Current Trends and Prospects for Application of Green Synthesized Metal Nanoparticles in Cancer and COVID-19 Therapies
Abstract
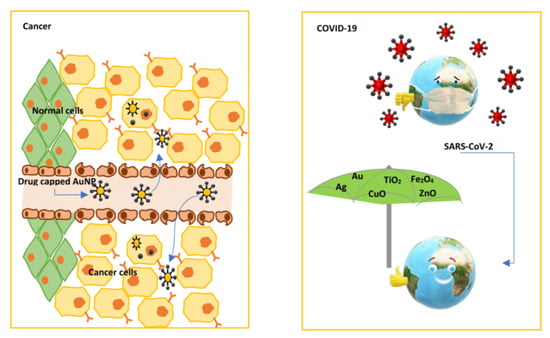
:1. Introduction
2. Nanotechnology
3. Synthesis of Metal Nanoparticles
Green Synthesis as An Ideal Approach
4. Metal Nanoparticles in Cancer Treatment
4.1. Anticancer Properties of Metal Nanoparticles and the Possible Mechanisms of Action
4.2. Metal Nanoparticles as Drug Delivery Systems
5. Metal Nanoparticles as Novel Antivirus Therapy: Effect against Various Viruses
| Metal | Synthesis Material/Conjugate | Physicochemical Properties | Virus | Antiviral Activity | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Silver | Biological (Moringa oleifera seed extract) | Spherical shape; 100 nm mean diameter size | Dengue virus | In vitro: C6/36 and Vero infected cells; IC50 value range of 10.24–21.17 ppm | Not available | [173] |
| Silver | Biological (Cinnamomum cassia H7N3) | Spherical shape; 25–55 nm diameter size range | Influenza A virus | In vitro: Vero infected cells; IC50 value of 125 μg/mL | Not available | [176] |
| Silver | Biological (Curcumin extract) | Spherical shape; 11–12 nm diameter size range | Respiratory syncytial virus | In vitro: Hep-2 infected cells; IC50 value of 0.008–0.12 nM | Reduction of cytopathic effects which led to the inactivation of the virus before entry into the host cell | [168] |
| Silver | Biological (Ginseng root extract) | Spherical shape; 2–50 nm diameter size range | Influenza A virus | In vitro: MDCK infected cells; IC50 value range of 0.02–0.25 M | Not available | [177] |
| Silver | Biological (Andrographis paniculate; Phyllanthus niruri; Tinospora cordifolia extracts) | Spherical shape; 70–95 nm (AP-AgNPs), 70–120 nm (PNAgNPs), 50–70 nm (TCAgNPs) diameter size range | Chikungunya virus | In vitro: Vero infected cells; IC50 values of 31.25 μg/mL (AP-AgNPs); 125 μg/mL (PN-AgNPs); 250 μg/mL (TC-AgNPs) | Not available | [179] |
| Silver | Biological (Lampranthus coccineus and Malephora lutea extracts) | Spherical shape; 10.12–27.89 nm diameter size range | Herpes simplex virus-1, Hepatitis A virus-10, and Coxsackie B4 virus | In vitro: Vero infected cells; IC50 value of 5.13 μg/mL | Not available | [186] |
| Silver | Biological (seaweed) | Spherical shape; 8–27 nm diameter size range | Herpes simplex virus-1 and 2 | In vitro: Vero infected cells; IC50 value of ID50–2.5 μL | Not available | [184] |
| Gold | Chemical (AgNPs coated with 100% and 50% density of the sulfated ligand) | Spherical shape; 1.7–2.6 nm diameter size range | Human immunodeficiency virus | In vitro: MT-2 infected cells; IC50 value of 1.29 and 2.32 μg/mL | Interaction with the HIVs envelop glycoprotein and prevent virus replication | [190] |
| Gold | Chemical (AuNPs coated with hyaluronic acid/interferon α) | Spherical shape; 46.03 nm diameter size range | Hepatitis C virus | In vitro: Daudi infected cells; IC50 not available | Enhancement of the expression of 2′, 5′- oligoadenylate synthetase 1 enzyme which activates innate immune responses to viral infection | [199] |
| Gold | Chemical (AuNPs conjugated with an HIV inhibitor, TAK-779) | Shape not available; 2.0 nm diameter size | Human immunodeficiency virus | In vitro: PBM infected cells; IC50 value of 10 nM | Inhibition of the viral replication | [189] |
| Gold | Chemical (AuNPs conjugated with carbohydrate and inhibitors lamivudine/abacavi) | Spherical, shape; 3 nm diameter size | Human immunodeficiency virus | In vitro: TZM-bl cells; IC50 value of 1 µM and 8 µM | Interaction with the cationic amino acids on the viral envelope glycoprotein gp120, thus blocking the replication of reverse transcriptase enzymes which prevents viral replication | [191] |
| Gold | Chemical (AuNPs coated with mercarptoethene sulfonate) | Spherical shape; 4 nm diameter size | Herpes simplex virus-1 | In vitro: VeroE6 infected cells; IC50 not available | Inhibition of viral attachment, entry, and cell-to-cell spread | [196] |
| Gold | Biological (Spirulina platensis extract) | Octahedral, pentagonal, and triangular shapes; 15.60–77.13 nm diameter size range | Herpes simplex virus-1 | In vitro: VeroE6 infected cells; IC50 value not available (90% cytopathic effect at 31.25 μL) | Inhibition of the viral replication | [197] |
| Gold | Chemical (AuNPs coated with hyaluronic acid and interferon) | Spherical shape; 29.16 nm diameter size | Hepatitis C virus | In vitro: Daudi infected cells; IC50 not available | Enhancement of the expression of 2’-5’ oligoadenylate synthetase 1 enzyme which activates innate immune responses to viral infection | [198] |
| Gold | Chemical (AuNPs coated with West Nile virus envelop protein) | Non-spherical, rod, and cube shapes 20–40 nm diameter size range | West Nile virus | In vitro: BMDCs; IC50 value of not available | Induction of IL-1β, IL-18, TNF-α, IL-6, IL-12 antibodies, and granulocyte– macrophage colony-stimulating factor | [200] |
| Gold | Chemical (AuNPs conjugated with thiosialoside) | Spherical shape; 5 nm and 20 nm diameter size | Influenza virus | In vitro: Chicken red blood cells (CRBCs); IC50 value not available | Inhibition of haemagglutin-in | [201] |
| Gold | Chemical (AuNPs coated with silicon dioxide; silicon) | Spherical shape; 5 nm and 100 nm diameter sizes | Adenovirus | In vitro: MDBK (the Madin-Darby bovine kidney line) and Hep-2 cells; IC50 value not available (66–86% virucidal effect) | Inhibition of virus reproduction due to field effects | [202] |
| Gold | Biological (garlic extract) | Spherical shape; 11 nm diameter size | MeV virus | In vitro: Vero infected cells; EC50 value of 8.829 µg/mL | Blockage of viral receptors resulting in a significant reduction of viral infection | [203] |
| Copper oxide | Chemical (CuONPs coated with cetyltrimethylammonium bromide) | Spherical shape; 45 nm diameter size | Hepatitis C virus | In vitro: Huh7 infected cells; IC50 value not available | Blockage of virus during the attachment and entry stages | [204] |
| Copper oxide | Biological (Syzygium alternifolium fruit extract) | Spherical shape; 2–69 nm diameter size | Newcastle disease virus | In ovo: Infected eggs; The embryo infectious dose (EID50) value was 106.5/mL and EC50 value of 50.98 μg/mL | Activation of oxidative stress; disruption of the genome and capsid of the virus | [211] |
5.1. Antiviral Properties of Metal Nanoparticles against COVID 19/SARS-CoV-2 and Possible Mechanisms of Action
| Metal | Conjugate/Adjuvant | Size | Antiviral Activity | Mechanism of Action | References |
|---|---|---|---|---|---|
| Gold | SARS-CoV S protein 40 | 40 nm and 100 nm | In vivo: infected BALB/c mice; IC50 value not available | Induction of strong antigen-specific IgG response | [153] |
| Gold | Triethyl phosphine (Drug, auronafin) | Not available | In vitro: Huh7 infected human cells; EC50 value of 1.4 μM | Inhibition of viral replication by suppressing virus associated cytokines | [29] |
| Zinc | Pyrithione | Not available | In vitro: VeroE6 infected cells; IC50 value of 1.4 mM | Inhibition of replication by hindering the virus’s RNA polymerase of the multiprotein replication and transcription complexes | [252] |
| Iron oxide (Fe2O3 and Fe3O4) | - | Not applicable | Not applicable | Binding with the S protein receptor-binding domain (S-RBD) | [254] |
| Silver | - | Around 10 nm | In vitro: VeroE6 and d Calu-3 infected cells; IC50 value not available | Inhibition of the viral entry, and disruption of viral integrity | [257] |
| Silver | Strawberry (Fragaria ananassa Duch) and ginger (Zingiber officinale); methanolic extracts | 5.89 nm and 5.77 nm | In vitro: Vero infected cells; IC50 of values of 0.0062 µg/mL (ginger extract) and 0.0989 µg/mL (strawberry extract) | Binding of various compounds of the extracts, neohesperidin to be specific, with both the SARVS-2 NSP16 viral protein and human AAK1 host protein | [260] |
5.2. Applications of Metal Nanoparticles against COVID-19/SARS-CoV-2
6. Limitations of Metal Nanoparticles in Clinical Applications
7. Prospects and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- W.H. Organization. Available online: https://apps.who.int/iris/bitstream/handle/10665/326369/WHO-NMH-NVI-16.10eng.pdf?sequence=1&isAllowed=y (accessed on 19 March 2022).
