Differential Impact of IL-32 Isoforms on the Functions of Coronary Artery Endothelial Cells: A Potential Link with Arterial Stiffness and Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Participants
2.2. Cells
2.3. Flow Cytometry Analysis
2.4. Cell Stimulation
2.5. ELISA
2.6. Quantitative Reverse Transcription Assays (RT-qPCR)
2.7. Transwell Assay
2.8. Carotid Artery Ultrasound Imaging and Image Analysis
2.9. Statistical Analysis
3. Results
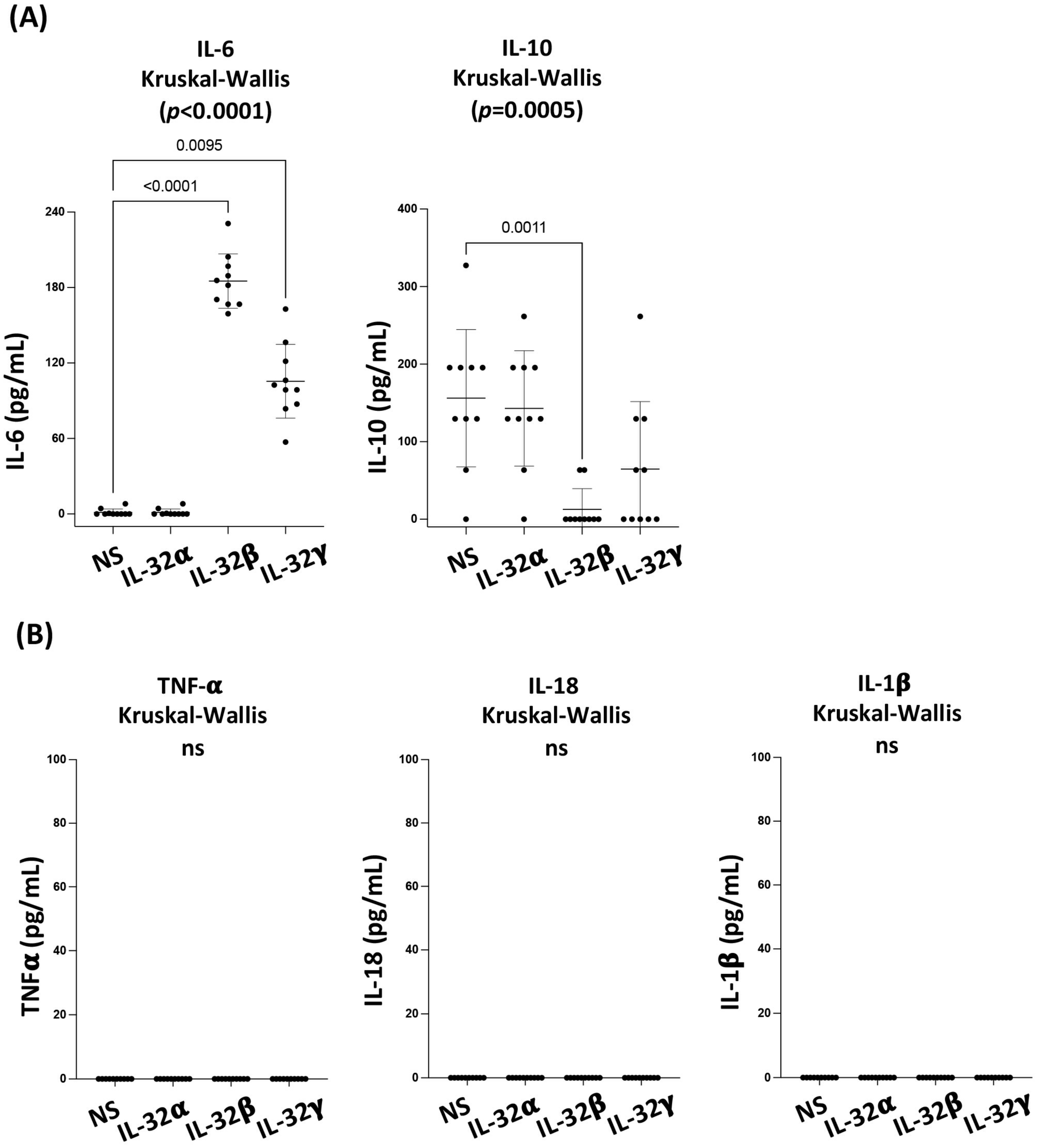
3.1. IL-32 Isoforms Exhibit a Differential Impact on Cytokine Production by the Primary Coronary Artery Endothelial Cells
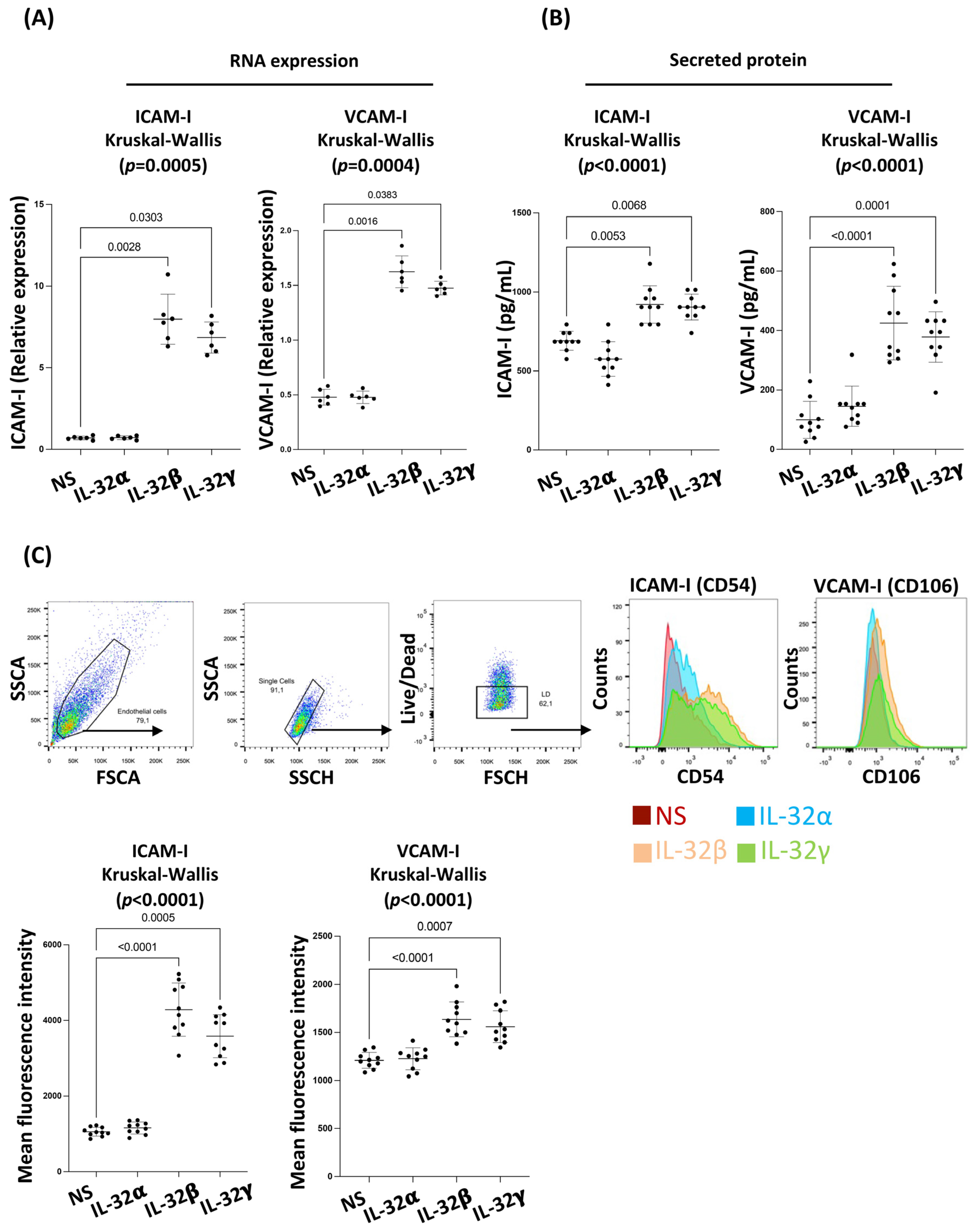
3.2. IL-32 β and γ Induce Coronary Artery Endothelial Cell Dysfunction
3.3. IL-32 β and γ Increase Chemokine Production in Coronary Artery Endothelial Cells
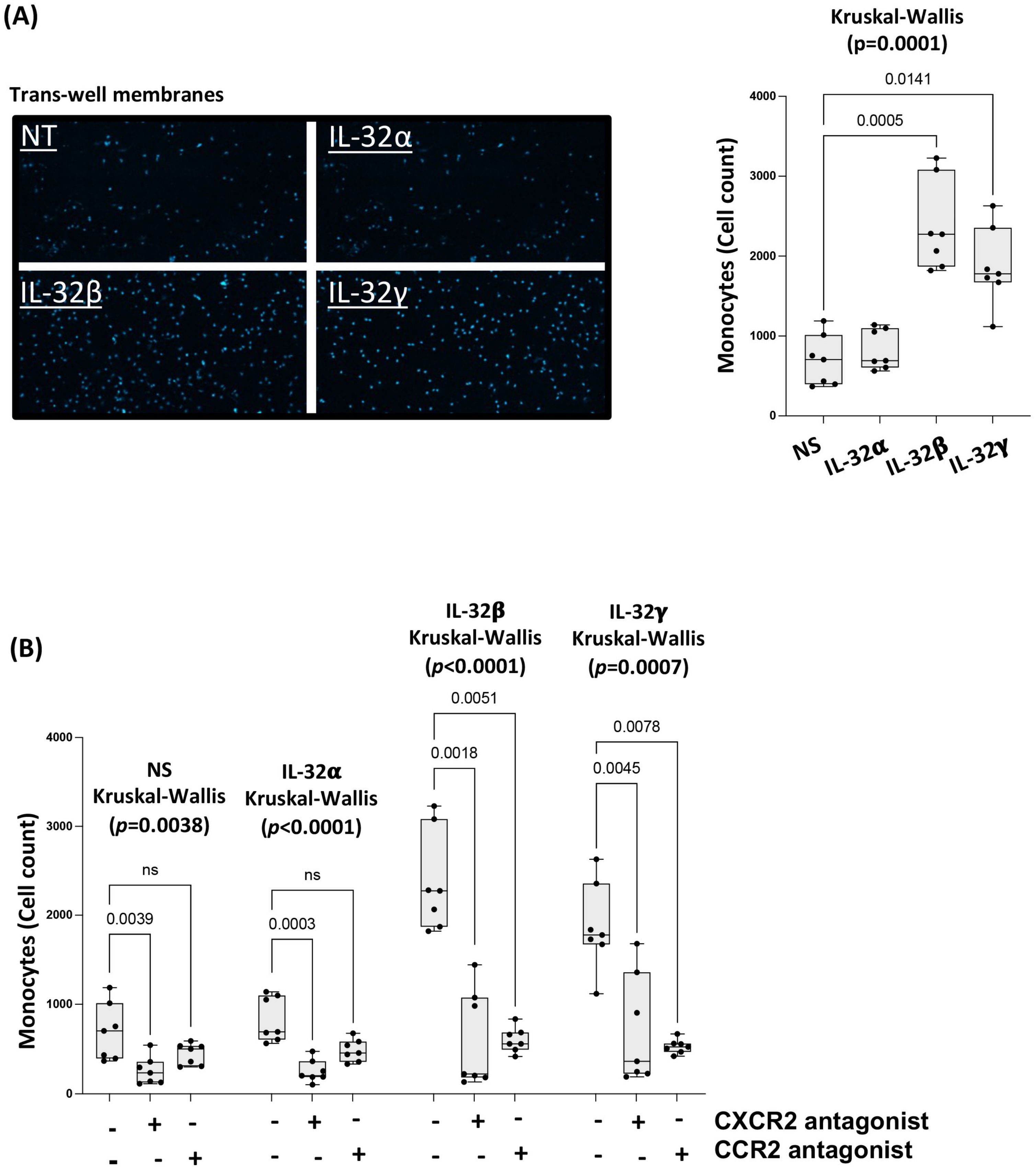
3.4. IL-32 β- and γ-Induced Chemokines Drive Monocyte Transmigration towards CAEC Supernatants
3.5. IL-32 Expression Is Associated with Carotid Artery Wall Stiffness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teeraananchai, S.; Kerr, S.; Amin, J.; Ruxrungtham, K.; Law, M. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med. 2017, 18, 256–266. [Google Scholar] [CrossRef]
- Legarth, R.A.; Ahlström, M.G.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Pedersen, G.; Mohey, R.; Gerstoft, J.; Obel, N. Long-Term Mortality in HIV-Infected Individuals 50 Years or Older: A Nationwide, Population-Based Cohort Study. J. Acquir. Immune. Defic. Syndr. 2016, 71, 213–218. [Google Scholar] [CrossRef]
- Wing, E.J. HIV and aging. Int. J. Infect. Dis. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Silverberg, M.J.; Chao, C.; Leyden, W.A.; Xu, L.; Tang, B.; Horberg, M.A.; Klein, D.; Quesenberry, C.P.; Towner, W.J.; Abrams, D.I. HIV infection and the risk of cancers with and without a known infectious cause. AIDS 2009, 23, 2337–2345. [Google Scholar] [CrossRef]
