Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation
Abstract
:1. Introduction
2. Post-Translational Modifications
2.1. Effects of Post-Translational Modifications on Protein Function
2.2. Effects of Post-Translational Modifications on Viruses
2.3. Identification of Post-Translational Modifications
3. N- and O-Glycosylation
3.1. N- and O-Glycosylation on Proteins
3.2. N- and O-Glycosylation on Viruses
3.3. Glycosylation on Noroviruses
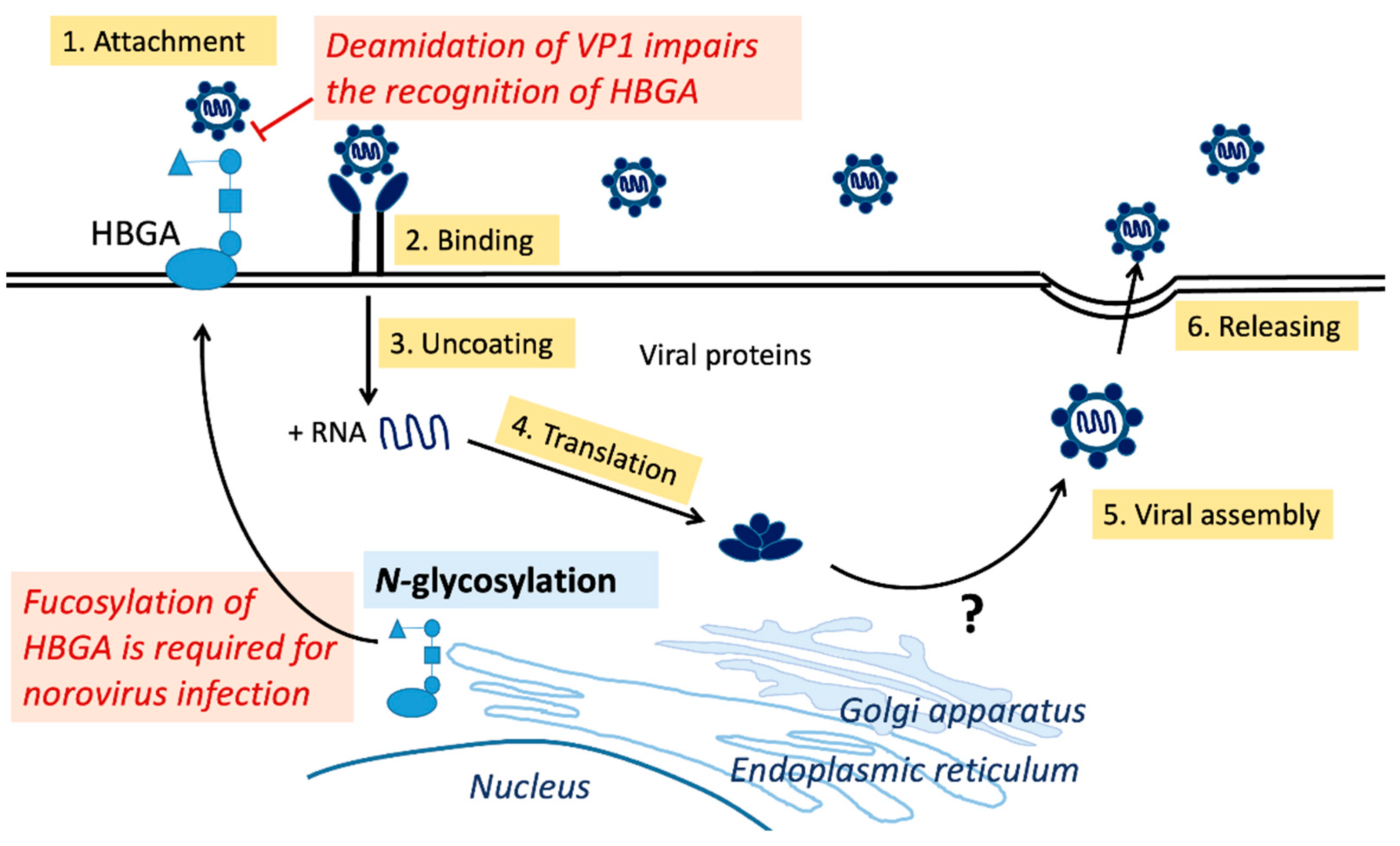
4. O-GlcNAcylation
4.1. O-GlcNAcylation on Proteins
4.2. O-GlcNAcylation on Viruses
4.3. O-GlcNAcylation on Noroviruses
5. Phosphorylation
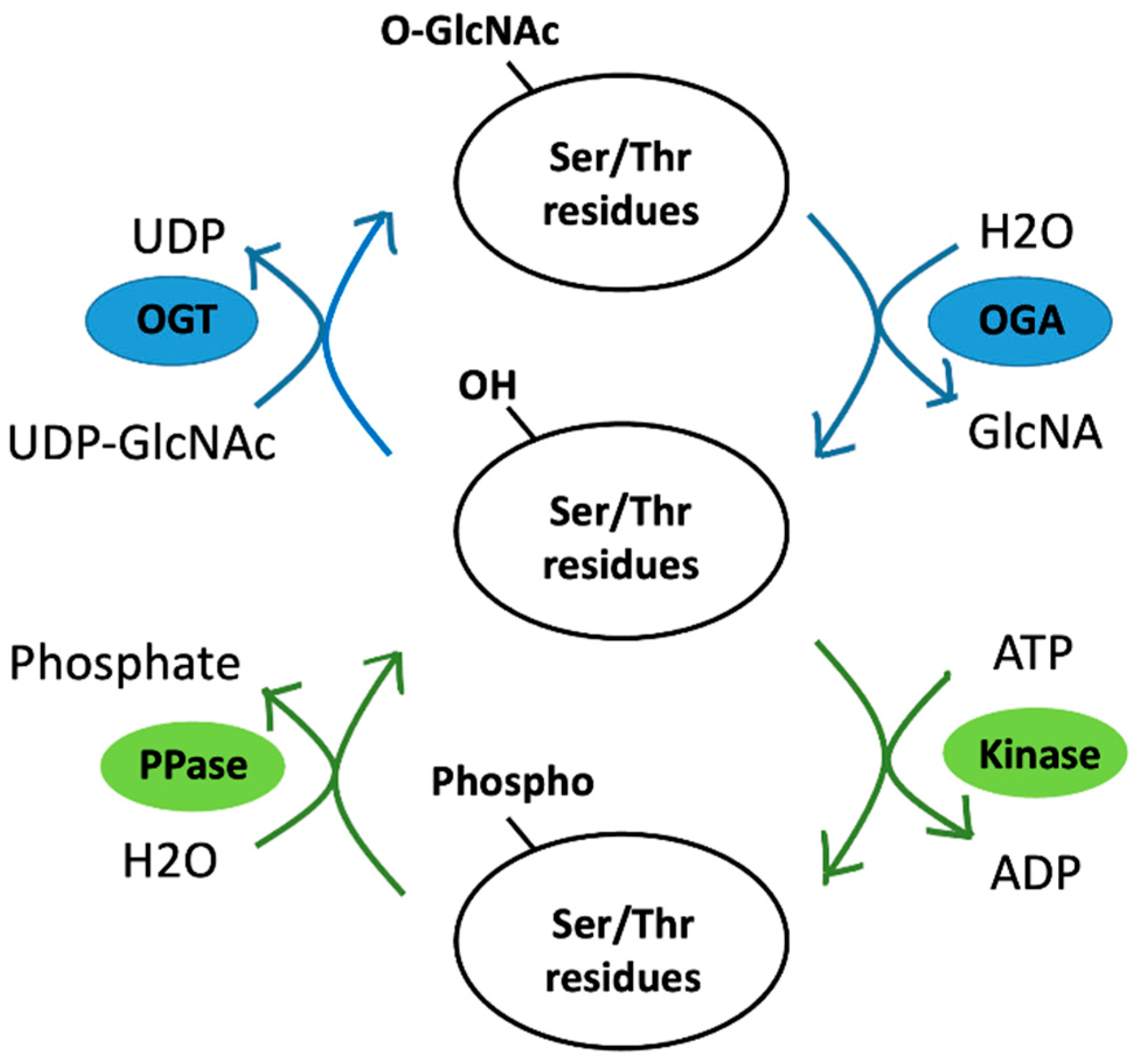
5.1. Phosphorylation vs. O-GlcNAcylation
5.2. Phosphorylation on Viruses
5.3. Phosphorylation on Noroviruses
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DENV | Dengue virus |
| DPAGT1 | Dolichol phosphate-dependent N-acetylglucosamine 1-phospho-transferase |
| ER | Endoplasmic reticulum |
| Endo H | Endoglycosidase H |
| Endo HF | Endo-β-N-acetylglucosaminidase H |
| FCV | Feline calicivirus |
| FUT2 | Fucosyltransferase 2 |
| GalNAc | N-acetylgalactosamine |
| GC-MS | Gas chromatography-mass spectrometry |
| HBGA | Histo-blood group antigen |
| HCV | Hepatitis C virus |
| HILIC | Hydrophilic interaction liquid chromatography |
| HIV-1 | Human immunodeficiency virus-1 |
| HuNoV | Human norovirus |
| LC-MS | Liquid chromatography-mass spectrometry |
| MBL | Mannose-binding lectin |
| MNV | Murine norovirus |
| MS | Mass spectrometry |
| MSE | Data-independent collection mode mass spectrometry |
| NS | Nonstructural protein |
| O-GlcNAc | O-linked N-acetylglucosamine |
| OGA | O-GlcNAcase |
| OGT | O-GlcNAc transferase |
| ORF | Open reading frame |
| OST | Oligosaccharyltransferase |
| PLGS | Protein Lynx Global Server |
| PNGase F | Peptide-N-glycosidase F |
| PTM | Post-translational modification |
| RdRp | RNA-dependent RNA polymerase |
| RNA | Ribonucleic acid |
| SARS-CoV | Severe acute respiratory syndrome-associated coronavirus |
| UDP-GlcNAc | Uridine diphosphate N-acetylglucosamine |
| UPLC | Ultraperformance liquid chromatography |
| WGA | Wheat germ agglutinin |
| ZIKV | Zika virus |
References
- Lucero, Y.; Matson, D.O.; Ashkenazi, S.; George, S.; O’Ryan, M. Norovirus: Facts and Reflections from Past, Present, and Future. Viruses 2021, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Pires, S.M.; Fischer-Walker, C.L.; Lanata, C.F.; Devleesschauwer, B.; Hall, A.J.; Kirk, M.D.; Duarte, A.S.; Black, R.E.; Angulo, F.J. Aetiology-Specific Estimates of the Global and Regional Incidence and Mortality of Diarrhoeal Diseases Commonly Transmitted through Food. PLoS ONE 2015, 10, e0142927. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Prasad, B.V.; Atmar, R.L. Noroviruses everywhere: Has something changed? Curr. Opin. Infect. Dis. 2006, 19, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.I.; Parashar, U.D.; Estes, M.K. Norovirus gastroenteritis. N. Engl. J. Med. 2009, 361, 1776–1785. [Google Scholar] [CrossRef] [Green Version]
- Netzler, N.E.; Enosi Tuipulotu, D.; White, P.A. Norovirus antivirals: Where are we now? Med. Res. Rev. 2019, 39, 860–886. [Google Scholar] [CrossRef] [PubMed]