- A.C. Society. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-4th-edition.pdf (accessed on 19 March 2022).
- N.C. Institute. Available online: https://www.cancer.gov/about-cancer/understanding/what-is-cancer#:~:text=Cancer%20is%20a%20disease%20caused,are%20also%20called%20genetic%20changes (accessed on 19 March 2022).
- Lokina, S.; Stephen, A.; Kaviyarasan, V.; Arulvasu, C.; Narayanan, V. Cytotoxicity and antimicrobial activities of green synthesized silver nanoparticles. Eur. J. Med. Chem. 2014, 76, 256–263. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Ovais, M.; Khan, A.; Raza, A. Docetaxel-loaded solid lipid nanoparticles: A novel drug delivery system. IET Nanobiotechnol. 2017, 11, 621–629. [Google Scholar] [CrossRef]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.; Pillat, M.M. CD147 as a target for COVID-19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Rev. Rep. 2020, 16, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Chen, C.S.; Chan, Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020, 83, 217. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M. Coronavirus Mutates into 40 Strains. How This Changes the Pandemic Outlook: Experts; Al Arabiya English: Dubai, UAE, 2020; Available online: https://english.alarabiya.net/en/features/2020/03/27/Coronavirus-mutates-into-40-strains-How-this-changes-the-pandemic-outlook-Exp (accessed on 19 March 2022).
- Bahrami, A.; Arabestani, M.R.; Taheri, M.; Farmany, A.; Norozzadeh, F.; Hosseini, S.M.; Nozari, H.; Nouri, F. Exploring the role of heavy metals and their derivatives on the pathophysiology of COVID-19. Biol. Trace Element Res. 2021, 200, 1–12. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate use of remdesivir for patients with severe covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef]
- Taylor, A.; Foo, S.S.; Bruzzone, R.; Vu Dinh, L.; King, N.J.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaid, P.; Raizada, P.; Saini, A.K.; Saini, R.V. Biogenic silver, gold and copper nanoparticles - A sustainable green chemistry approach for cancer therapy. Sustain. Chem. Pharm. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral properties of flavonoids and delivery strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- Malinga, T.; Kudanga, T.; Mbatha, L.S. Stealth doxorubicin conjugated bimetallic selenium/silver nanoparticles for targeted cervical cancer therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 045006. [Google Scholar] [CrossRef]
- Hu, M.-L. Dietary polyphenols as antioxidants and anticancer agents: More questions than answers. Chang. Gung Med. J. 2011, 34, 449–460. [Google Scholar]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Karthik, C.; Punnaivalavan, K.; Prabha, S.P.; Caroline, D. Multifarious global flora fabricated phytosynthesis of silver nanoparticles: A green nanoweapon for antiviral approach including SARS-CoV-2. Int. Nano Lett. 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Simard, J.M.; Worrall, J.W.; Rotello, V.M. Tunable reactivation of nanoparticle-inhibited β-galactosidase by glutathione at intracellular concentrations. J. Am. Chem. Soc. 2004, 126, 13987–13991. [Google Scholar] [CrossRef]
- Liu, C.L.; Wu, H.T.; Hsiao, Y.H.; Lai, C.W.; Shih, C.W.; Peng, Y.K.; Tang, K.C.; Chang, H.W.; Chien, Y.C.; Hsiao, J.K.; et al. Insulin-directed synthesis of fluorescent gold nanoclusters: Preservation of insulin bioactivity and versatility in cell imaging. Angew. Chem. Int. Ed. 2011, 50, 7056–7060. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothan, H.A.; Stone, S.; Natekar, J.; Kumari, P.; Arora, K.; Kumar, M. The FDA-approved gold drug auranofin inhibits novel coronavirus (SARS-COV-2) replication and attenuates inflammation in human cells. Virology 2020, 547, 7–11. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the inhibition of viral infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kruger, H.G.; Maguire, G.E.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2017, 15, 93–114. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Karmous, I.; Pandey, A.; Haj, K.B.; Chaoui, A. Efficiency of the green synthesized nanoparticles as new tools in cancer therapy: Insights on plant-based bioengineered nanoparticles, biophysical properties, and anticancer roles. Biol. Trace Element Res. 2019, 196, 330–342. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Shankar, P.D.; Shobana, S.; Karuppusamy, I.; Pugazhendhi, A.; Ramkumar, V.S.; Arvindnarayan, S.; Kumar, G. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: Formation mechanism and applications. Enzym. Microb. Technol. 2016, 95, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2017, 25, 10362–10370. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Rajendran, R.B. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Sisubalan, N.; Ramkumar, V.S.; Pugazhendhi, A.; Karthikeyan, C.; Indira, K.; Gopinath, K.; Hameed, A.S.H.; Basha, M.H.G. ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ. Sci. Pollut. Res. 2017, 25, 10482–10492. [Google Scholar] [CrossRef]
- Indira, K.; Mudali, U.K.; Nishimura, T.; Rajendran, N. A review on TiO2 nanotubes: Influence of anodization parameters, formation mechanism, properties, corrosion behavior, and biomedical applications. J. Bio Tribo Corros. 2015, 1, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Ramdath, S.; Mellem, J.; Mbatha, L.S. Anticancer and antimicrobial activity evaluation of cowpea-porous-starch-formulated silver nanoparticles. J. Nanotechnol. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Sarkar, P.K.; Das Mukhopadhyay, C. Ayurvedic metal nanoparticles could be novel antiviral agents against SARS-CoV-2. Int. Nano Lett. 2021, 11, 197–203. [Google Scholar] [CrossRef]
- Shu, T.; Huang, M.; Wu, D.; Ren, Y.; Zhang, X.; Han, Y.; Mu, J.; Wang, R.; Qiu, Y.; Zhang, D.-Y.; et al. SARS-coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 2020, 35, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Wei, L.J.; Gan, S.H. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxidative Med. Cell. Longev. 2016, 2016, 3685671. [Google Scholar] [CrossRef] [Green Version]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.A.; Sadighi, A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv. Colloid Interface Sci. 2013, 189, 1–20. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Patel, N.; Kasumbwe, K.; Mohanlall, V. Antibacterial screening of Gunnera perpensa-mediated silver nanoparticles. J. Nanotechnol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Sharma, S.; Ahmad, N.; Ghosh, A.; Sinha, P. Bacteria mediated extracellular synthesis of metallic nanoparticles. Int. Res. J. Biotechnol. 2010, 1, 071–079. Available online: http://www.interesjournals.org/IRJOB (accessed on 19 March 2022).
