Antibody Immunity to Zika Virus among Young Children in a Flavivirus-Endemic Area in Nicaragua
Abstract
1. Introduction
2. Methods
Study Design and Biospecimens
3. Results
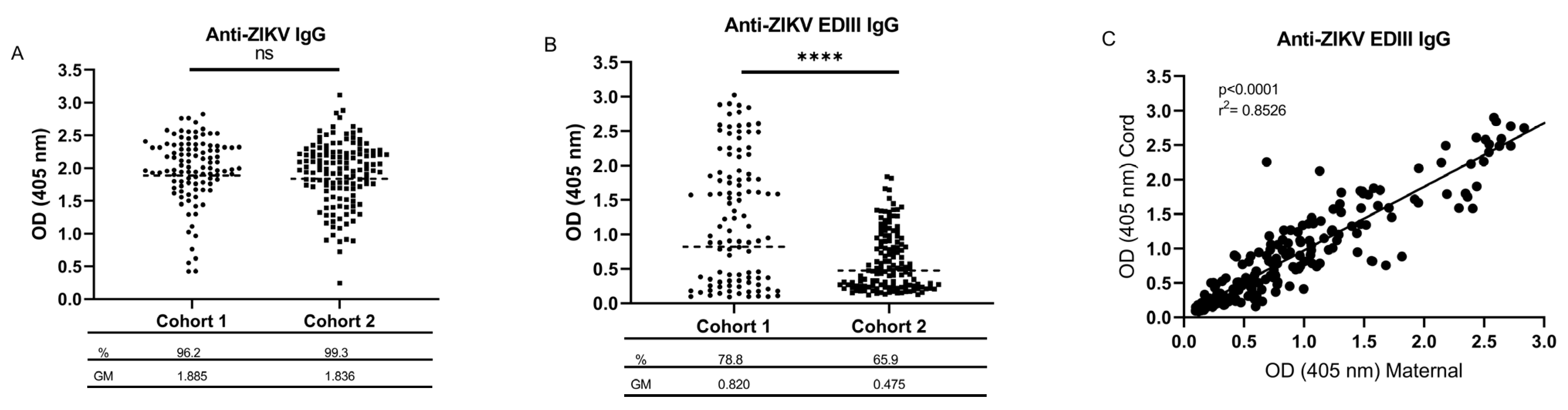
3.1. Seroprevalence of Maternal Flavivirus Cross-Reactive and ZIKV-Specific IgG in Cord Blood
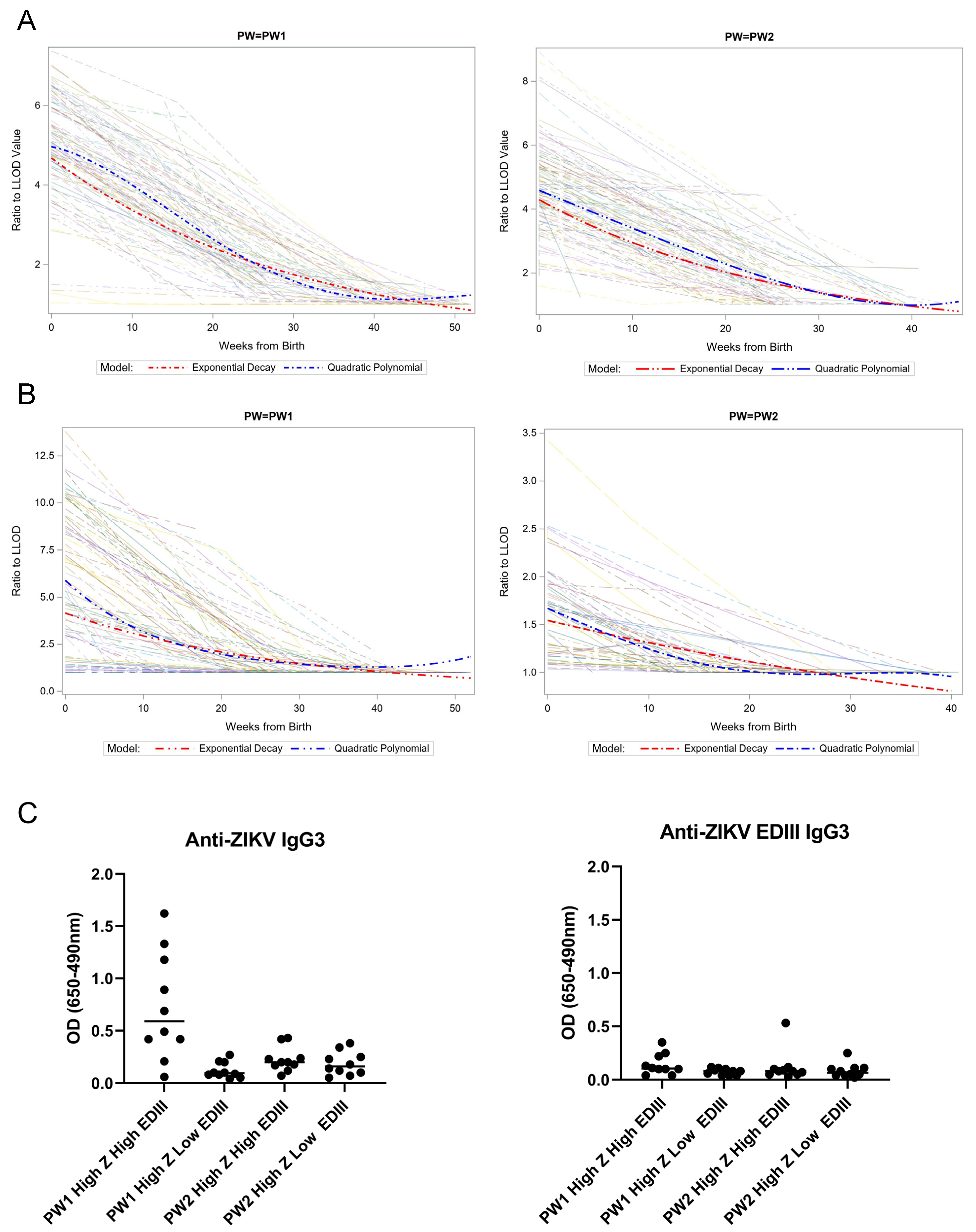
3.2. Kinetics and Composition of Maternal-Derived ZIKV-Reactive Antibodies
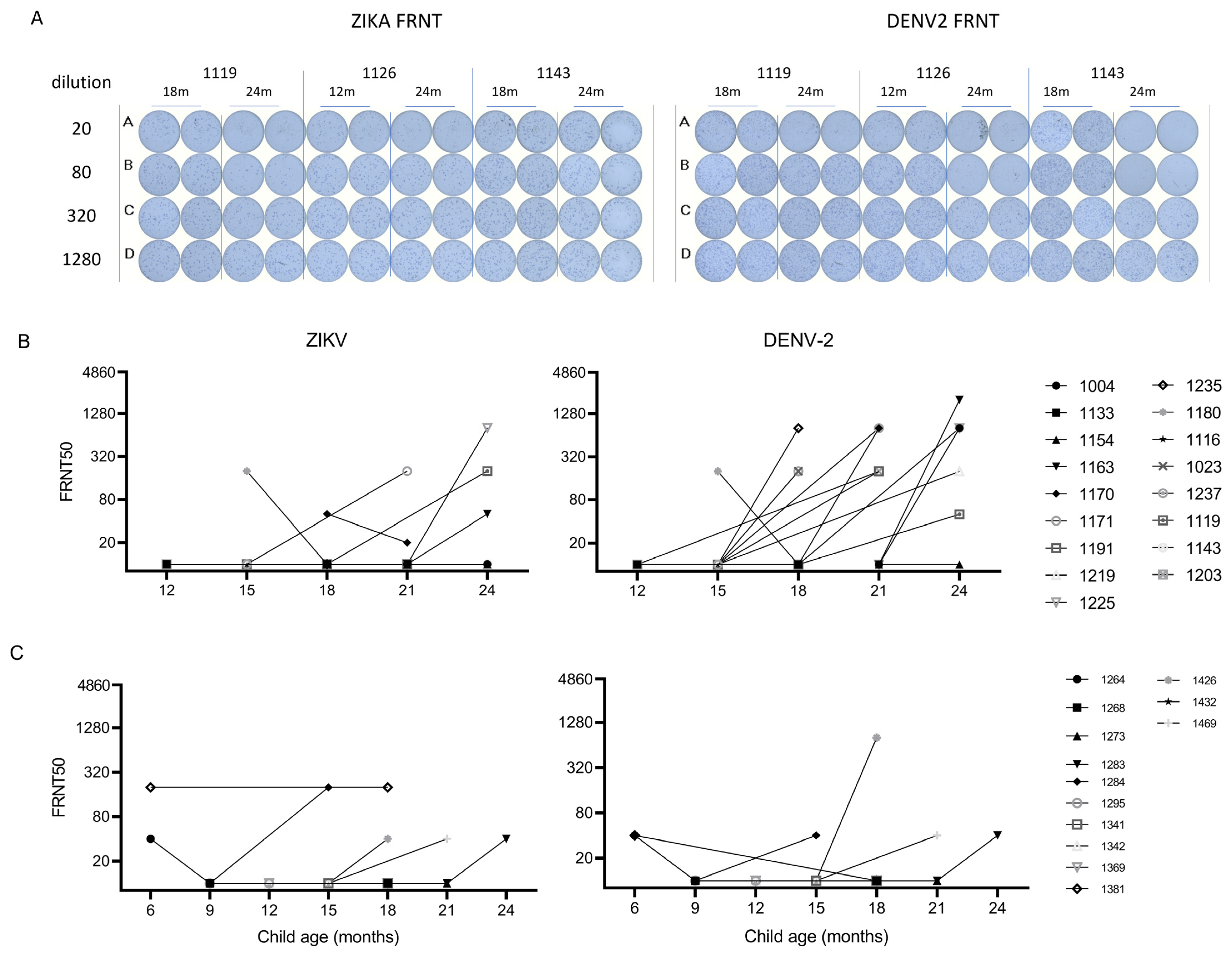
3.3. Incident Dengue Infection after Maternal-Derived Antibody Decay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sampathkumar, P.; Sanchez, J.L. Zika Virus in the Americas: A Review for Clinicians. Mayo Clin. Proc. 2016, 91, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.H.