- Edén, A.; Marcotte, T.D.; Heaton, R.K.; Nilsson, S.; Zetterberg, H.; Fuchs, D.; Franklin, D.; Price, R.W.; Grant, I.; Letendre, S.L.; et al. Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS ONE 2016, 11, e0157160. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.M.; Wu, J.; Jansson, J.; Wilson, D.P. Relative risk of cardiovascular disease among people living with HIV: A systematic review and meta-analysis. HIV Med. 2012, 13, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Gokce, N.; Keaney, J.F., Jr.; Vita, J.A. The clinical implications of endothelial dysfunction. J. Am. Coll. Cardiol. 2003, 42, 1149–1160. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A "set up" for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevicius, P.V.; Fleg, J.L.; Engel, J.H.; O’Connor, F.C.; Wright, J.G.; E Lakatta, L.; Yin, F.C.; Lakatta, E.G. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993, 88 4 Pt 1, 1456–1462. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Grover-Páez, F.; Zavalza-Gómez, A.B. Endothelial dysfunction and cardiovascular risk factors. Diabetes Res. Clin. Pract. 2009, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Garcia-Zepeda, E.A.; Lim, Y.-C.; Yoshida, M.; Ding, H.A.; Gimbrone, M.A., Jr.; Luster, A.D.; Luscinskas, F.W.; Rosenzweig, A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999, 398, 718–723. [Google Scholar] [CrossRef]
- Meerschaert, J.; Furie, M.B. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J. Immunol. 1995, 154, 4099–4112. [Google Scholar] [CrossRef]
- Woollard, K.J.; Geissmann, F. Monocytes in atherosclerosis: Subsets and functions. Nat. Rev. Cardiol. 2010, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V. Teasing out monocyte trafficking mechanisms. Blood 2008, 112, 929–930. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mehta, J.L.; Saldeen, T.G.; Rand, K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J. Am. Coll. Cardiol. 1998, 31, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Mohammad-Rezaei, M.; Ahmadi, R.; Rafiei, A.; Khaledifar, A.; Fattahi, S.; Samiei-Sefat, A.; Emami, S.; Bagheri, N. Serum levels of IL-32 in patients with coronary artery disease and its relationship with the serum levels of IL-6 and TNF-alpha. Mol. Biol. Rep. 2021, 48, 4263–4271. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Xue, Y.; Zeng, T.; Shi, Y.; Lin, Y.; Liu, L. Interleukin-32 increases in coronary arteries and plasma from patients with coronary artery disease. Clin. Chim. Acta 2019, 497, 104–109. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Kim, S.H. IL-32, a novel cytokine with a possible role in disease. Ann. Rheum. Dis. 2006, 65 (Suppl. 3), iii61–iii64. [Google Scholar] [CrossRef]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar] [CrossRef]