- Thorne, L.G.; Goodfellow, I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014, 95 Pt 2, 278–291. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Cao, S.; Lou, Z.; Tan, M.; Chen, Y.; Liu, Y.; Zhang, Z.; Zhang, X.C.; Jiang, X.; Li, X.; Rao, Z. Structural basis for the recognition of blood group trisaccharides by norovirus. J. Virol. 2007, 81, 5949–5957. [Google Scholar] [CrossRef] [Green Version]
- Hutson, A.M.; Atmar, R.L.; Marcus, D.M.; Estes, M.K. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J. Virol. 2003, 77, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Mallagaray, A.; Creutznacher, R.; Dülfer, J.; Mayer, P.H.O.; Grimm, L.L.; Orduña, J.M.; Trabjerg, E.; Stehle, T.; Rand, K.D.; Blaum, B.S.; et al. A post-translational modification of human Norovirus capsid protein attenuates glycan binding. Nat. Commun. 2019, 10, 1320. [Google Scholar] [CrossRef] [Green Version]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Bailey, D.; Putics, A.; Goodfellow, I. Model systems for the study of human norovirus Biology. Future Virol. 2009, 4, 353–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef] [PubMed]
- Olspert, A.; Hosmillo, M.; Chaudhry, Y.; Peil, L.; Truve, E.; Goodfellow, I. Protein-RNA linkage and posttranslational modifications of feline calicivirus and murine norovirus VPg proteins. PeerJ 2016, 4, e2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eden, J.S.; Sharpe, L.J.; White, P.A.; Brown, A.J. Norovirus RNA-dependent RNA polymerase is phosphorylated by an important survival kinase, Akt. J. Virol. 2011, 85, 10894–10898. [Google Scholar] [CrossRef] [Green Version]
- Ettayebi, K.; Tenge, V.R.; Cortes-Penfield, N.W.; Crawford, S.E.; Neill, F.H.; Zeng, X.L.; Yu, X.; Ayyar, B.V.; Burrin, D.; Ramani, S.; et al. New Insights and Enhanced Human Norovirus Cultivation in Human Intestinal Enteroids. mSphere 2021, 6, e01136-20. [Google Scholar] [CrossRef]
- Lin, S.C.; Qu, L.; Ettayebi, K.; Crawford, S.E.; Blutt, S.E.; Robertson, M.J.; Zeng, X.L.; Tenge, V.R.; Ayyar, B.V.; Karandikar, U.C.; et al. Human norovirus exhibits strain-specific sensitivity to host interferon pathways in human intestinal enteroids. Proc. Natl. Acad. Sci. USA 2020, 117, 23782–23793. [Google Scholar] [CrossRef]
- Van Dycke, J.; Ny, A.; Conceicao-Neto, N.; Maes, J.; Hosmillo, M.; Cuvry, A.; Goodfellow, I.; Nogueira, T.C.; Verbeken, E.; Matthijnssens, J.; et al. A robust human norovirus replication model in zebrafish larvae. PLoS Pathog. 2019, 15, e1008009. [Google Scholar] [CrossRef] [Green Version]
- Mirabelli, C.; Santos-Ferreira, N.; Gillilland, M.G., 3rd; Cieza, R.J.; Colacino, J.A.; Sexton, J.Z.; Neyts, J.; Taube, S.; Rocha-Pereira, J.; Wobus, C.E. Human Norovirus Efficiently Replicates in Differentiated 3D-Human Intestinal Enteroids. J. Virol. 2022, 96, e0085522. [Google Scholar] [CrossRef]
- Haga, K.; Ettayebi, K.; Tenge, V.R.; Karandikar, U.C.; Lewis, M.A.; Lin, S.C.; Neill, F.H.; Ayyar, B.V.; Zeng, X.L.; Larson, G.; et al. Genetic Manipulation of Human Intestinal Enteroids Demonstrates the Necessity of a Functional Fucosyltransferase 2 Gene for Secretor-Dependent Human Norovirus Infection. mBio 2020, 11, e00251-20. [Google Scholar] [CrossRef] [Green Version]