-O.; Bohlin, J.; Dupont-Rouzeyrol, M.; Brynildsrud, O.B.; Alfsnes, K.; Cao-Lormeau, V.-M.; Gaunt, M.W.; Falconar, A.K.; de Lamballerie, X.; Eldholm, V.; et al. Re-visiting the evolution, dispersal and epidemiology of Zika virus in Asia. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef]
- Netto, E.M.; Moreira-Soto, A.; Pedroso, C.; Höser, C.; Funk, S.; Kucharski, A.J.; Rockstroh, A.; Kümmerer, B.M.; Sampaio, G.S.; Luz, E.; et al. High Zika Virus Seroprevalence in Salvador, Northeastern Brazil Limits the Potential for Further Outbreaks. MBio 2017, 8, e01390-17. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, J.V.; Bustos Carrillo, F.; Burger-Calderon, R.; Collado, D.; Sanchez, N.; Ojeda, S.; Carey Monterrey, J.; Plazaola, M.; Lopez, B.; Arguello, S.; et al. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc. Natl. Acad. Sci. USA 2018, 115, 9294–9299. [Google Scholar] [CrossRef]
- Siraj, A.S.; Perkins, T.A. Assessing the population at risk of Zika virus in Asia—Is the emergency really over? BMJ Glob. Health 2017, 2, e000309. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Brent, S.; Watts, A.G.; Hay, S.I.; Kulkarni, M.A.; Brownstein, J.S.; et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: A modelling study. Lancet Infect. Dis. 2016, 16, 1237–1245. [Google Scholar] [CrossRef]
- Statistics and Maps | Zika virus | CDC. Available online: https://www.cdc.gov/zika/reporting/index.html (accessed on 10 March 2023).