- Zaidan, S.M.; Leyre, L.; Bunet, R.; Larouche-Anctil, E.; Turcotte, I.; Sylla, M.; Chamberland, A.; Chartrand-Lefebvre, C.; Ancuta, P.; Routy, J.-P.; et al. Upregulation of IL-32 Isoforms in Virologically Suppressed HIV-Infected Individuals: Potential Role in Persistent Inflammation and Transcription From Stable HIV-1 Reservoirs. J. Acquir. Immune. Defic. Syndr. 2019, 82, 503–513. [Google Scholar] [CrossRef]
- Rasool, S.T.; Tang, H.; Wu, J.; Li, W.; Mukhtar, M.M.; Zhang, J.; Mu, Y.; Xing, H.X.; Wu, J.; Zhu, Y. Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol. Lett. 2008, 117, 161–167. [Google Scholar] [CrossRef]
- El-Far, M.; Investigators of the Canadian HIV+ Slow Progressor Cohort; Kouassi, P.; Sylla, M.; Zhang, Y.; Fouda, A.; Fabre, T.; Goulet, J.-P.; van Grevenynghe, J.; Lee, T.; et al. Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci. Rep. 2016, 6, 22902. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Hanna, D.B.; Durand, M.; Larouche-Anctil, E.; Sylla, M.; Chartrand-Lefebvre, C.; Cloutier, G.; Goulet, J.P.; Kassaye, S.; Karim, R.; et al. Brief Report: Subclinical Carotid Artery Atherosclerosis Is Associated With Increased Expression of Peripheral Blood IL-32 Isoforms Among Women Living With HIV. J. Acquir. Immune. Defic. Syndr. 2021, 88, 186–191. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; Durand, M.; Turcotte, I.; Larouche-Anctil, E.; Sylla, M.; Zaidan, S.; Chartrand-Lefebvre, C.; Bunet, R.; Ramani, H.; Sadouni, M.; et al. Upregulated IL-32 Expression And Reduced Gut Short Chain Fatty Acid Caproic Acid in People Living With HIV With Subclinical Atherosclerosis. Front. Immunol. 2021, 12, 664371. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; For the investigators of the Canadian HIV and Aging Cohort Study; Chartrand-Lefebvre, C.; Baril, J.-G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; et al. The Canadian HIV and aging cohort study—Determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: Rationale and study protocol. BMC. Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-M.; Shieh, J.-M.; Chen, C.-L.; Tsou, C.-J.; Wu, W.-B. Vascular endothelial growth factor induces CXCL1 chemokine release via JNK and PI-3K-dependent pathways in human lung carcinoma epithelial cells. Int. J. Mol. Sci. 2013, 14, 10090–10106. [Google Scholar] [CrossRef]