- Jakubiec, A.; Jupin, I. Regulation of positive-strand RNA virus replication: The emerging role of phosphorylation. Virus Res. 2007, 129, 73–79. [Google Scholar] [CrossRef]
- Mann, M.; Jensen, O.N. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. Engl. 2005, 44, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.; Lin, S.; Deng, W.; Peng, D.; Cui, Q.; Xue, Y. PTMD: A Database of Human Disease-associated Post-translational Modifications. Genom. Proteom. Bioinform. 2018, 16, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ryšlavá, H.; Doubnerová, V.; Kavan, D.; Vaněk, O. Effect of posttranslational modifications on enzyme function and assembly. J. Proteom. 2013, 92, 80–109. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Luan, X.; Melamed, J.; Brockhausen, I. Role of Glycans on Key Cell Surface Receptors That Regulate Cell Proliferation and Cell Death. Cells 2021, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Chang, C.F.; Lu, P.L.; Chu, C.S.; Lai, W.T.; Shin, S.J.; Liu, F.T.; Chen, C.H. Increased APOE glycosylation plays a key role in the atherogenicity of L5 low-density lipoprotein. FASEB J. 2020, 34, 9802–9813. [Google Scholar] [CrossRef]
- Leutert, M.; Entwisle, S.W.; Villen, J. Decoding Post-Translational Modification Crosstalk With Proteomics. Mol. Cell. Proteom. 2021, 20, 100129. [Google Scholar] [CrossRef]
- Kumar, R.; Mehta, D.; Mishra, N.; Nayak, D.; Sunil, S. Role of Host-Mediated Post-Translational Modifications (PTMs) in RNA Virus Pathogenesis. Int. J. Mol. Sci. 2020, 22, 323. [Google Scholar] [CrossRef]
- Ikram, A.; Rauff, B.; Alzahrani, B.; Awan, F.M.; Obaid, A.; Naz, A.; Kakar, S.J.; Janjua, H.A. Integrated analysis to study the interplay between post-translational modifications (PTM) in hepatitis C virus proteins and hepatocellular carcinoma (HCC) development. Sci. Rep. 2022, 12, 15648. [Google Scholar] [CrossRef] [PubMed]
- Reynard, O.; Borowiak, M.; Volchkova, V.A.; Delpeut, S.; Mateo, M.; Volchkov, V.E. Ebolavirus glycoprotein GP masks both its own epitopes and the presence of cellular surface proteins. J. Virol. 2009, 83, 9596–9601. [Google Scholar] [CrossRef] [Green Version]
- Binley, J.M.; Ban, Y.E.; Crooks, E.T.; Eggink, D.; Osawa, K.; Schief, W.R.; Sanders, R.W. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J. Virol. 2010, 84, 5637–5655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, G.; Harvey, D.J.; Feldmann, F.; Stroeher, U.; Feldmann, H.; Royle, L.; Dwek, R.A.; Rudd, P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology 2010, 399, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloor, A.; Aradhya, R.; Venugopal, P.; Gopalakrishnan Nair, B.; Suravajhala, R. Glycosylation in SARS-CoV-2 variants: A path to infection and recovery. Biochem. Pharmacol. 2022, 206, 115335. [Google Scholar] [CrossRef] [PubMed]
- Flamand, M.; Megret, F.; Mathieu, M.; Lepault, J.; Rey, F.A.; Deubel, V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 1999, 73, 6104–6110. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Liang, L.; Wang, S.; Nakao, T.; Li, Y.; Liu, L.; Guan, Y.; Fukuyama, S.; Bu, Z.; Kawaoka, Y.; et al. Glycosylation of the Hemagglutinin Protein of H5N1 Influenza Virus Increases Its Virulence in Mice by Exacerbating the Host Immune Response. J. Virol. 2017, 91, e02215-16. [Google Scholar] [CrossRef] [Green Version]