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, O.; Beltrán, M.; Nelson, C.A.; Valencia, D.; Tolosa, N.; Farr, S.L.; Padilla, A.V.; Tong, V.T.; Cuevas, E.L.; Espinosa-Bode, A.; et al. Zika Virus Disease in Colombia—Preliminary Report. N. Engl. J. Med. 2016, 383, e44. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Lazear, H.M. Zika virus-reigniting the TORCH. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef]
- Melo, A.S.d.O.; Aguiar, R.S.; Amorim, M.M.R.; Arruda, M.B.; Melo, F.d.O.; Ribeiro, S.T.C.; Batista, A.G.M.; Ferreira, T.; dos Santos, M.P.; Sampaio, V.V.; et al. Congenital Zika Virus Infection. JAMA Neurol. 2016, 73, 1407. [Google Scholar] [CrossRef]
- Stringer, E.M.; Martinez, E.; Blette, B.; Toval Ruiz, C.E.; Boivin, M.; Zepeda, O.; Stringer, J.S.A.; Morales, M.; Ortiz-Pujols, S.; Familiar, I.; et al. Neurodevelopmental Outcomes of Children Following In Utero Exposure to Zika in Nicaragua. Clin. Infect. Dis. 2021, 72, e146–e153. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; Du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr. 2020, 174, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Subissi, L.; Dub, T.; Besnard, M.; Mariteragi-Helle, T.; Nhan, T.; Lutringer-Magnin, D.; Barboza, P.; Gurry, C.; Brindel, P.; Nilles, E.J.; et al. Zika virus infection during pregnancy and effects on early childhood development, French Polynesia, 2013–2016. Emerg. Infect. Dis. 2018, 24, 1850–1858. [Google Scholar] [CrossRef]
- Simmons, C.P.; Chau, T.N.B.; Thuy, T.T.; Tuan, N.M.; Hoang, D.M.; Thien, N.T.; Lien, L.B.; Quy, N.T.; Hieu, N.T.; Hien, T.T.; et al. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J. Infect. Dis. 2007, 196, 416–424. [Google Scholar] [CrossRef]
- Chau, T.N.B.; Hieu, N.T.; Anders, K.L.; Wolbers, M.; Lien, L.B.; Hieu, L.T.M.; Hien, T.T.; Hung, N.T.; Farrar, J.; Whitehead, S.; et al. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J. Infect. Dis. 2009, 200, 1893–1900. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Mercado, B.L.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Carillo, F.B.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Dussupt, V.; Modjarrad, K.; Krebs, S.J. Landscape of Monoclonal Antibodies Targeting Zika and Dengue: Therapeutic Solutions and Critical Insights for Vaccine Development. Front. Immunol. 2021, 11, 3687. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Zika Vaccine Development: Current Status. Mayo Clin. Proc. 2019, 94, 2572–2586. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Zepeda, O.; Blette, B.; Jadi, R.; Morales, M.; Pérez, R.; Liou, G.-J.A.; Montoya-Cruz, M.; Harris, E.; Becker-Dreps, S.; et al. Serologic surveillance of maternal Zika infection in a prospective cohort in Leon, Nicaragua during the peak of the Zika epidemic. PLoS ONE 2020, 15, e0230692. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; Tu, H.A.; Gimblet-Ochieng, C.; Liou, G.-J.A.; Jadi, R.S.; Metz, S.W.; Thomas, A.; McElvany, B.D.; Davidson, E.; Doranz, B.J.; et al. Human antibody response to Zika targets type-specific quaternary structure epitopes. JCI Insight 2019, 4, e124588. [Google Scholar] [CrossRef] [PubMed]
- Brien, J.D.; Lazear, H.M.; Diamond, M.S. Propagation, quantification, detection, and storage of West Nile virus. Curr. Protoc. Microbiol. 2013, 31, 15D.3.1–15D.3.18. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; McGowan, E.; Jadi, R.; Young, E.; Lopez, C.A.; Baric, R.S.; Lazear, H.M.; de Silva, A.M. Lack of durable cross-neutralizing antibodies against zika virus from dengue virus infection. Emerg. Infect. Dis. 2017, 23, 773–781. [Google Scholar] [CrossRef]