- Filewod, N.C.; Pistolic, J.; Hancock, R.E. Low concentrations of LL-37 alter IL-8 production by keratinocytes and bronchial epithelial cells in response to proinflammatory stimuli. FEMS Immunol Med. Microbiol. 2009, 56, 233–240. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Zhu, R.; Li, H.; Han, Q.; Zhao, R.C. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway. J. Hematol. Oncol. 2016, 9, 42. [Google Scholar] [CrossRef]
- Jang, H.; Kwak, S.-Y.; Park, S.; Kim, K.; Kim, Y.-H.; Na, J.; Kim, H.; Jang, W.-S.; Lee, S.-J.; Kim, M.J.; et al. Pravastatin Alleviates Radiation Proctitis by Regulating Thrombomodulin in Irradiated Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 1897. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, M.-H.R.; Durand, M.; Chartrand-Lefebvre, C.; Fortin, C.; Baril, J.-G.; Trottier, B.; Routy, J.-P.; Soulez, G.; Tremblay, C.; Cloutier, G. Increased carotid artery wall stiffness and plaque prevalence in HIV infected patients measured with ultrasound elastography. Eur. Radiol. 2020, 30, 3178–3187. [Google Scholar] [CrossRef]
- Zegeye, M.M.; Lindkvist, M.; Fälker, K.; Kumawat, A.K.; Paramel, G.; Grenegård, M.; Sirsjö, A.; Ljungberg, L.U. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Constans, J.; Conri, C. Circulating markers of endothelial function in cardiovascular disease. Clin. Chim. Acta 2006, 368, 33–47. [Google Scholar] [CrossRef]
- Appay, V.; Rowland-Jones, S.L. RANTES: A versatile and controversial chemokine. Trends Immunol. 2001, 22, 83–87. [Google Scholar] [CrossRef]
- Lin, C.I.; Chen, C.-N.; Chen, J.H.; Lee, H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J. Cell. Biochem. 2006, 99, 1216–1232. [Google Scholar] [CrossRef]
- Fox, S.E.; Lu, W.; Maheshwari, A.; Christensen, R.D.; Calhoun, D.A. The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine 2005, 29, 135–140. [Google Scholar] [CrossRef]