- Nurdin, J.A.; Kotaki, T.; Kawagishi, T.; Sato, S.; Yamasaki, M.; Nouda, R.; Minami, S.; Kanai, Y.; Kobayashi, T. N-Glycosylation of Rotavirus NSP4 Protein Affects Viral Replication and Pathogenesis. J. Virol. 2023, 97, e01861-22. [Google Scholar] [CrossRef]
- Carbaugh, D.L.; Baric, R.S.; Lazear, H.M. Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis. J. Virol. 2019, 93, e00113-19. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Berndsen, Z.T.; Raghwani, J.; Seabright, G.E.; Allen, J.D.; Pybus, O.G.; McLellan, J.S.; Wilson, I.A.; Bowden, T.A.; Ward, A.B.; et al. Vulnerabilities in coronavirus glycan shields despite extensive glycosylation. Nat. Commun. 2020, 11, 2688. [Google Scholar] [CrossRef]
- Stewart-Jones, G.B.; Soto, C.; Lemmin, T.; Chuang, G.Y.; Druz, A.; Kong, R.; Thomas, P.V.; Wagh, K.; Zhou, T.; Behrens, A.J.; et al. Trimeric HIV-1-Env Structures Define Glycan Shields from Clades A, B, and G. Cell 2016, 165, 813–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommerstein, R.; Flatz, L.; Remy, M.M.; Malinge, P.; Magistrelli, G.; Fischer, N.; Sahin, M.; Bergthaler, A.; Igonet, S.; Ter Meulen, J.; et al. Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection. PLoS Pathog. 2015, 11, e1005276. [Google Scholar] [CrossRef] [PubMed]
- Casas-Sanchez, A.; Romero-Ramirez, A.; Hargreaves, E.; Ellis, C.C.; Grajeda, B.I.; Estevao, I.L.; Patterson, E.I.; Hughes, G.L.; Almeida, I.C.; Zech, T.; et al. Inhibition of Protein N-Glycosylation Blocks SARS-CoV-2 Infection. mBio 2022, 13, e0371821. [Google Scholar] [CrossRef] [PubMed]
- Grant, O.C.; Montgomery, D.; Ito, K.; Woods, R.J. Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition. Sci. Rep. 2020, 10, 14991. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Bowden, T.A.; Wilson, I.A.; Crispin, M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1480–1497. [Google Scholar] [CrossRef]
- Hanisch, F.G. Recombinant norovirus capsid protein VP1 (GII.4) expressed in H5 insect cells exhibits post-translational modifications with potential impact on lectin activity and vaccine design. Glycobiology 2022, 32, 496–505. [Google Scholar] [CrossRef]
- González, S.A.; Burrone, O.R. Rotavirus NS26 is modified by addition of single O-linked residues of N-acetylglucosamine. Virology 1991, 182, 8–16. [Google Scholar] [CrossRef]
- Whitford, M.; Faulkner, P. A structural polypeptide of the baculovirus Autographa californica nuclear polyhedrosis virus contains O-linked N-acetylglucosamine. J. Virol. 1992, 66, 3324–3329. [Google Scholar] [CrossRef] [Green Version]
- Caillet-Boudin, M.L.; Strecker, G.; Michalski, J.C. O-linked GlcNAc in serotype-2 adenovirus fibre. Eur. J. Biochem. 1989, 184, 205–211. [Google Scholar] [CrossRef]
- Greis, K.D.; Gibson, W.; Hart, G.W. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J. Virol. 1994, 68, 8339–8349. [Google Scholar] [CrossRef] [Green Version]