- Yazdi, M.H.; Sepehrizadeh, Z.; Mahdavi, M.; Shahverdi, A.R.; Faramarzi, M.A. Metal, metalloid, and oxide nanoparticles for therapeutic and diagnostic oncology. Nano Biomed. Eng. 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.B.; Zhuang, L.; Li, T.; Chen, N.H.; Wu, Z.N.; Li, P.; Li, Y.L.; Wang, G.C. Diterpenoid alkaloids from Delphinium ajacis and their anti-RSV activities. Planta Medica 2016, 83, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ti, H.; Zhuang, Z.; Yu, Q.; Wang, S. Progress of plant medicine derived extracts and alkaloids on modulating viral infections and inflammation. Drug Des. Dev. Ther. 2021, 15, 1385–1408. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mater. Res. 2013, 832, 350–355. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Phougat, N.; Kumar, M.; Saini, R.V.; Chhillar, A.K. Green chemistry approach towards nanoparticle synthesis, metabolic engineering for bioactive compounds; Springer: Berlin, Germany, 2017; pp. 249–268. [Google Scholar] [CrossRef]
- Murphy, C.J. Sustainability as an emerging design criterion in nanoparticle synthesis and applications. J. Mater. Chem. 2008, 18, 2173–2176. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Mbatha, L.S.; Singh, M. Starburst poly(amidoamine) dendrimer grafted gold nanoparticles as a scaffold for folic acid-targeted plasmid DNA delivery in vitro. J. Nanosci. Nanotechnol. 2019, 19, 1959–1970. [Google Scholar] [CrossRef]
- Zimmermann, S.; Dziadziuszko, R.; Peters, S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat. Rev. 2014, 40, 716–722. [Google Scholar] [CrossRef]
- Basiuk, V.A.; Basiuk, E.V. Green Processes for Nanotechnology; Springer: Berlin, Germany, 2015; p. 446. [Google Scholar] [CrossRef]
- Koren, E.; Fuchs, Y. Modes of regulated cell death in cancer. Cancer Discov. 2021, 11, 245–265. [Google Scholar] [CrossRef]
- Hengartner, M.O. Apoptosis: Corralling the corpses. Cell 2001, 104, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Schneider, P.; Tschopp, J. Apoptosis induced by death receptors. Pharmacochem. Libr. 2000, 31, 281–286. [Google Scholar] [CrossRef]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Kirby, R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.; Golstein, P.; Peter, M.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.; Malorni, W.; A Knight, R.; et al. Classification of cell death: Recommendations of the nomenclature committee on cell death. Cell Death Differ. 2005, 12, 1463–1467. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.-Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, F.; Gao, S.; Shao, J.; Chang, H. The applications of gold nanoparticle-initialed chemiluminescence in biomedical detection. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Duncan, B.; Creran, B.; Rotello, V.M. Triggered nanoparticles as therapeutics. Nano Today 2013, 8, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraj, M.; Arun, R.; Sathishkumar, G.; MubarakAli, D.; Rajesh, M.; Sivanandhan, G.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Ganapathi, A. An evidence on G2/M arrest, DNA damage and caspase mediated apoptotic effect of biosynthesized gold nanoparticles on human cervical carcinoma cells (HeLa). Mater. Res. Bull. 2014, 52, 15–24. [Google Scholar] [CrossRef]
- Klekotko, M.; Matczyszyn, K.; Siednienko, J.; Olesiak-Banska, J.; Pawlik, K.; Samoc, M. Bio-mediated synthesis, characterization and cytotoxicity of gold nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 29014–29019. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera gold nanoparticles modulate oncogenes, tumor suppressor genes, and caspase-9 splice variants in A549 cells. J. Cell. Biochem. 2016, 117, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Ngabire, D.; Thi, H.H.P.; Kim, M.D.; Kim, G.D. Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 2016, 28, 119–132. [Google Scholar] [CrossRef]
- Barai, A.C.; Paul, K.; Dey, A.; Manna, S.; Roy, S.; Bag, B.G.; Mukhopadhyay, C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pei, Q.; Shou, T.; Zhang, W.; Hu, J.; Li, W. Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomed. 2019, 14, 4091. [Google Scholar] [CrossRef] [Green Version]
- El-Borady, O.M.; Ayat, M.S.; Shabrawy, M.A.; Millet, P. Green synthesis of gold nanoparticles using parsley leaves extract and their applications as an alternative catalytic, antioxidant, anticancer, and antibacterial agents. Adv. Powder Technol. 2020, 31, 4390–4400. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Rajkuberan, C.; Santhiya, T.; Krejcar, O.; Kuča, K.; Periakaruppan, R.; Prabukumar, S. Green synthesis of gold nanoparticles using Polianthes tuberosa L. floral extract. Plants 2021, 10, 2370. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M.; El-Badry, Y.A.; Hussein, E.E.; Eltaweil, A.S. Plant-assisted synthesis of gold nanoparticles for photocatalytic, anticancer, and antioxidant applications. J. Saudi Chem. Soc. 2022, 26, 101419. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Nakkala, J.R.; Mata, R.; Bhagat, E.; Sadras, S.R. Green synthesis of silver and gold nanoparticles from Gymnema sylvestre leaf extract: Study of antioxidant and anticancer activities. J. Nanopart. Res. 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Abel, E.E.; Poonga, P.R.; Panicker, S.G. Characterization and in vitro studies on anticancer, antioxidant activity against colon cancer cell line of gold nanoparticles capped with Cassia tora SM leaf extract. Appl. Nanosci. 2015, 6, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, T.; Sambandam, B.; Aravinthan, A.; Sastry, T.P.; Kim, J.-H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process. Biochem. 2016, 51, 384–391. [Google Scholar] [CrossRef]
- Mishra, P.; Ray, S.; Sinha, S.; Das, B.; Khan, M.I.; Behera, S.K.; Yun, S.-I.; Tripathy, S.K.; Mishra, A. Facile biosynthesis of gold nanoparticles by using extract of Hibiscus sabdariffa and evaluation of its cytotoxicity against U87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016, 105, 264–272. [Google Scholar] [CrossRef]
- Rajan, A.; Rajan, A.R.; Philip, D. Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. Opennano 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Dhayalan, M.; Denison, M.I.J.; Ayyar, M.; Gandhi, N.N.; Krishnan, K.; Abdulhadi, B. Biogenic synthesis, characterization of gold and silver nanoparticles from Coleus forskohlii and their clinical importance. J. Photochem. Photobiol. B Biol. 2018, 183, 251–257. [Google Scholar] [CrossRef]
- Khandanlou, R.; Murthy, V.; Saranath, D.; Damani, H. Synthesis and characterization of gold-conjugated Backhousia citriodora nanoparticles and their anticancer activity against MCF-7 breast and HepG2 liver cancer cell lines. J. Mater. Sci. 2017, 53, 3106–3118. [Google Scholar] [CrossRef]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2017, 46, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.; Su, W.; Wang, Y.; Dang, M.; Zhang, W.; Wang, C. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Duan, X.; Hu, C.; Wu, C.; Chen, X.; Huang, J.; Liu, J.; Cui, S. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Sunderam, V.; Thiyagarajan, D.; Lawrence, A.V.; Mohammed, S.S.S.; Selvaraj, A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J. Biol. Sci. 2018, 26, 455–459. [Google Scholar] [CrossRef]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef] [Green Version]
- Vinosha, M.; Palanisamy, S.; Muthukrishnan, R.; Selvam, S.; Kannapiran, E.; You, S.; Prabhu, N.M. Biogenic synthesis of gold nanoparticles from Halymenia dilatata for pharmaceutical applications: Antioxidant, anti-cancer and antibacterial activities. Process. Biochem. 2019, 85, 219–229. [Google Scholar] [CrossRef]
- Adewale, O.B.; Anadozie, S.O.; Potts-Johnson, S.S.; Onwuelu, J.O.; Obafemi, T.O.; Osukoya, O.A.; Fadaka, A.O.; Davids, H.; Roux, S. Investigation of bioactive compounds in Crassocephalum rubens leaf and in vitro anticancer activity of its biosynthesized gold nanoparticles. Biotechnol. Rep. 2020, 28, e00560. [Google Scholar] [CrossRef] [PubMed]