- Borne, P.V.D.; Quax, P.H.A.; Hoefer, I.E.; Pasterkamp, G. The multifaceted functions of CXCL10 in cardiovascular disease. Biomed. Res. Int. 2014, 2014, 893106. [Google Scholar]
- Gouwy, M.; Struyf, S.; Noppen, S.; Schutyser, E.; Springael, J.-Y.; Parmentier, M.; Proost, P.; Van Damme, J. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol. Pharmacol. 2008, 74, 485–495. [Google Scholar] [CrossRef]
- Damen, M.S.; Popa, C.D.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Interleukin-32 in chronic inflammatory conditions is associated with a higher risk of cardiovascular diseases. Atherosclerosis 2017, 264, 83–91. [Google Scholar] [CrossRef]
- Moschen, A.R.; Fritz, T.; Clouston, A.D.; Rebhan, I.; Bauhofer, O.; Barrie, H.D.; Powell, E.E.; Kim, S.; Dinarello, C.A.; Bartenschlager, R.; et al. Interleukin-32: A new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology 2011, 53, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.C.; Monteleone, G.; MacDonald, T.T. New players in the cytokine orchestra of inflammatory bowel disease. Inflamm. Bowel. Dis. 2007, 13, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Baraldo, S.; Bazzan, E.; Lunardi, F.; Rea, F.; Maestrelli, P.; Turato, G.; Lokar-Oliani, K.; Papi, A.; Zuin, R.; et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 178, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Santinelli, L.; Statzu, M.; Pierangeli, A.; Frasca, F.; Bressan, A.; Pinacchio, C.; Nonne, C.; Turriziani, O.; Antonelli, G.; D’Ettorre, G.; et al. Increased expression of IL-32 correlates with IFN-gamma, Th1 and Tc1 in virologically suppressed HIV-1-infected patients. Cytokine 2019, 120, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Fearon, W.F.; Fearon, D.T. Fearon, Inflammation and cardiovascular disease: Role of the interleukin-1 receptor antagonist. Circulation 2008, 117, 2577–2579. [Google Scholar] [CrossRef]
- Xiao, H.; Li, H.; Wang, J.J.; Zhang, J.S.; Shen, J.; An, X.B.; Zhang, C.C.; Wu, J.M.; Song, Y.; Wang, X.Y. IL-18 cleavage triggers cardiac inflammation and fibrosis upon beta-adrenergic insult. Eur. Heart J. 2018, 39, 60–69. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Leeuwenberg, J.F.; Smeets, E.F.; Neefjes, J.J.; A Shaffer, M.; Cinek, T.; Jeunhomme, T.M.; Ahern, T.J.; A Buurman, W. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 1992, 77, 543–549. [Google Scholar] [PubMed]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Dansky, H.M.; Barlow, C.B.; Lominska, C.; Sikes, J.L.; Kao, C.; Weinsaft, J.; Cybulsky, M.I.; Smith, J.D. Adhesion of monocytes to arterial endothelium and initiation of atherosclerosis are critically dependent on vascular cell adhesion molecule-1 gene dosage. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Mestas, J.; Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Newby, A.C. Macrophage heterogeneity in atherosclerotic plaques. Curr. Opin. Lipidol. 2009, 20, 370–378. [Google Scholar] [CrossRef]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef]
- Packard, T.A.; Schwarzer, R.; Herzig, E.; Rao, D.; Luo, X.; Egedal, J.H.; Hsiao, F.; Widera, M.; Hultquist, J.F.; Grimmett, Z.W.; et al. CCL2: A Chemokine Potentially Promoting Early Seeding of the Latent HIV Reservoir. mBio 2022, 13, e0189122. [Google Scholar] [CrossRef]
- Lane, B.R.; Strieter, R.M.; Coffey, M.J.; Markovitz, D.M. Human immunodeficiency virus type 1 (HIV-1)-induced GRO-alpha production stimulates HIV-1 replication in macrophages and T lymphocytes. J. Virol. 2001, 75, 5812–5822. [Google Scholar] [CrossRef]
- Haarmann, A.; Schuhmann, M.K.; Silwedel, C.; Monoranu, C.-M.; Stoll, G.; Buttmann, M. Human Brain Endothelial CXCR2 is Inflammation-Inducible and Mediates CXCL5- and CXCL8-Triggered Paraendothelial Barrier Breakdown. Int. J. Mol. Sci. 2019, 20, 602. [Google Scholar] [CrossRef]
- Choi, J.-D.; Bae, S.-Y.; Hong, J.-W.; Azam, T.; Dinarello, C.A.; Her, E.; Choi, W.-S.; Kim, B.-K.; Lee, C.-K.; Yoon, D.-Y.; et al. Identification of the most active interleukin-32 isoform. Immunology 2009, 126, 535–542. [Google Scholar] [CrossRef]
- Son, D.J.; Jung, Y.Y.; Seo, Y.S.; Park, H.; Lee, D.H.; Kim, S.; Roh, Y.-S.; Han, S.B.; Yoon, D.Y.; Hong, J.T. Interleukin-32alpha Inhibits Endothelial Inflammation, Vascular Smooth Muscle Cell Activation, and Atherosclerosis by Upregulating Timp3 and Reck through suppressing microRNA-205 Biogenesis. Theranostics 2017, 7, 2186–2203. [Google Scholar] [CrossRef] [PubMed]