- Adams, C.; Jadi, R.; Segovia-Chumbez, B.; Daag, J.; Ylade, M.; Medina, F.A.; Sharp, T.M.; Munoz-Jordan, J.L.; Yoon, I.K.; Deen, J.; et al. Novel Assay to Measure Seroprevalence of Zika Virus in the Philippines. Emerg. Infect. Dis. 2021, 27, 3073–3081. [Google Scholar] [CrossRef]
- Grifoni, A.; Costa-Ramos, P.; Pham, J.; Tian, Y.; Rosales, S.L.; Seumois, G.; Sidney, J.; de Silva, A.D.; Premkumar, L.; Collins, M.H.; et al. Cutting Edge:Transcriptional profiling reveals multifunctional and cytotoxic antiviral responses of zika virus-specific CD8 + T cells. J. Immunol. 2018, 201, 3487–3491. [Google Scholar] [CrossRef]
- Reller, M.E.; de Silva, A.M.; Miles, J.J.; Jadi, R.S.; Broadwater, A.; Walker, K.; Woods, C.; Mayorga, O.; Matute, A. Unsuspected Dengue as a Cause of Acute Febrile Illness in Children and Adults in Western Nicaragua. PLoS Negl. Trop. Dis. 2016, 10, e0005026. [Google Scholar] [CrossRef][Green Version]
- Speer, S.D.; Pierson, T.C. VIROLOGY. Diagnostics for Zika virus on the horizon. Science 2016, 353, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Allwinn, R.; Doerr, H.W.; Emmerich, P.; Schmitz, H.; Preiser, W. Cross-reactivity in flavivirus serology: New implications of an old finding? Med. Microbiol. Immunol. 2002, 190, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Jordan, J.L. Diagnosis of Zika Virus Infections: Challenges and Opportunities. J. Infect. Dis. 2017, 216, S951–S956. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.B.; Endy, T.P.; Thomas, S.J. Finding the Signal Among the Noise in the Serologic Diagnosis of Flavivirus Infections. J. Infect. Dis. 2018, 218, 516–518. [Google Scholar] [CrossRef]
- Collins, M.H. Serologic tools and strategies to support intervention trials to combat Zika virus infection and disease. Trop. Med. Infect. Dis. 2019, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.; Collins, M.; Graham, S.; Liou, G.A.; Lopez, C.A.; Jadi, R.; Balmaseda, A.; Brackbill, J.A.; Dietze, R.; Camacho, E.; et al. Development of Envelope Protein Antigens To Serologically Differentiate Zika Virus Infection from Dengue Virus Infection. J. Clin. Microbiol. 2018, 56, 1504–1521. [Google Scholar]
- Calvert, A.E.; Boroughs, K.L.; Laven, J.; Stovall, J.L.; Luy, B.E.; Kosoy, O.I.; Huang, C.Y.-H. Incorporation of IgG Depletion in a Neutralization Assay Facilitates Differential Diagnosis of Zika and Dengue in Secondary Flavivirus Infection Cases. J. Clin. Microbiol. 2018, 56, e00234-18. [Google Scholar] [CrossRef]
- Balmaseda, A.; Stettler, K.; Medialdea-Carrera, R.; Collado, D.; Jin, X.; Zambrana, J.V.; Jaconi, S.; Cameroni, E.; Saborio, S.; Rovida, F.; et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 201704984. [Google Scholar] [CrossRef]
- Wong, S.J.; Furuya, A.; Zou, J.; Xie, X.; Dupuis, A.P.; Kramer, L.D.; Shi, P.-Y. A Multiplex Microsphere Immunoassay for Zika Virus Diagnosis. EBioMedicine 2017, 16, 136–140. [Google Scholar] [CrossRef]
- Tokarz, R.; Mishra, N.; Tagliafierro, T.; Sameroff, S.; Caciula, A.; Chauhan, L.; Patel, J.; Sullivan, E.; Gucwa, A.; Fallon, B.; et al. A multiplex serologic platform for diagnosis of tick-borne diseases. Sci. Rep. 2018, 8, 3158. [Google Scholar] [CrossRef]
- Bosch, I.; de Puig, H.; Hiley, M.; Carré-Camps, M.; Perdomo-Celis, F.; Narváez, C.F.; Salgado, D.M.; Senthoor, D.; O’Grady, M.; Phillips, E.; et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017, 9, eaan1589. [Google Scholar] [CrossRef]