- Takamatsu, Y.; Yoshikawa, T.; Kurosu, T.; Fukushi, S.; Nagata, N.; Shimojima, M.; Ebihara, H.; Saijo, M.; Noda, T. Role of VP30 Phosphorylation in Ebola Virus Nucleocapsid Assembly and Transport. J. Virol. 2022, 96, e0108322. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Tsai, C.N.; Lee, K.Y.; Pan, T.C.; Lo, C.W.; Yu, M.J. Sequential S232/S235/S238 Phosphorylation of the Hepatitis C Virus Nonstructural Protein 5A. J. Virol. 2018, 92, e01295-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, J.X.; Park, E.; Schultz, K.L.W.; Griffin, D.E. NF-κB Activation Promotes Alphavirus Replication in Mature Neurons. J. Virol. 2019, 93, e01071-19. [Google Scholar] [CrossRef]
- Surjit, M.; Kumar, R.; Mishra, R.N.; Reddy, M.K.; Chow, V.T.; Lal, S.K. The severe acute respiratory syndrome coronavirus nucleocapsid protein is phosphorylated and localizes in the cytoplasm by 14-3-3-mediated translocation. J. Virol. 2005, 79, 11476–11486. [Google Scholar] [CrossRef] [Green Version]
- Mazzon, M.; Jones, M.; Davidson, A.; Chain, B.; Jacobs, M. Dengue virus NS5 inhibits interferon-alpha signaling by blocking signal transducer and activator of transcription 2 phosphorylation. J. Infect. Dis. 2009, 200, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, C.M.; Eisenhauer, P.; Manuelyan, I.; Weir, M.E.; Bruce, E.A.; Ballif, B.A.; Botten, J. Host-Driven Phosphorylation Appears to Regulate the Budding Activity of the Lassa Virus Matrix Protein. Pathogens 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Klemic, J.F.; Chang, S.; Bertone, P.; Casamayor, A.; Klemic, K.G.; Smith, D.; Gerstein, M.; Reed, M.A.; Snyder, M. Analysis of yeast protein kinases using protein chips. Nat. Genet. 2000, 26, 283–289. [Google Scholar] [CrossRef]
- Reimer, U.; Reineke, U.; Schneider-Mergener, J. Peptide arrays: From macro to micro. Curr. Opin. Biotechnol. 2002, 13, 315–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Jensen, O.N. Modification-specific proteomics: Strategies for characterization of post-translational modifications using enrichment techniques. Proteomics 2009, 9, 4632–4641. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.Y.; Lu, C.T.; Kao, H.J.; Chen, Y.J.; Chen, Y.J.; Lee, T.Y. Characterization and identification of protein O-GlcNAcylation sites with substrate specificity. BMC Bioinform. 2014, 15 (Suppl. 16), S1. [Google Scholar] [CrossRef] [Green Version]
- Hargett, A.A.; Renfrow, M.B. Glycosylation of viral surface proteins probed by mass spectrometry. Curr. Opin. Virol. 2019, 36, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Cheng, N.; Trejo, J. Post-Translational Modifications of G Protein-Coupled Receptors Control Cellular Signaling Dynamics in Space and Time. Pharmacol. Rev. 2021, 73, 120–151. [Google Scholar] [CrossRef] [PubMed]
- Piacente, F.; Gaglianone, M.; Laugieri, M.E.; Tonetti, M.G. The Autonomous Glycosylation of Large DNA Viruses. Int. J. Mol. Sci. 2015, 16, 29315–29328. [Google Scholar] [CrossRef] [Green Version]
- Varelas, X.; Bouchie, M.P.; Kukuruzinska, M.A. Protein N-glycosylation in oral cancer: Dysregulated cellular networks among DPAGT1, E-cadherin adhesion and canonical Wnt signaling. Glycobiology 2014, 24, 579–591. [Google Scholar] [CrossRef] [Green Version]
- Klement, E.; Lipinszki, Z.; Kupihar, Z.; Udvardy, A.; Medzihradszky, K.F. Enrichment of O-GlcNAc modified proteins by the periodate oxidation-hydrazide resin capture approach. J. Proteome Res. 2010, 9, 2200–2206. [Google Scholar] [CrossRef] [Green Version]