- Al Saqr, A.; Khafagy, E.-S.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; Anwer, M.; Khan, S.; Lila, A.S.A.; Arab, H.H.; Hegazy, W.A. Synthesis of gold nanoparticles by using green machinery: Characterization and In vitro toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Botteon, C.; Silva, L.; Ccana-Ccapatinta, G.; Silva, T.; Ambrosio, S.; Veneziani, R.; Bastos, J.; Marcato, P. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, S.R.; Durgawale, P.P.; Patil, M.N.; Hinge, D.D.; Jagdale, N.J.; Deshmukh, V.N.; More, A.L. Biogenic synthesis of gold nanoparticles using Argemone mexicana L. and their cytotoxic and genotoxic effects on human colon cancer cell line (HCT-15). J. Genet. Eng. Biotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yas, R.M.; Ghafoor, A.; Saeed, M.A. Anticancer effect of green synthesized gold nanoparticles using orchid extract and their characterizations on breast cancer AMJ-13 cell line. Syst. Rev. Pharm. 2021, 12, 500–505. [Google Scholar] [CrossRef]
- Mukundan, D.; Mohankumar, R.; Vasanthakumari, R. Green synthesis of silver nanoparticles using leaves extract of Bauhinia tomentosa Linn and it’s in vitro anticancer potential. Mater. Today Proc. 2015, 2, 4309–4316. [Google Scholar] [CrossRef]
- Salehi, S.; Shandiz, S.A.S.; Ghanbar, F.; Darvish, M.R.; Ardestani, M.S.; Mirzaie, A.; Jafari, M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016, 11, 1835. [Google Scholar] [CrossRef] [Green Version]
- Kelkawi, A.H.A.; Abbasi Kajani, A.; Bordbar, A.K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017, 11, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Palem, R.R.; Ganesh, S.D.; Kroneková, Z.; Sláviková, M.; Saha, N.; Sáha, P. Green synthesis of silver nanoparticles and biopolymer nanocomposites: A comparative study on physico-chemical, antimicrobial and anticancer activity. Bull. Mater. Sci. 2018, 41, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PloS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [Green Version]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.; Arasu, M.V.; Al-Dhabi, N.A.; Ponnusamy, V.K.; Agastian, P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-mediated green synthesis of silver nanoparticles exhibiting antioxidant and anticancer activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.; Mahmood, A.; Alsharidah, M.; Mohammed, H.A.; Alenize, S.K.; Bouazzaoui, A.; Al Rugaie, O.; Alnuqaydan, A.M.; Ahmad, R.; Vaali-Mohammad, M.-A.; et al. Bioactivities of the green synthesized silver nanoparticles reduced using Allium cepa L aqueous extracts induced apoptosis in colorectal cancer cell lines. J. Nanomater. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.R.; Álvarez-Sánchez, A.R.; Romo-Quiñonez, C.R.; Barraza, A.; Magallón-Barajas, F.J.; Chávez-Sánchez, A.; García-Ramos, J.C.; Toledano-Magaña, Y.; Bogdanchikova, N.; Pestryakov, A.; et al. Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol. 2019, 84, 1083–1089. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K.; et al. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomed. 2016, 11, 1879. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.-Y.; Jin, H.; Park, Y. Green synthesis and biological activities of silver nanoparticles prepared by Carpesium cernuum extract. Arch. Pharmacal. Res. 2019, 42, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Cyril, N.; George, J.B.; Joseph, L.; Raghavamenon, A.; Sylas, V.P. Assessment of antioxidant, antibacterial and anti-proliferative (lung cancer cell line A549) activities of green synthesized silver nanoparticles from Derris trifoliata. Toxicol. Res. 2019, 8, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, T.; Nabiuni, M.; Baharara, J.; Parivar, K.; Namvar, F. Sensitization of resistance ovarian cancer cells to cisplatin by biogenic synthesized silver nanoparticles through p53 activation. Iran. J. Pharm. Res. Int. J. Psychother. Pract. Res. 2019, 18, 222. [Google Scholar]
- Palle, S.R.; Penchalaneni, J.; Lavudi, K.; Gaddam, S.A.; Kotakadi, V.S.; Challagundala, V.N. Green synthesis of silver nanoparticles by leaf extracts of Boerhavia erecta and spectral characterization and their antimicrobial, antioxidant ad cytotoxic studies on ovarian cancer cell lines. Lett. Appl. NanoBioSci. 2020, 9, 1165–1176. [Google Scholar] [CrossRef]
- Saber, M.M.; Mirtajani, S.B.; Karimzadeh, K. Green synthesis of silver nanoparticles using Trapa natans extract and their anticancer activity against A431 human skin cancer cells. J. Drug Deliv. Sci. Technol. 2018, 47, 375–379. [Google Scholar] [CrossRef]
- KPJ, H.; Shantakani, S.; Botcha, S. Green synthesis of silver nanoparticles using aqueous fruit and tuber extracts of Momordica cymbalaria. J. Plant Biochem. Biotechnol. 2021, 30, 196–204. [Google Scholar] [CrossRef]
- Adebayo, I.A.; Arsad, H.; Gagman, H.A.; Ismail, N.Z.; Samian, M.R. Inhibitory effect of eco-friendly naturally synthesized silver nanoparticles from the leaf extract of medicinal Detarium microcarpum plant on pancreatic and cervical cancer cells. Asian Pac. J. Cancer Prev. 2020, 21, 1247. [Google Scholar] [CrossRef]
- Katragadda, C.S.; Choudhury, P.K.; Murthy, P. Nanoparticles as non-viral gene delivery vectors. Indian J. Pharm. Educ. Res. 2010, 44, 109–111. [Google Scholar]
- Zhu, H.; Jiang, R.; Xiao, L.; Zeng, G. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, B.; Qiao, Y. Fe3O4 nanoparticles in targeted drug/gene delivery systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namvar, F.; Rahman, H.S.; Mohammad, R.; Baharara, J.; Mahdavi, M.; Amini, E.; Chartrand, M.S.; Yeap, S.K. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int. J. Nanomed. 2014, 9, 2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagajyothi, P.; Pandurangan, M.; Kim, D.H.; Sreekanth, T.; Shim, J. Green synthesis of iron oxide nanoparticles and their catalytic and in vitro anticancer activities. J. Clust. Sci. 2017, 28, 245–257. [Google Scholar] [CrossRef]
- Farshchi, H.K.; Azizi, M.; Jaafari, M.R.; Nemati, S.H.; Fotovat, A. Green synthesis of iron nanoparticles by rosemary extract and cytotoxicity effect evaluation on cancer cell lines. Biocatal. Agric. Biotechnol. 2018, 16, 54–62. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.-W.; Teow, S.-Y. Evaluating anticancer activity of plant-mediated synthesized iron oxide nanoparticles using Punica granatum fruit peel extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- Sarala, E.; Madhukara Naik, M.; Vinuth, M.; Rami Reddy, Y.; Sujatha, H. Green synthesis of Lawsonia inermis-mediated zinc ferrite nanoparticles for magnetic studies and anticancer activity against breast cancer (MCF-7) cell lines. J. Mater. Sci. Mater. Electron. 2020, 31, 8589–8596. [Google Scholar] [CrossRef]
- Yoonus, J.; Resmi, R.; Beena, B. Evaluation of antibacterial and anticancer activity of green synthesized iron oxide (α-Fe2O3) nanoparticles. Mater. Today Proc. 2021, 46, 2969–2974. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Yee, O.S.; Teow, S.-Y.; Hedayatnasab, Z.; Jahangirian, H.; Webster, T.J.; Kuča, K. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomed. 2021, 16, 2515. [Google Scholar] [CrossRef]
- Ansari, M.A.; Asiri, S.M.M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. Int. J. Biol. Macromol. 2021, 171, 44–58. [Google Scholar] [CrossRef]
- Kulkarni, S.; Jadhav, M.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U. Green synthesized multifunctional Ag@Fe2O3 nanocomposites for effective antibacterial, antifungal and anticancer properties. New J. Chem. 2017, 41, 9513–9520. [Google Scholar] [CrossRef]
- Bagyalakshmi, B.; Priyadarshini, S.L.; Balamurugan, A. Anticancer activity of bee venom against lung cancer cell line (A549 cells) enhanced by iron oxide nanoparticles synthesized from Syzygium aromaticum. J. Drug Deliv. Ther. 2019, 9, 248–254. [Google Scholar] [CrossRef]