- Rojas, A.; Natrajan, M.S.; Weber, J.; Cardozo, F.; Cantero, C.; Ananta, J.S.; Kost, J.; Tang, M.; Lopez, S.; Bernal, C.; et al. Comparison of Anti-Dengue and Anti-Zika IgG on a Plasmonic Gold Platform with Neutralization Testing. Am. J. Trop. Med. Hyg. 2021, 104, 1729. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Peeling, R.W.; Chu, M.C.; Gubler, D.J.; de Silva, A.M.; Harris, E.; Murtagh, M.; Chua, A.; Rodriguez, W.; Kelly, C.; et al. Innovative and new approaches to laboratory diagnosis of Zika and dengue: A meeting report. J. Infect. Dis. 2017, 217, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.M.; Fernandes, J.D.C.P.; Yamamoto, A.Y.; Negrini, S.F.B.d.M.; Negrini, B.V.d.M.; Teixeira, S.R.; Amaral, F.R.; da Motta, M.S.F.; Bárbaro, A.A.T.; Aragon, D.C.; et al. Persistence of anti-zikv-igg over time is not a useful congenital infection marker in infants born to zikv-infected mothers: The natzig cohort. Viruses 2021, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Sesay, S.S.S.; Giorgi, E.; Diggle, P.J.; Schellenberg, D.; Lalloo, D.G.; Terlouw, D.J. Surveillance in easy to access population subgroups as a tool for evaluating malaria control progress: A systematic review. PLoS ONE 2017, 12, e0183330. [Google Scholar] [CrossRef] [PubMed]
- Damelang, T.; Rogerson, S.J.; Kent, S.J.; Chung, A.W. Role of IgG3 in Infectious Diseases. Trends Immunol. 2019, 40, 197–211. [Google Scholar] [CrossRef]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef]
- Borghi, S.; Bournazos, S.; Thulin, N.K.; Li, C.; Gajewski, A.; Sherwood, R.W.; Zhang, S.; Harris, E.; Jagannathan, P.; Wang, L.X.; et al. FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 12943–12951. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Goldfarb, I.; Dolatshahi, S.; Cosgrove, C.; Noelette, F.J.; Krykbaeva, M.; Das, J.; Sarkar, A.; Gorman, M.J.; Fischinger, S.; et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell 2019, 178, 202–215.e14. [Google Scholar] [CrossRef]
- Kam, Y.W.; Simarmata, D.; Chow, A.; Her, Z.; Teng, T.S.; Ong, E.K.S.; Rénia, L.; Leo, Y.S.; Ng, L.F.P. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J. Infect. Dis. 2012, 205, 1147–1154. [Google Scholar] [CrossRef]
- Montoya, M.; Collins, M.; Dejnirattisai, W.; Katzelnick, L.C.; Puerta-Guardo, H.; Jadi, R.; Schildhauer, S.; Supasa, P.; Vasanawathana, S.; Malasit, P.; et al. Longitudinal analysis of antibody cross-neutralization following zika virus and dengue virus infection in Asia and the Americas. J. Infect. Dis. 2018, 218, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Ospina, J.A.; Trujillo, A.M.; Jiménez-Posada, E.V.; Sepúlveda-Arias, J.C.; Tabares-Villa, F.A.; Altieri-Rivera, J.S.; Monsalve, A.; Restrepo-Chica, J.; Osorio, D.; Espinoza, D.; et al. Susceptibility to endemic Aedes-borne viruses among pregnant women in Risaralda, Colombia. Int. J. Infect. Dis. 2022, 122, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.G.; Atyeo, C.; Loos, C.; Montoya, M.; Roy, V.; Bos, S.; Narvekar, P.; Singh, T.; Katzelnick, L.C.; Kuan, G.; et al. Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection. Sci. Transl. Med. 2022, 14, eabm3151. [Google Scholar] [CrossRef]
- Irvine, E.B.; Alter, G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 2020, 30, 241. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Pathogenesis of Dengue: Dawn of a New Era. F1000Research 2015, 4, 1353. [Google Scholar] [CrossRef]
- Abbink, P.; Larocca, R.A.; Visitsunthorn, K.; Boyd, M.; De La Barrera, R.A.; Gromowski, G.D.; Kirilova, M.; Peterson, R.; Li, Z.; Nanayakkara, O.; et al. Durability and Correlates of Vaccine Protection against Zika Virus in Rhesus Monkeys. Sci. Transl. Med. 2017, 9, eaao4163. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, S.; Ruckwardt, T.J.; Morabito, K.M.; Foreman, B.M.; Burgomaster, K.E.; Gordon, D.N.; Pelc, R.S.; DeMaso, C.R.; Ko, S.Y.; Fisher, B.E.; et al. Distinct neutralizing antibody correlates of protection among related Zika virus vaccines identify a role for antibody quality. Sci. Transl. Med. 2020, 12, eaaw9066. [Google Scholar] [CrossRef] [PubMed]