- Ohyama, Y.; Nakajima, K.; Renfrow, M.B.; Novak, J.; Takahashi, K. Mass spectrometry for the identification and analysis of highly complex glycosylation of therapeutic or pathogenic proteins. Expert. Rev. Proteom. 2020, 17, 275–296. [Google Scholar] [CrossRef]
- Zhao, S.; Walsh, I.; Abrahams, J.L.; Royle, L.; Nguyen-Khuong, T.; Spencer, D.; Fernandes, D.L.; Packer, N.H.; Rudd, P.M.; Campbell, M.P. GlycoStore: A database of retention properties for glycan analysis. Bioinformatics 2018, 34, 3231–3232. [Google Scholar] [CrossRef] [Green Version]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Lu, J.; Marathe, G.K.; Chu, C.S.; Chan, H.C.; Wang, C.Y.; Tung, Y.C.; McIntyre, T.M.; et al. Enhanced Sphingomyelinase Activity Contributes to the Apoptotic Capacity of Electronegative Low-Density Lipoprotein. J. Med. Chem. 2016, 59, 1032–1040. [Google Scholar] [CrossRef]
- Eggink, D.; Melchers, M.; Wuhrer, M.; van Montfort, T.; Dey, A.K.; Naaijkens, B.A.; David, K.B.; Le Douce, V.; Deelder, A.M.; Kang, K.; et al. Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology 2010, 401, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Tate, M.D.; Job, E.R.; Deng, Y.M.; Gunalan, V.; Maurer-Stroh, S.; Reading, P.C. Playing hide and seek: How glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses 2014, 6, 1294–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiemmeca, S.; Tamdet, C.; Punyadee, N.; Prommool, T.; Songjaeng, A.; Noisakran, S.; Puttikhunt, C.; Atkinson, J.P.; Diamond, M.S.; Ponlawat, A.; et al. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization. J. Immunol. 2016, 197, 4053–4065. [Google Scholar] [CrossRef] [Green Version]
- Ruvoën-Clouet, N.; Mas, E.; Marionneau, S.; Guillon, P.; Lombardo, D.; Le Pendu, J. Bile-salt-stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem. J. 2006, 393 Pt 3, 627–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakakhel, S.; Khan, H.; Nigar, K.; Khan, A. Genomic stratification and differential natural selection signatures among human norovirus genogroup II isolates. Arch. Virol. 2022, 167, 1235–1245. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Matsushima, Y.; Motoya, T.; Mizukoshi, F.; Ueki, Y.; Sakon, N.; Murakami, K.; Shimizu, T.; Okabe, N.; Nagata, N.; et al. Genetic Analysis of Human Norovirus Strains in Japan in 2016-2017. Front. Microbiol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niendorf, S.; Jacobsen, S.; Faber, M.; Eis-Hubinger, A.M.; Hofmann, J.; Zimmermann, O.; Hohne, M.; Bock, C.T. Steep rise in norovirus cases and emergence of a new recombinant strain GII.P16-GII.2, Germany, winter 2016. Euro Surveill. 2017, 22, 30447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Fang, L.; Sun, L.; Zeng, H.; Li, Y.; Zheng, H.; Wu, S.; Yang, F.; Song, T.; Lin, J.; et al. Association of GII.P16-GII.2 Recombinant Norovirus Strain with Increased Norovirus Outbreaks, Guangdong, China, 2016. Emerg. Infect. Dis. 2017, 23, 1188–1190. [Google Scholar] [CrossRef] [Green Version]
- Kokkinidis, M.; Glykos, N.M.; Fadouloglou, V.E. Catalytic activity regulation through post-translational modification: The expanding universe of protein diversity. Adv. Protein Chem. Struct. Biol. 2020, 122, 97–125. [Google Scholar]