- Shejawal, K.P.; Randive, D.S.; Bhinge, S.D.; Bhutkar, M.A.; Wadkar, G.H.; Jadhav, N.R. Green synthesis of silver and iron nanoparticles of isolated proanthocyanidin: Its characterization, antioxidant, antimicrobial, and cytotoxic activities against COLO320DM and HT29. J. Genet. Eng. Biotechnol. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Saquib, Q.; Siddiqui, M.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J. Anti-cancer efficacy of Aloe vera capped hematite nanoparticles in human breast cancer (MCF-7) cells. J. Drug Deliv. Sci. Technol. 2020, 60, 102052. [Google Scholar] [CrossRef]
- Rajendran, A.; Alsawalha, M.; Alomayri, T. Biogenic synthesis of husked rice-shaped iron oxide nanoparticles using coconut pulp (Cocos nucifera L.) extract for photocatalytic degradation of rhodamine B dye and their in vitro antibacterial and anticancer activity. J. Saudi Chem. Soc. 2021, 25, 101307. [Google Scholar] [CrossRef]
- Raveesha, H.; Bharath, H.; Vasudha, D.; Sushma, B.; Pratibha, S.; Dhananjaya, N. Antibacterial and antiproliferation activity of green synthesized nanoparticles from rhizome extract of Alpinia galangal (L.) Wild. Inorg. Chem. Commun. 2021, 132, 108854. [Google Scholar] [CrossRef]
- Dylla, A.G.; Xiao, P.; Henkelman, G.; Stevenson, K.J. Morphological dependence of lithium insertion in nanocrystalline TiO2(B) nanoparticles and nanosheets. J. Phys. Chem. Lett. 2012, 3, 2015–2019. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Yin, M.; Ju, E.; Chen, Z.; Li, Z.; Ren, J.; Qu, X. Upconverting nanoparticles with a mesoporous TiO2 shell for near-infrared-triggered drug delivery and synergistic targeted cancer therapy. Chem.–Eur. J. 2014, 20, 14012–14017. [Google Scholar] [CrossRef]
- Akasaka, H.; Mukumoto, N.; Nakayama, M.; Wang, T.; Yada, R.; Shimizu, Y.; Inubushi, S.; Kyotani, K.; Okumura, K.; Miyamoto, M.; et al. Investigation of the potential of using TiO2 nanoparticles as a contrast agent in computed tomography and magnetic resonance imaging. Appl. Nanosci. 2020, 10, 3143–3148. [Google Scholar] [CrossRef]
- Hasanzadeh Kafshgari, M.; Kah, D.; Mazare, A.; Nguyen, N.T.; Distaso, M.; Peukert, W.; Goldmann, W.H.; Schmuki, P.; Fabry, B. Anodic titanium dioxide nanotubes for magnetically guided therapeutic delivery. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renuka, M.; Soundhari, C. Antibacterial and anticancer activity of green synthesised titanium dioxide nanoparticle from Terminalia chebula. World J. Pharm. Res. 2017, 7, 1164–1179. [Google Scholar] [CrossRef]
- He, F.; Yu, W.; Fan, X.; Jin, B. In vitro cytotoxicity of biosynthesized titanium dioxide nanoparticles in human prostate cancer cell lines. Trop. J. Pharm. Res. 2017, 16, 2793–2799. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, D.; Thangamuniyandi, P.; Selvakumar, P.; Devan, U.; Pugazhendhi, A.; Vasantharaja, R.; Nehru, L. Green approach synthesis of Pd@TiO2 nanoparticles: Characterization, visible light active picric acid degradation and anticancer activity. Process. Biochem. 2019, 87, 83–88. [Google Scholar] [CrossRef]
- Rao, T.N.; Babji, P.; Ahmad, N.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; Husain, F.M. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J. Biol. Sci. 2019, 26, 1385–1391. [Google Scholar] [CrossRef]
- Rehman, S.; Farooq, R.; Jermy, R.; Asiri, S.M.; Ravinayagam, V.; Al Jindan, R.; AlSalem, Z.; Shah, M.A.; Reshi, Z.; Sabit, H.; et al. A wild fomes fomentarius for biomediation of one pot synthesis of titanium oxide and silver nanoparticles for antibacterial and anticancer application. Biomolecules 2020, 10, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute respiratory syndrome-related coronavirus infection but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2020, 64, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Aswini, R.; Murugesan, S.; Kannan, K. Bio-engineered TiO2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial and anticancer activity. Int. J. Environ. Anal. Chem. 2021, 101, 2926–2936. [Google Scholar] [CrossRef]
- Saeidi, J.; Dolatabadi, S.; Esfahani, M.B.; Saeidi, M.; Mohtashami, M.; Mokhtari, K.; Ghasemi, A. Anticancer potential of doxorubicin in combination with green-synthesized silver nanoparticle and its cytotoxicity effects on cardio-myoblast normal cells. Anti Cancer Agents Med. Chem. 2021, 21, 1842–1849. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Desai, N.; Momin, M.; Khan, T.; Gharat, S.; Ningthoujam, R.S.; Omri, A. Metallic nanoparticles as drug delivery system for the treatment of cancer. Expert Opin. Drug Deliv. 2021, 18, 1261–1290. [Google Scholar] [CrossRef]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keles, E.; Song, Y.; Du, D.; Dong, W.J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Sun, D.; Wang, R. A review of the ligands and related targeting strategies for active targeting of paclitaxel to tumours. J. Drug Target. 2016, 24, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.S.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C.R. Green synthesis and characterization of monodispersed gold nanoparticles: Toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef]
- Pooja, D.; Panyaram, S.; Kulhari, H.; Reddy, B.; Rachamalla, S.S.; Sistla, R. Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int. J. Biol. Macromol. 2015, 80, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Firdhouse, J.; Lalitha, P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 2015, 4, 113. [Google Scholar] [CrossRef] [Green Version]
- Gul, A.R.; Shaheen, F.; Rafique, R.; Bal, J.; Waseem, S.; Park, T.J. Grass-mediated biogenic synthesis of silver nanoparticles and their drug delivery evaluation: A biocompatible anti-cancer therapy. Chem. Eng. J. 2021, 407, 127202. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Iku, S.I.; Ntwasa, M.; Nkambule, T.T.; Mamba, B.B.; Msagati, T.A. Doxorubicin conjugated hydrophilic AuPt bimetallic nanoparticles fabricated from Phragmites australis: Characterization and cytotoxic activity against human cancer cells. J. Drug Deliv. Sci. Technol. 2020, 57, 101749. [Google Scholar] [CrossRef]
- Zadeh, F.A.; Jasim, S.A.; Atakhanova, N.E.; Majdi, H.S.; Jawad, M.A.; Hasan, M.K.; Borhani, F.; Khatami, M. Drug delivery and anticancer activity of biosynthesised mesoporous Fe2O3 nanoparticles. IET Nanobiotechnol. 2022, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. Metal-based nanoparticles for the treatment of infectious diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, D.; Kothari, S. Green synthesis of silver nanoparticles and their application in plant virus inhibition. J. Mycol. Plant Pathol. 2014, 44, 21–24. [Google Scholar]
- Elbeshehy, E.K.; Elazzazy, A.M.; Aggelis, G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against bean yellow mosaic virus and human pathogens. Front. Microbiol. 2015, 6, 453. [Google Scholar] [CrossRef] [Green Version]
- Noha, K.; Bondok, A.; El-Dougdoug, K. Evaluation of silver nanoparticles as antiviral agent against ToMV and PVY in tomato plants. Sciences 2018, 8, 100–111. [Google Scholar]
- Sujitha, V.; Murugan, K.; Paulpandi, M.; Panneerselvam, C.; Suresh, U.; Roni, M.; Nicoletti, M.; Higuchi, A.; Madhiyazhagan, P.; Subramaniam, J.; et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef]
- Chen, Y.N.; Hsueh, Y.H.; Hsieh, C.T.; Tzou, D.Y.; Chang, P.L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [Green Version]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnology 2005, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fatima, M.; Sadaf Zaidi, N.-u.S.; Amraiz, D.; Afzal, F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza A virus. J. Microbiol. Biotechnol. 2016, 26, 151–159. [Google Scholar] [CrossRef]
- Sreekanth, T.; Nagajyothi, P.; Muthuraman, P.; Enkhtaivan, G.; Vattikuti, S.; Tettey, C.; Kim, D.H.; Shim, J.; Yoo, K. Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities. J. Photochem. Photobiol. B Biol. 2018, 188, 6–11. [Google Scholar] [CrossRef]
