo-EM structure of the cetacean morbillivirus nucleoprotein-RNA complex. J. Struct. Biol. 2021, 213, 107750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shan, H.; Liu, M.; Li, T.; Luo, R.; Yang, L.; Qi, L.; Chu, X.; Su, X.; Wang, R.; et al. Structure and assembly of double-headed Sendai virus nucleocapsids. Commun. Biol. 2021, 4, 494. [Google Scholar] [CrossRef]
- Li, T.; Shen, Q.-T. Insights into Paramyxovirus Nucleocapsids from Diverse Assemblies. Viruses 2021, 13, 2479. [Google Scholar] [CrossRef]
- Bloyet, L.-M. The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template. Viruses 2021, 13, 2465. [Google Scholar] [CrossRef] [PubMed]
- Pringle, C.R. Genetics and Genome Segment Reassortment. In The Bunyaviridae; Elliott, R.M., Ed.; Springer US: Boston, MA, USA, 1996; pp. 189–226. [Google Scholar]
- Weber, M.; Weber, F. Segmented negative-strand RNA viruses and RIG-I: Divide (your genome) and rule. Curr. Opin. Microbiol. 2014, 20, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, R.W.H.; Crépin, T.; Kolakofsky, D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr. Opin. Microbiol. 2011, 14, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; Kormelink, R.; Kortekaas, J. Genome packaging of the Bunyavirales. Curr. Opin. Virol. 2018, 33, 151–155. [Google Scholar] [CrossRef]
- Leventhal, S.S.; Wilson, D.; Feldmann, H.; Hawman, D.W. A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins. Viruses 2021, 13, 314. [Google Scholar] [CrossRef]
- Klempa, B. Reassortment events in the evolution of hantaviruses. Virus Genes 2018, 54, 638–646. [Google Scholar] [CrossRef]
- Battisti, A.J.; Chu, Y.-K.; Chipman, P.R.; Kaufmann, B.; Jonsson, C.B.; Rossmann, M.G. Structural Studies of Hantaan Virus. J. Virol. 2011, 85, 835–841. [Google Scholar] [CrossRef]
- Arragain, B.; Reguera, J.; Desfosses, A.; Gutsche, I.; Schoehn, G.; Malet, H. High resolution cryo-EM structure of the helical RNA-bound Hantaan virus nucleocapsid reveals its assembly mechanisms. eLife 2019, 8, e43075. [Google Scholar] [CrossRef]
- Kaukinen, P.; Kumar, V.; Tulimäki, K.; Engelhardt, P.; Vaheri, A.; Plyusnin, A. Oligomerization of Hantavirus N protein: C-terminal alpha-helices interact to form a shared hydrophobic space. J. Virol. 2004, 78, 13669–13677. [Google Scholar] [CrossRef]
- Hopkins, F.R.; Álvarez-Rodríguez, B.; Heath, G.R.; Panayi, K.; Hover, S.; Edwards, T.A.; Barr, J.N.; Fontana, J. The Native Orthobunyavirus Ribonucleoprotein Possesses a Helical Architecture. mBio 2022, 13, e01405-22. [Google Scholar] [CrossRef] [PubMed]
- Wichgers Schreur, P.J.; Kortekaas, J. Single-Molecule FISH Reveals Non-selective Packaging of Rift Valley Fever Virus Genome Segments. PLoS Pathog. 2016, 12, e1005800. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, W.; Ji, W.; Deng, M.; Sun, Y.; Zhou, H.; Yang, C.; Deng, F.; Wang, H.; Hu, Z.; et al. Crimean–Congo hemorrhagic fever virus nucleoprotein reveals endonuclease activity in bunyaviruses. Proc. Natl. Acad. Sci. USA 2012, 109, 5046–5051. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Guo, Y.; Shen, S.; Zhao, L.; Zhang, P.; Sun, Y.; Sui, S.-F.; Deng, F.; Lou, Z. Molecular basis for the formation of ribonucleoprotein complex of Crimean-Congo hemorrhagic fever virus. J. Struct. Biol. 2016, 196, 455–465. [Google Scholar] [CrossRef]
- Reguera, J.; Malet, H.; Weber, F.; Cusack, S. Structural basis for encapsidation of genomic RNA by La Crosse Orthobunyavirus nucleoprotein. Proc. Natl. Acad. Sci. USA 2013, 110, 7246–7251. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Gao, G.F.; Tien, P.; Liu, W. Bunyavirales ribonucleoproteins: The viral replication and transcription machinery. Crit. Rev. Microbiol. 2018, 44, 522–540. [Google Scholar] [CrossRef]
- Compans, R.W.; Content, J.; Duesberg, P.H. Structure of the Ribonucleoprotein of Influenza Virus. J. Virol. 1972, 10, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Martín-Benito, J.; Zürcher, T.; Valpuesta, J.M.; Carrascosa, J.L.; Ortín, J. Ultrastructural and Functional Analyses of Recombinant Influenza Virus Ribonucleoproteins Suggest Dimerization of Nucleoprotein during Virus Amplification. J. Virol. 2000, 74, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Martín-Benito, J.; Area, E.; Ortega, J.; Llorca, O.; Valpuesta, J.M.; Carrascosa, J.L.; Ortín, J. Three-dimensional reconstruction of a recombinant influenza virus ribonucleoprotein particle. EMBO Rep. 2001, 2, 313–317. [Google Scholar] [CrossRef]
- Coloma, R.; Valpuesta, J.M.; Arranz, R.; Carrascosa, J.L.; Ortín, J.; Martín-Benito, J. The Structure of a Biologically Active Influenza Virus Ribonucleoprotein Complex. PLoS Pathog. 2009, 5, e1000491. [Google Scholar] [CrossRef]