- Nold-Petry, C.A.; Nold, M.F.; Zepp, J.A.; Kim, S.H.; Voelkel, N.F.; Dinarello, C.A. IL-32-dependent effects of IL-1beta on endothelial cell functions. Proc. Natl. Acad. Sci. USA 2009, 106, 3883–3888. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Lacolley, P. Structural and genetic bases of arterial stiffness. Hypertension 2005, 45, 1050–1055. [Google Scholar] [CrossRef]
- Giraldo-Grueso, M.; Echeverri, D. From Endothelial Dysfunction to Arterial Stiffness in Diabetes Mellitus. Curr. Diabetes. Rev. 2020, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Duprez, D.A. Arterial stiffness/elasticity in the contribution to progression of heart failure. Heart Fail. Clin. 2012, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Huang, W.; Wang, R.; Chen, C.; Chen, Y.; Wang, Y.; Tan, X. Elevated circulating IL-32 presents a poor prognostic outcome in patients with heart failure after myocardial infarction. Int. J. Cardiol. 2017, 243, 367–373. [Google Scholar] [CrossRef]
- Neuhaus, J.; Jacobs, D.R., Jr.; Baker, J.V.; Calmy, A.; Duprez, D.; La Rosa, A.; Kuller, L.H.; Pett, S.L.; Ristola, M.; Ross, M.J.; et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J. Infect. Dis. 2010, 201, 1788–1795. [Google Scholar] [CrossRef]
- Borges, H.; O’Connor, J.L.; Phillips, A.N.; Rönsholt, F.F.; Pett, S.; Vjecha, M.J.; French, M.; Lundgren, J. Factors Associated with Plasma IL-6 Levels During HIV Infection. J. Infect. Dis. 2015, 212, 585–595. [Google Scholar] [CrossRef]
- Deeks, S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011, 62, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Heinhuis, B.; Popa, C.D.; van Tits, B.L.; Kim, S.-H.; Zeeuwen, P.L.; Berg, W.B.V.D.; van der Meer, J.W.; van der Vliet, J.A.; Stalenhoef, A.F.; Dinarello, C.A.; et al. Towards a role of interleukin-32 in atherosclerosis. Cytokine 2013, 64, 433–440. [Google Scholar] [CrossRef] [PubMed]


| Variable | Controls | PLWH | p Value |
|---|---|---|---|
| Number of participants (Female/Male) Age (Years) | 53 (5/48) 55.9 ± 8.32 | 60 (1/59) 57.6 ± 7.63 | NS |
| Predicted 10 years Framingham Risk score (number of individuals with available data) | 11.08 ± 4.63 (50/53) | 11.12 ± 6.77 (58/60) | NS |
| D-dimer (mg/L) (number of individuals with available data) | 0.301 ± 0.107 (18/53) | 0.292 ± 0.155 (39/60) | NS |
| Body Mass Index (BMI) (number of individuals with available data) | 27.11 ± 4.83 (17/53) | 24.98 ± 4.49 (48/60) | 0.034 |