- Kaushik, S.; Sharma, V.; Chhikara, S.; Yadav, J.; Kaushik, S. Anti-chikungunya activity of green synthesized silver nanoparticles using Carica papaya leaves in animal cell culture model. Asian J. Pharm. Clin. Res. 2019, 12, 170–174. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, M.; Gaikwad, S.; Ingle, A. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303. [Google Scholar] [CrossRef] [Green Version]
- El-Mohamady, R.S.; Ghattas, T.; Zawrah, M.; Abd El-Hafeiz, Y. Inhibitory effect of silver nanoparticles on bovine herpesvirus-1. Int. J. Vet. Sci. Med. 2018, 6, 296–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Li, S.; Kong, F.; Hou, R.; Guan, X.; Guo, F. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet. Mol. Res. 2014, 13, 7022–7028. [Google Scholar] [CrossRef] [PubMed]
- Bekele, A.Z.; Gokulan, K.; Williams, K.M.; Khare, S. Dose and size-dependent antiviral effects of silver nanoparticles on feline calicivirus, a human norovirus surrogate. Foodborne Pathog. Dis. 2016, 13, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Dhanasezhian, A.; Srivani, S.; Govindaraju, K.; Parija, P.; Sasikala, S.; Kumar, M. Anti-herpes simplex virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Geo Mar. Sci. 2019, 48, 1252–1257. [Google Scholar]
- Govindaraju, K.; Kiruthiga, V.; Kumar, V.G.; Singaravelu, G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2009, 9, 5497–5501. [Google Scholar] [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217. [Google Scholar] [CrossRef] [Green Version]
- Avilala, J.; Golla, N. Antibacterial and antiviral properties of silver nanoparticles synthesized by marine actinomycetes. Int. J. Pharm. Sci. Res. 2019, 10, 1223–1228. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.C.; Ballard, T.E.; Ackerson, C.J.; Feldheim, D.L.; Margolis, D.M.; Melander, C. Inhibition of HIV fusion with multivalent gold nanoparticles. J. Am. Chem. Soc. 2008, 130, 6896–6897. [Google Scholar] [CrossRef] [Green Version]
- Di Gianvincenzo, P.; Marradi, M.; Martínez-Ávila, O.M.; Bedoya, L.M.; Alcami, J.; Penadés, S. Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorganic Med. Chem. Lett. 2010, 20, 2718–2721. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, F.; Marradi, M.; Calvo, J.; Yuste, E.; Penadés, S. Glycosystems in nanotechnology: Gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein J. Org. Chem. 2014, 10, 1339–1346. [Google Scholar] [CrossRef]
- Garrido, C.; Simpson, C.A.; Dahl, N.P.; Bresee, J.; Whitehead, D.C.; Lindsey, E.A.; Harris, T.L.; Smith, C.A.; Carter, C.J.; Feldheim, D.L.; et al. Gold nanoparticles to improve HIV drug delivery. Futur. Med. Chem. 2015, 7, 1097–1107. [Google Scholar] [CrossRef]
- Kesarkar, R.; Shroff, S.; Yeole, M.; Chowdhary, A. L-cysteine functionalized gold nanocargos potentiates anti-HIV activity of azidothymydine against HIV-1. System 2015, 3, 10. [Google Scholar] [CrossRef]
- Kesarkar, R.; Oza, G.; Pandey, S.; Dahake, R.; Mukherjee, S.; Chowdhary, A.; Sharon, M. Gold nanoparticles: Effective as both entry inhibitors and virus neutralizing agents against HIV. J. Microbiol. Biotechnol. Res. 2012, 2, 276–283. [Google Scholar]
- Bastian, A.R.; Nangarlia, A.; Bailey, L.D.; Holmes, A.; Sundaram, R.V.K.; Ang, C.; Moreira, D.R.; Freedman, K.; Duffy, C.; Contarino, M.; et al. Mechanism of multivalent nanoparticle encounter with HIV-1 for potency enhancement of peptide triazole virus inactivation. J. Biol. Chem. 2015, 290, 529–543. [Google Scholar] [CrossRef] [Green Version]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against herpes simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Heal. Res. 2022, 32, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Yang, J.A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef] [PubMed]
- Yasri, S.; Wiwanitkit, V. Effect of gold nanoparticle on viral load of hepatitis C virus. J. Coast. Life Med. 2014, 2, 2754–2761. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Sakoda, Y.; Ohyanagi, T.; Nagahori, N.; Shibuya, H.; Okamastu, M.; Miura, N.; Kida, H.; Nishimura, S.-I. Novel thiosialosides tethered to metal nanoparticles as potent influenza a virus haemagglutinin blockers. Antivir. Chem. Chemother. 2013, 23, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Meléndez-Villanueva, M.A.; Morán-Santibañez, K.; Martínez-Sanmiguel, J.J.; Rangel-López, R.; Garza-Navarro, M.A.; Rodríguez-Padilla, C.; Zarate-Triviño, D.G.; Trejo-Ávila, L.M. Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 2019, 11, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral activity of cuprous oxide nanoparticles against hepatitis C virus in vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Majbauddin, A.; Kodani, I.; Ryoke, K. The effect of bamboo leaf extract solution and sodium copper chlorophyllin solution on growth and volatile sulfur compounds production of oral malodor associated some anaerobic periodontal bacteria. Yonago Acta Med. 2015, 58, 129. [Google Scholar]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human coronavirus 229E remains infectious on common touch surface materials. MBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Tsuneki, A.; Yoshida, Y.; Ryoke, K.; Kaidoh, T.; Kageyama, S. In vitro inhibition of cytopathic effect of influenza virus and human immunodeficiency virus by bamboo leaf extract solution and sodium copper chlorophyllin. Yonago Acta Med. 2016, 59, 61. [Google Scholar] [PubMed]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zerbib, S.; Vallet, L.; Muggeo, A.; de Champs, C.; Lefebvre, A.; Jolly, D.; Kanagaratnam, L. Copper for the prevention of outbreaks of health care–associated infections in a long-term care facility for older adults. J. Am. Med. Dir. Assoc. 2020, 21, 68–71.e61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, A.A.; Zuñiga, J.M. The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115176. [Google Scholar] [CrossRef] [PubMed]
- Yugandhar, P.; Vasavi, T.; Rao, Y.J.; Devi, P.U.M.; Narasimha, G.; Savithramma, N. Cost effective, green synthesis of copper oxide nanoparticles using fruit extract of Syzygium alternifolium (Wt.) Walp: Characterization and evaluation of antiviral activity. J. Clust. Sci. 2018, 29, 743–755. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; DiTaranto, N.; Cioffi, N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [Green Version]
- Fouad, G.I. A proposed insight into the anti-viral potential of metallic nanoparticles against novel coronavirus disease-19 (COVID-19). Bull. Natl. Res. Cent. 2021, 45, 1–22. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Guo, M.; Xiao, M.; Wang, C.; Zhao, M.; Xu, T.; Xia, Y.; Zhu, B. Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Adv. 2017, 7, 35290–35296. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.X.; Li, C.M.; Li, Y.F.; Wang, J.; Huang, C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale 2017, 9, 16086–16092. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.; Topno, R.; Madhukar, M.; Das, P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Qin, T.; Ma, R.; Yin, Y.; Miao, X.; Chen, S.; Fan, K.; Xi, J.; Liu, Q.; Gu, Y.; Yin, Y.; et al. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 2019, 9, 6920. [Google Scholar] [CrossRef] [PubMed]
- Shelby, T.; Banerjee, T.; Zegar, I.; Santra, S. Highly sensitive, engineered magnetic nanosensors to investigate the ambiguous activity of zika virus and binding receptors. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadavalli, T.; Shukla, D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Jamal, W.T.; Kostarelos, K. Liposome–nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine 2007, 2, 85–98. [Google Scholar] [CrossRef]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Wei, J.C.; Lee, Y.L.; Hsu, S.H.; Lin, J.J.; Lin, Y.L. Surfactant-modified nanoclay exhibits an antiviral activity with high potency and broad spectrum. J. Virol. 2014, 88, 4218–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.C.; Davis-Poynter, N.; Nguyen, C.T.; Peters, A.A.; Monteith, G.R.; Strounina, E.; Popat, A.; Ross, B.P. GAG mimetic functionalised solid and mesoporous silica nanoparticles as viral entry inhibitors of herpes simplex type 1 and type 2 viruses. Nanoscale 2016, 8, 16192–16196. [Google Scholar] [CrossRef] [Green Version]