- Arranz, R.; Coloma, R.; Chichón, F.J.; Conesa, J.J.; Carrascosa, J.L.; Valpuesta, J.M.; Ortín, J.; Martín-Benito, J. The Structure of Native Influenza Virion Ribonucleoproteins. Science 2012, 338, 1634–1637. [Google Scholar] [CrossRef]
- Moeller, A.; Kirchdoerfer, R.N.; Potter, C.S.; Carragher, B.; Wilson, I.A. Organization of the Influenza Virus Replication Machinery. Science 2012, 338, 1631–1634. [Google Scholar] [CrossRef]
- Noda, T.; Sagara, H.; Yen, A.; Takada, A.; Kida, H.; Cheng, R.H.; Kawaoka, Y. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 2006, 439, 490–492. [Google Scholar] [CrossRef]
- Nakatsu, S.; Sagara, H.; Sakai-Tagawa, Y.; Sugaya, N.; Noda, T.; Kawaoka, Y. Complete and Incomplete Genome Packaging of Influenza A and B Viruses. mBio 2016, 7, e01248-16. [Google Scholar] [CrossRef] [PubMed]
- Noda, T.; Sugita, Y.; Aoyama, K.; Hirase, A.; Kawakami, E.; Miyazawa, A.; Sagara, H.; Kawaoka, Y. Three-dimensional analysis of ribonucleoprotein complexes in influenza A virus. Nat. Commun. 2012, 3, 639. [Google Scholar] [CrossRef] [PubMed]
- Martín-Benito, J.; Ortín, J. Influenza Virus Transcription and Replication. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 87, pp. 113–137. [Google Scholar]
- Villa, T.G.; Abril, A.G.; Sánchez, S.; de Miguel, T.; Sánchez-Pérez, A. Animal and human RNA viruses: Genetic variability and ability to overcome vaccines. Arch. Microbiol. 2021, 203, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, P.; Noueiry, A.O.; Lee, W.-M.; Kushner, D.B.; Dye, B.T. Host Factors in Positive-Strand RNA Virus Genome Replication. J. Virol. 2003, 77, 8181–8186. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M.; Dewantari, A.K.; Wiyatno, A. Molecular biology of coronaviruses: Current knowledge. Heliyon 2020, 6, e04743. [Google Scholar] [CrossRef]
- Macnaughton, M.R.; Davies, H.A.; Nermut, M.V. Ribonucleoprotein-like Structures from Coronavirus Particles. J. Gen. Virol. 1978, 39, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Neuman, B.W.; Adair, B.D.; Yoshioka, C.; Quispe, J.D.; Orca, G.; Kuhn, P.; Milligan, R.A.; Yeager, M.; Buchmeier, M.J. Supramolecular Architecture of Severe Acute Respiratory Syndrome Coronavirus Revealed by Electron Cryomicroscopy. J. Virol. 2006, 80, 7918–7928. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, M.; Oostergetel, G.T.; Bartelink, W.; Faas, F.G.A.; Verkleij, A.; Rottier, P.J.M.; Koster, A.J.; Bosch, B.J. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 2009, 106, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Liu, X.; Guo, D.; Zhang, Z.; Yin, C.-C.; Chen, Y.; Xiang, Y. Electron microscopy studies of the coronavirus ribonucleoprotein complex. Protein Cell 2017, 8, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Cortese, M.; Winter, S.L.; Wachsmuth-Melm, M.; Neufeldt, C.J.; Cerikan, B.; Stanifer, M.L.; Boulant, S.; Bartenschlager, R.; Chlanda, P. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Nat. Commun. 2020, 11, 5885. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738.e13. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Galaz-Montoya, J.G.; Sherman, M.B.; Sun, S.Y.; Goldsmith, C.S.; O’Toole, E.T.; Ackerman, L.; Carlson, L.-A.; Weaver, S.C.; Chiu, W.; et al. Neutralizing Antibodies Inhibit Chikungunya Virus Budding at the Plasma Membrane. Cell Host Microbe 2018, 24, 417–428.e5. [Google Scholar] [CrossRef]
- Calder, L.J.; Calcraft, T.; Hussain, S.; Harvey, R.; Rosenthal, P.B. Electron cryotomography of SARS-CoV-2 virions reveals cylinder-shaped particles with a double layer RNP assembly. Commun. Biol. 2022, 5, 1210. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrego, A.; Carlero, D.; Arranz, R.; Martín-Benito, J. CryoEM of Viral Ribonucleoproteins and Nucleocapsids of Single-Stranded RNA Viruses. Viruses 2023, 15, 653. https://doi.org/10.3390/v15030653
Modrego A, Carlero D, Arranz R, Martín-Benito J. CryoEM of Viral Ribonucleoproteins and Nucleocapsids of Single-Stranded RNA Viruses. Viruses. 2023; 15(3):653. https://doi.org/10.3390/v15030653
Chicago/Turabian StyleModrego, Andrea, Diego Carlero, Rocío Arranz, and Jaime Martín-Benito. 2023. "CryoEM of Viral Ribonucleoproteins and Nucleocapsids of Single-Stranded RNA Viruses" Viruses 15, no. 3: 653. https://doi.org/10.3390/v15030653
APA StyleModrego, A., Carlero, D., Arranz, R., & Martín-Benito, J. (2023). CryoEM of Viral Ribonucleoproteins and Nucleocapsids of Single-Stranded RNA Viruses. Viruses, 15(3), 653. https://doi.org/10.3390/v15030653