| LDL–C (mmol/L) (number of individuals with available data) | 3.16 ± 0.77 (51/53) | 2.86 ± 1.06 (55/60) | 0.017 |
| HDL–C (mmol/L) (number of individuals with available data) | 1.38 ± 0.39 (53/53) | 1.24 ± 0.33 (58/60) | 0.056 (NS) |
| Duration of infection (Years) | N/A | 17.68 ± 7.9 | |
| Duration of ART (Years) | N/A | 14.36 ± 6.8 | |
| Viral load (Log10 copies/mL) | N/A | 1.6 ± 0.01 | |
| Nadir CD4 count (cells/mm3) | N/A | 215 ± 161 | |
| CD4 count (cells/mm3) | NA | 595 ± 228 | |
| CD4/CD8 ratio | NA | 0.9 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunet, R.; Roy-Cardinal, M.-H.; Ramani, H.; Cleret-Buhot, A.; Durand, M.; Chartrand-Lefebvre, C.; Routy, J.-P.; Thomas, R.; Trottier, B.; Ancuta, P.; et al. Differential Impact of IL-32 Isoforms on the Functions of Coronary Artery Endothelial Cells: A Potential Link with Arterial Stiffness and Atherosclerosis. Viruses 2023, 15, 700. https://doi.org/10.3390/v15030700
Bunet R, Roy-Cardinal M-H, Ramani H, Cleret-Buhot A, Durand M, Chartrand-Lefebvre C, Routy J-P, Thomas R, Trottier B, Ancuta P, et al. Differential Impact of IL-32 Isoforms on the Functions of Coronary Artery Endothelial Cells: A Potential Link with Arterial Stiffness and Atherosclerosis. Viruses. 2023; 15(3):700. https://doi.org/10.3390/v15030700
Chicago/Turabian StyleBunet, Rémi, Marie-Hélène Roy-Cardinal, Hardik Ramani, Aurélie Cleret-Buhot, Madeleine Durand, Carl Chartrand-Lefebvre, Jean-Pierre Routy, Réjean Thomas, Benoît Trottier, Petronela Ancuta, and et al. 2023. "Differential Impact of IL-32 Isoforms on the Functions of Coronary Artery Endothelial Cells: A Potential Link with Arterial Stiffness and Atherosclerosis" Viruses 15, no. 3: 700. https://doi.org/10.3390/v15030700
APA StyleBunet, R., Roy-Cardinal, M.-H., Ramani, H., Cleret-Buhot, A., Durand, M., Chartrand-Lefebvre, C., Routy, J.-P., Thomas, R., Trottier, B., Ancuta, P., Hanna, D. B., Landay, A. L., Cloutier, G., Tremblay, C. L., & El-Far, M. (2023). Differential Impact of IL-32 Isoforms on the Functions of Coronary Artery Endothelial Cells: A Potential Link with Arterial Stiffness and Atherosclerosis. Viruses, 15(3), 700. https://doi.org/10.3390/v15030700