- Chunduri, L.; Kurdekar, A.; Haleyurgirisetty, M.K.; Bulagonda, E.P.; Kamisetti, V.; Hewlett, I.K. Femtogram level sensitivity achieved by surface engineered silica nanoparticles in the early detection of HIV infection. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A. Green synthesized ZnO nanoparticles mediated by Mentha spicata extract induce plant systemic resistance against tobacco mosaic virus. Appl. Sci. 2020, 10, 5054. [Google Scholar] [CrossRef]
- Zholobak, N.; Ivanov, V.; Shcherbakov, A.; Shaporev, A.; Polezhaeva, O.; Baranchikov, A.; Spivak, N.; Tretyakov, Y. UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J. Photochem. Photobiol. B Biol. 2011, 102, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, N.; Chuan, Y.P.; Seth, A.; Cordoba, Y.; Lua, L.H.; Middelberg, A.P. Co-administration of non-carrier nanoparticles boosts antigen immune response without requiring protein conjugation. Vaccine 2014, 32, 3664–3669. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.B.; Reukov, V.V.; Yakimansky, A.V.; Krasnopeeva, E.L.; Ivanova, O.S.; Popov, A.L.; Ivanov, V.K. CeO2 nanoparticle-containing polymers for biomedical applications: A review. Polymers 2021, 13, 924. [Google Scholar] [CrossRef]
- Mazumder, S.; Dewangan, A.K.; Pavurala, N. Enhanced dissolution of poorly soluble antiviral drugs from nanoparticles of cellulose acetate based solid dispersion matrices. Asian J. Pharm. Sci. 2017, 12, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.S.; Repkova, M.N.; Mazurkova, N.A.; Makarevich, E.V.; Ismagilov, Z.R.; Zarytova, V.F. Knockdown of different influenza A virus subtypes in cell culture by a single antisense oligodeoxyribonucleotide. Int. J. Antimicrob. Agents 2015, 46, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Sexton, N.R.; Smith, E.C.; Blanc, H.; Vignuzzi, M.; Peersen, O.B.; Denison, M.R. Homology-based identification of a mutation in the coronavirus RNA-dependent RNA polymerase that confers resistance to multiple mutagens. J. Virol. 2016, 90, 7415–7428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Zhao, K.; Shi, Z.-L.; Zhou, P. Bat coronaviruses in China. Viruses 2019, 11, 210. [Google Scholar] [CrossRef] [Green Version]
- Mainardes, R.M.; Diedrich, C. The potential role of nanomedicine on COVID-19 therapeutics. Ther. Deliv. 2020, 11, 411–414. [Google Scholar] [CrossRef]
- Pandey, A.; Nikam, A.N.; Shreya, A.B.; Mutalik, S.P.; Gopalan, D.; Kulkarni, S.; Padya, B.S.; Fernandes, G.; Mutalik, S.; Prassl, R. Potential therapeutic targets for combating SARS-CoV-2: Drug repurposing, clinical trials and recent advancements. Life Sci. 2020, 256, 117883. [Google Scholar] [CrossRef]
- Pal, A.; Pawar, A.; Goswami, K.; Sharma, P.; Prasad, R. Hydroxychloroquine and covid-19: A cellular and molecular biology based update. Indian J. Clin. Biochem. 2020, 35, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangali, G.R. Antimicrobial activity of orthosiphon aristatus (Balbas pusa) nanoparticle and leaf extract against E. coli and S. aureus. World J. Pharm. Pharm. Sci. 2019, 9, 174–199. [Google Scholar] [CrossRef]
- Maduray, K.; Parboosing, R. Metal nanoparticles: A promising treatment for viral and arboviral infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176. [Google Scholar] [CrossRef]
- Medhi, B.; Prajapat, M.; Sarma, P.; Shekhar, N.; Prakash, A.; Avti, P.; Bhattacharyya, A.; Kaur, H.; Kumar, S.; Bansal, S.; et al. Update on the target structures of SARS-nCoV-2: A systematic review. Indian J. Pharmacol. 2020, 52, 142. [Google Scholar] [CrossRef]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P. Nanoparticle vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Cojocaru, F.D.; Botezat, D.; Gardikiotis, I.; Uritu, C.-M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.-I.; Mihai, C.-T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, M.A.; Martina, B.; Prakash, J. Nanomedicine strategies to target coronavirus. Nano Today 2020, 35, 100961. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. Why have nanotechnologies been underutilized in the global uprising against the coronavirus pandemic? Nanomedicine 2020, 15, 1719–1734. [Google Scholar] [CrossRef] [PubMed]
- Atal, S.; Fatima, Z. IL-6 inhibitors in the treatment of serious COVID-19: A promising therapy? Pharm. Med. 2020, 34, 223–231. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Volkov, A.A.; Mezhenny, P.V.; Domnitsky, I.Y.; Fomin, A.S.; Kozlov, S.V.; Dykman, L.A.; Guliy, O.I. Prospects for the use of spherical gold nanoparticles in immunization. Appl. Microbiol. Biotechnol. 2019, 103, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, W.; Zhai, Z.; Gao, C. Antiviral activity of nanomaterials against coronaviruses. Macromol. Biosci. 2020, 20, 2000196. [Google Scholar] [CrossRef] [PubMed]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; Van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Derwand, R.; Scholz, M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Ziem, B.; Azab, W.; Gholami, M.F.; Rabe, J.P.; Osterrieder, N.; Haag, R. Size-dependent inhibition of herpesvirus cellular entry by polyvalent nanoarchitectures. Nanoscale 2017, 9, 3774–3783. [Google Scholar] [CrossRef]
- Palmieri, V.; Papi, M. Can graphene take part in the fight against COVID-19? Nano Today 2020, 33, 100883. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Medhi, R.; Srinoi, P.; Ngo, N.; Tran, H.-V.; Lee, T.R. Nanoparticle-based strategies to combat COVID-19. ACS Appl. Nano Mater. 2020, 3, 8557–8580. [Google Scholar] [CrossRef]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-López, I.; Alvarado-Vera, M.; Patrón-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, M.M.; Abelyan, N.; Abdelgawad, M.A.; Musa, A.; Ghoneim, M.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Alnusaire, T.S.; Abdelwahab, S.F.; et al. Strawberry and ginger silver nanoparticles as potential inhibitors for SARS-CoV-2 assisted by in silico modeling and metabolic profiling. Antibiotics 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C. Diagnosing COVID-19: The disease and tools for detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020, 11, 748–787. [Google Scholar] [CrossRef]
- Kumar, A.; Nath, K.; Parekh, Y.; Enayathullah, M.G.; Bokara, K.K.; Sinhamahapatra, A. Antimicrobial silver nanoparticle-photodeposited fabrics for SARS-CoV-2 destruction. Colloid Interface Sci. Commun. 2021, 45, 100542. [Google Scholar] [CrossRef]
- Ahmed, T.; Ogulata, R.T.; Bozok, S.S. Silver nanoparticles against SARS-CoV-2 and its potential application in medical protective clothing – A review. J. Text. Inst. 2021, 113, 1–14. [Google Scholar] [CrossRef]
- Takeda, Y.; Jamsransuren, D.; Nagao, T.; Fukui, Y.; Matsuda, S.; Ogawa, H. Application of copper iodide nanoparticle-doped film and fabric to inactivate SARS-CoV-2 via the virucidal activity of cuprous ions (Cu+). Appl. Environ. Microbiol. 2021, 87, e0182421. [Google Scholar] [CrossRef]
- Sarkar, S. Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. J. Curr. Med Res. Opin. 2020, 3, 449–450. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, A.A.; Tawfeek, H.M.; Abdelfattah, A.; Batiha, G.E.S.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Kumar, B. A review on nanotechnology-based innovations in diagnosis and treatment of multiple sclerosis. J. Cell. Immunother. 2018, 4, 56–64. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Reece, S.P.; Brown, J.M. Immunotoxicological impact of engineered nanomaterial exposure: Mechanisms of immune cell modulation. Toxicol. Mech. Methods 2013, 23, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, R.; Kaushik, N.K.; Kaushik, N.; Choi, E.H.; Umar, A.; Dwivedi, S.; Musarrat, J.; Al-Khedhairy, A. ZnO nanoparticles induces cell death in malignant human T98G gliomas, KB and non-malignant HEK cells. J. Biomed. Nanotechnol. 2013, 9, 1181–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—A comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Kim, J.-K.; Seo, S.-J.; Kim, K.-H.; Kim, T.-J.; Chung, M.-H.; Kim, K.-R.; Yang, T.-K. Therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect. Nanotechnology 2010, 21, 425102. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef]