- Singh, B.K.; Leuthold, M.M.; Hansman, G.S. Human noroviruses’ fondness for histo-blood group antigens. J. Virol. 2015, 89, 2024–2040. [Google Scholar] [CrossRef] [Green Version]
- Graziano, V.R.; Wei, J.; Wilen, C.B. Norovirus Attachment and Entry. Viruses 2019, 11, 495. [Google Scholar] [CrossRef] [Green Version]
- Hassan, E.; Baldridge, M.T. Norovirus encounters in the gut: Multifaceted interactions and disease outcomes. Mucosal Immunol. 2019, 12, 1259–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell. Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Laarse, S.A.M.; Leney, A.C.; Heck, A.J.R. Crosstalk between phosphorylation and O-GlcNAcylation: Friend or foe. FEBS J. 2018, 285, 3152–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, H.; Yi, W. O-GlcNAcylation, a sweet link to the pathology of diseases. J. Zhejiang Univ. Sci. B 2019, 20, 437–448. [Google Scholar] [CrossRef]
- Hart, G.W.; Slawson, C.; Ramirez-Correa, G.; Lagerlof, O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011, 80, 825–858. [Google Scholar] [CrossRef] [Green Version]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. Embo J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Vercoutter-Edouart, A.S.; El Yazidi-Belkoura, I.; Guinez, C.; Baldini, S.; Leturcq, M.; Mortuaire, M.; Mir, A.M.; Steenackers, A.; Dehennaut, V.; Pierce, A.; et al. Detection and identification of O-GlcNAcylated proteins by proteomic approaches. Proteomics 2015, 15, 1039–1050. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.; Ye, M.; Zou, H. Enrichment and separation techniques for large-scale proteomics analysis of the protein post-translational modifications. J. Chromatogr. A 2014, 1372c, 1–17. [Google Scholar] [CrossRef]
- Butkinaree, C.; Park, K.; Hart, G.W. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 2010, 1800, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Liu, Y.; Yao, H.; Zhao, Y. NMR-based investigation into protein phosphorylation. Int. J. Biol. Macromol. 2020, 145, 53–63. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, S.J.; Kim, E.J.; Kim, T.E.; Moon, J.S.; Kim, G.W.; Lee, S.H.; Cho, K.; Yoo, J.S.; Son, W.S.; et al. Phosphorylation of hepatitis C virus RNA polymerases ser29 and ser42 by protein kinase C-related kinase 2 regulates viral RNA replication. J. Virol. 2014, 88, 11240–11252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, N.M.; Bertozzi, C.R.; Pitteri, S.J. A Pragmatic Guide to Enrichment Strategies for Mass Spectrometry-Based Glycoproteomics. Mol. Cell. Proteom. 2021, 20, 100029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Muir, T.W.; Kent, S.B.; Tischer, E.; Scardina, J.M.; Chait, B.T. Mapping protein-protein interactions by affinity-directed mass spectrometry. Proc. Natl. Acad. Sci. USA 1996, 93, 4020–4024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlin, D.T.; Chait, B.T. Analysis of phosphorylated proteins and peptides by mass spectrometry. Curr. Opin. Chem. Biol. 2001, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Thingholm, T.E.; Jorgensen, T.J.; Jensen, O.N.; Larsen, M.R. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat. Protoc. 2006, 1, 1929–1935. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, J.H.; Kim, Y.G.; Lim, H.S.; Oh, J.W. Protein kinase C-related kinase 2 regulates hepatitis C virus RNA polymerase function by phosphorylation. J. Biol. Chem. 2004, 279, 50031–50041. [Google Scholar] [CrossRef] [Green Version]