- Eckels, K.H.; De, R.A.; Barrera, L.; Putnak, J.R. Immunological Assays used to Support Efficacy of Zika Virus Vaccines. Trop. Med. Infect. Dis. 2019, 4, 97. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Young, E.F.; Baric, T.J.; Yount, B.L.; Metz, S.W.; Begley, M.C.; de Silva, A.M.; Baric, R.S. Role of Zika Virus Envelope Protein Domain III as a Target of Human Neutralizing Antibodies. MBio 2019, 10, e01485-19. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.L.; Wahala, W.M.P.B.; Orozco, S.; de Silva, A.M.; Harris, E. Antibodies targeting dengue virus envelope domain III are not required for serotype-specific protection or prevention of enhancement in vivo. Virology 2012, 429, 12–20. [Google Scholar] [CrossRef]
- Semmes, E.C.; Chen, J.L.; Goswami, R.; Burt, T.D.; Permar, S.R.; Fouda, G.G. Understanding Early-Life Adaptive Immunity to Guide Interventions for Pediatric Health. Front. Immunol. 2021, 11, 3544. [Google Scholar] [CrossRef] [PubMed]
- Lyerly, A.D.; Robin, S.G.; Jaffe, E. Rubella and Zika Vaccine Research-A Cautionary Tale About Caution. JAMA Pediatr. 2017, 171, 719–720. [Google Scholar] [CrossRef] [PubMed]
| Mother’s Characteristics | No. of Subjects (%) a | |
|---|---|---|
| Cohort 1 n = 102 | Cohort 2 n = 134 | |
| Delivery dates (range) | 5 February 2017 to 9 October 2017 | 15 November 2017 to 4 July 2018 |
| Mean mother’s age at delivery (year) (±SD) | 23.8 (±7.1) | 24.1 (±5.5) |
| Mean gestational week at birth (week) (±SD) | 38.4 (±1.9) | 38.6 (±1.4) |
| <37 weeks | 10 (10%) | 12 (9%) |
| Cesarean delivery | 47 (46%) | 56 (42%) |
| Previous diagnosis of chikungunya | 39 (38%) | 54 (40%) |
| Previous diagnosis of dengue | 7 (7%) | 12 (9%) |
| Previous diagnosis of Zika | 3 (3%) | 1 (1%) |
| Children’s characteristics at birth | ||
| Gender b | ||
| Female | 49 (47%) | 78 (58%) |
| Male | 55 (53%) | 57 (42%) |
| Mean head circumference (cm) ±SD | 33.9 ± 1.5 | 33.9 ± 1.6 |
| Mean child length (cm) ±SD | 49.8 ± 3.2 | 50.1 ± 2.6 |
| Mean birth weight (g) ±SD | 3087 ± 464.8 | 3102 ± 488.8 |
| Birth defects c | 2 (2%) | 2 (1%) |
| Microcephaly | 1 (1%) | --- |
| Household characteristics | ||
| Brick wall | 79 (78%) | 114 (85%) |
| Municipal piped water supply | 76 (75%) | 114 (85%) |
| Indoor toilet | 62 (61%) | 88 (66%) |
| Cement or ceramic floor | 62 (61%) | 82 (61%) |
| Electricity | 97 (95%) | 133 (99%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zepeda, O.; Espinoza, D.O.; Martinez, E.; Cross, K.A.; Becker-Dreps, S.; de Silva, A.M.; Bowman, N.M.; Premkumar, L.; Stringer, E.M.; Bucardo, F.; et al. Antibody Immunity to Zika Virus among Young Children in a Flavivirus-Endemic Area in Nicaragua. Viruses 2023, 15, 796. https://doi.org/10.3390/v15030796
Zepeda O, Espinoza DO, Martinez E, Cross KA, Becker-Dreps S, de Silva AM, Bowman NM, Premkumar L, Stringer EM, Bucardo F, et al. Antibody Immunity to Zika Virus among Young Children in a Flavivirus-Endemic Area in Nicaragua. Viruses. 2023; 15(3):796. https://doi.org/10.3390/v15030796
Chicago/Turabian StyleZepeda, Omar, Daniel O. Espinoza, Evelin Martinez, Kaitlyn A. Cross, Sylvia Becker-Dreps, Aravinda M. de Silva, Natalie M. Bowman, Lakshmanane Premkumar, Elizabeth M. Stringer, Filemón Bucardo, and et al. 2023. "Antibody Immunity to Zika Virus among Young Children in a Flavivirus-Endemic Area in Nicaragua" Viruses 15, no. 3: 796. https://doi.org/10.3390/v15030796
APA StyleZepeda, O., Espinoza, D. O., Martinez, E., Cross, K. A., Becker-Dreps, S., de Silva, A. M., Bowman, N. M., Premkumar, L., Stringer, E. M., Bucardo, F., & Collins, M. H. (2023). Antibody Immunity to Zika Virus among Young Children in a Flavivirus-Endemic Area in Nicaragua. Viruses, 15(3), 796. https://doi.org/10.3390/v15030796