- Docea, A.O.; Calina, D.; Buga, A.M.; Zlatian, O.; Paoliello, M.; Mogosanu, G.D.; Streba, C.T.; Popescu, E.L.; Stoica, A.E.; Bîrcă, A.C.; et al. The effect of silver nanoparticles on antioxidant/pro-oxidant balance in a murine model. Int. J. Mol. Sci. 2020, 21, 1233. [Google Scholar] [CrossRef] [Green Version]
- Elbialy, N.S.; Aboushoushah, S.F.; Alshammari, W.W. Long-term biodistribution and toxicity of curcumin capped iron oxide nanoparticles after single-dose administration in mice. Life Sci. 2019, 230, 76–83. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale 2016, 8, 12444–12470. [Google Scholar] [CrossRef]
- Fischer, H.C.; Chan, W.C. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Patra, C.R. Biologically synthesized metal nanoparticles: Recent advancement and future perspectives in cancer theranostics. Futur. Sci. OA 2017, 3, FSO203. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Plant Source | Physicochemical Properties | In Vitro Anticancer Activity | References |
|---|---|---|---|
| 1. Podophyllum hexadrum L. (Leaf extract, H2O) | Spherical shape; 5–35 nm diameter size range | HeLa cells; IC50 of 20 μg/mL | [80] |
| 2. Mentha piperita (Leaf extract, H2O) | Spherical, hexagonal, and triangular shape; 10–300 nm diameter size range | HEK293 cells; IC50 value not available | [81] |
| 3. Meringa oleifa (Leaf extract, H2O) | Spherical and polyhedral shape; 10–20 nm diameter size range | A549 cells and SNO cells; IC50 values of 98.46 and 92.01 μg/mL | [82] |
| 4. Rhus chinesis (Galls extract, H2O) | Spherical and oval shape; 20–40 nm diameter size range | Hep3B cells, MG-63 cells, and MKN-28 cells; IC50 value of 150 µg/mL | [83] |
| 5. Nerium oleander (Stem bark extract, H2O) | Spherical, hexagonal, triangular, rod, and flower-like shape; 10–100 nm diameter size range | MCF-7 cells; IC50 value of 74 μg/mL | [84] |
| 6. Curcuma wenyujin (Rhizome extract, H2O) | Spherical shape; 200 nm mean diameter size | A498 cells and SW156 cells; IC50 values of 25 µg/mL and 40 µg/mL | [85] |
| 7. Retroselinum crispum (Leaf extract, H2O) | Spherical, semi-rod, and flower-like shape; 20–80 nm diameter size range | CO-II cells; IC50 value of 84.39 µg/mL | [86] |
| 8. Polianthes tuberosa L. (Floral extract, H2O) | Spherical, triangular, rod, hexagon, and pentagon shape; 10–100 nm diameter size range | MCF-7 cells; IC50 value not available | [87] |
| 9. Tecoma capensis (Leaf extract, H2O) | Spherical shape; 10–35 nm diameter size range | MCF-7 cells; IC50 value of 9.6 µg/mL | [88] |
| Plant Source | Physicochemical Properties | In Vitro Anticancer Activity | References |
|---|---|---|---|
| 1. Bauhinia tomentosa Linn (Leaf extract, H2O) | Spherical shape; 16.7 nm mean diameter size | A549 cells; IC50 value of 28.125 μg/mL | [109] |
| 2. Artemisia marschalliana (Aerial extract, H2O) | Spherical shape; 5–50 nm diameter size range | AGS cells; IC50 value of 21.05 µg/mL | [110] |
| 3. Mentha pulegium (Leaf extracts, H2O and MeOH) | Anisotropic shape; 5–50 nm diameter size range | HeLa cells and MCF-7 cells; IC50 value of approximately 100 µg/mL | [111] |
| 4. Rheun Rhabarbarum Rhubarb (Stem extract, H2O) | Spherical shape; 5–50 nm diameter size range | HeLa cells; IC50 value of 10 mg/mL | [112] |
| 5. Cynara scolymus (Leaf extract, H2O) | Spherical shape; 200–223 nm diameter size range | MCF-7 cells; IC50 value of 10 μg/mL | [113] |
| 6. Curcuma longa and Zingiber officinale (Rhizome extracts, H2O) | Spherical shape; 42–61 nm diameter size range | HT-29 cells; IC50 value of 150.8 µg/mL | [114] |
| 7. Hypericum Perforatum L. (Aerial extract, H2O) | Spherical shape; 20–50 nm diameter size range | HeLa cells, HepG2 cells, and A549 cells; IC50 values of 6.72 μg/mL, 6.88 μg/mL), 6.08 μg/mL | [115] |
| 8. Cowpea starch (Seed extract, H2O) | Spherical shape; 40–70 nm diameter size range | HEK293 cells, MCF-7 cells, and A549 cells; IC50 values of 41.7, 56.3, and 63.8 μg/mL | [42] |
| 9. Allium cepa L. (Shallot extract, H2O) | Cubic shape; 150–250 nm diameter size range | HT-29 cells and SW620 cells; IC50 values not available | [116] |
| Plant Source | Physicochemical Properties | In Vitro Anticancer Activity | References |
|---|---|---|---|
| 1. Sargassum muticum (Seaweed extract, H2O) | Cubic shape; 18 nm mean diameter size | MCF-7 cells, Jurkat cells, HepG2 cells, and HeLa cells; IC50 values of 18.75 μg/mL, 6.40 μg/mL, 23.83 μg/mL, and 12.50 μg/mL | [129] |
| 2. Psoralea corylifolia (Seed extract, H2O) | Spherical, rod, hexagonal, cubic, crystalline shape; 39 nm mean diameter size | Caki-2 cells; IC50 value of 0.8 mg/mL | [130] |
| 3. Rosmarinus officinalis L. (Leaf extract, H2O) | Spherical shape; 100 nm mean diameter size | 4T1 cells and C26 cells; IC50 values of 44 µg/mL and 100 µg/mL | [131] |
| 4. Punica granatum (Fruit peel extract, H2O) | Spherical and cubic shape; 26.5 nm mean diameter size | NPC cells and HONE1 cells; IC50 values of 197.46 and 85.06 μg/mL | [132] |
| 5. Lawsonia inermis (Leaf extract, H2O) | Spherical and irregular shape; 45.8 nm mean diameter size | MCF-7 cells; IC50 value not available | [133] |
| 6. Piper betel (Leaf extract, H2O) | Cubic shape; 25.8 nm mean diameter size | A549 cells; IC50 value of 104.6 mg/mL | [134] |
| 7. Garcinia mangostana (Fruit peel extract, crude) | Spherical shape; 13.4 nm mean diameter size | HCT116 cells and CCD112 cells; IC50 values of 99.80 µg/mL and 140.80 µg/mL | [135] |
| Plant Source | Physicochemical Properties | In Vitro Anticancer Activity | References |
|---|---|---|---|
| 1. Terminalia chebula (Fruit rind extract) | Irregular shape; 80–100 nm diameter size range | A549 cells; IC50 value of 62.5μg/mL | [148] |
| 2. Cinnamomum tamala, (Leaf extract, H2O) | Irregular shape; 23 nm mean diameter size | D145 cells; IC50 value not available | [149] |
| 3. Aloe vera (Gel extract) | Irregular shape; 11 nm mean diameter size | A549 cells; IC50 of values of 165 μg/mL and 210 μg/mL | [150] |
| 4. Acacia nilotia (Aerial extract, H2O) | Spherical shape; 20–40 nm diameter size range | MCF-7 cells; IC50 value not available | [151] |
| 5. Fomes fomentarius (Mushroom extract, H2O) | Irregular shape; 100–120 nm diameter size range | HCT-116 cells; IC50 value not available | [152] |
| 6. Withania somnifera (Root extract, H2O) | Spherical shape; 50–90 nm diameter size range | HepG2 cells; IC50 value of 53.65 µg/mL | [153] |
| 7. Ledebouria revoluta (Bulb extract, H2O) | Spherical and tetragonal shape; 47 nm mean diameter size | A549 cells; IC50 value of 53.65 µg/mL | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbatha, L.S.; Akinyelu, J.; Chukwuma, C.I.; Mokoena, M.P.; Kudanga, T. Current Trends and Prospects for Application of Green Synthesized Metal Nanoparticles in Cancer and COVID-19 Therapies. Viruses 2023, 15, 741. https://doi.org/10.3390/v15030741
Mbatha LS, Akinyelu J, Chukwuma CI, Mokoena MP, Kudanga T. Current Trends and Prospects for Application of Green Synthesized Metal Nanoparticles in Cancer and COVID-19 Therapies. Viruses. 2023; 15(3):741. https://doi.org/10.3390/v15030741
Chicago/Turabian StyleMbatha, Londiwe Simphiwe, Jude Akinyelu, Chika Ifeanyi Chukwuma, Mduduzi Paul Mokoena, and Tukayi Kudanga. 2023. "Current Trends and Prospects for Application of Green Synthesized Metal Nanoparticles in Cancer and COVID-19 Therapies" Viruses 15, no. 3: 741. https://doi.org/10.3390/v15030741
APA StyleMbatha, L. S., Akinyelu, J., Chukwuma, C. I., Mokoena, M. P., & Kudanga, T. (2023). Current Trends and Prospects for Application of Green Synthesized Metal Nanoparticles in Cancer and COVID-19 Therapies. Viruses, 15(3), 741. https://doi.org/10.3390/v15030741