- Ahlquist, P.; Noueiry, A.O.; Lee, W.M.; Kushner, D.B.; Dye, B.T. Host factors in positive-strand RNA virus genome replication. J. Virol. 2003, 77, 8181–8186. [Google Scholar] [CrossRef] [Green Version]
- Jakubiec, A.; Tournier, V.; Drugeon, G.; Pflieger, S.; Camborde, L.; Vinh, J.; Héricourt, F.; Redeker, V.; Jupin, I. Phosphorylation of viral RNA-dependent RNA polymerase and its role in replication of a plus-strand RNA virus. J. Biol. Chem. 2006, 281, 21236–21249. [Google Scholar] [CrossRef] [Green Version]
- Goodfellow, I. The genome-linked protein VPg of vertebrate viruses—a multifaceted protein. Curr. Opin. Virol. 2011, 1, 355–362. [Google Scholar] [CrossRef] [Green Version]

| PTM | Effect | Virus | Method | Year | Reference |
|---|---|---|---|---|---|
| N- and O-glycosylation | Attachment and entry | N-glycosylation: Ebola, HIV-1; N- and O-glycosylation: SARS-CoV2 | PNGase F, Endo HF, HILIC-UPLC, MALDI TOF | 2009, 2010, 2010, 2022 | [32,33,34,35] |
| Viral replication and maturation | N-glycosylation: influenza, DENV, rotavirus; N- and O-glycosylation: SARS-CoV2 | PNGase F, Western blot, HILIC-UPLC, MALDI TOF | 2022, 1999 2017, 2023 | [35,36,37,38] | |
| Viral pathology | N-glycosylation: DENV, influenza, ZIKV | PNGase F, Western blot, lectins | 1999, 2017 2023 | [36,37,39] | |
| Immune evasion by glycan shielding | N-glycosylation: Ebola, influenza, coronavirus, HIV-1, arenavirus, SARS-CoV2 | PNGase F, Western blot, Synapt G2S, Orbitrap MS, HILIC-UPLC | 2009, 2017 2020, 2016 2015, 2022 2022 | [32,37,40,41,42,43,44] | |
| Release of new virus particles | N-glycosylation: influenza; N- and O-glycosylation: SARS-CoV2 | PNGase F, LC-MS | 2022 1999 | [35,45] | |
| O-GlcNAcylation | Attachment and entry into cells | Norovirus | Lectins, antibodies, GC-MS | 2022 | [46] |
| RNA polymerase II transcription factors | Rotavirus | Enzyme | 1991 | [47] | |
| Viral protein stability | Adenovirus, baculovirus | WGA, [14C] GlcN radiolabeled fiber | 1992 1989 | [48,49] | |
| Viral pathology | Human cytomegalovirus | Electrospray-MS | 1994 | [50] | |
| Phosphorylation | Viral replication and maturation | Alphavirus, Ebola, HCV | Antibodies, Western blot | 2022, 2018 2019 | [51,52,53] |
| Viral protein synthesis | Norovirus, FCV | Electrophoresis | 2016, 2011 | [15,16] | |
| Inhibition of immune pathways | DENV, ZIKV, yellow fever virus, SARS-CoV | Metabolic labeling immunoblot | 2005 2009 | [54,55] | |
| Release of new virus particles | Lassa virus | Gel electrophoresis LC-MS/MS | 2018 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-C.; Ke, G.-M.; Chu, P.-Y.; Ke, L.-Y. Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation. Viruses 2023, 15, 798. https://doi.org/10.3390/v15030798
Cheng C-C, Ke G-M, Chu P-Y, Ke L-Y. Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation. Viruses. 2023; 15(3):798. https://doi.org/10.3390/v15030798
Chicago/Turabian StyleCheng, Chia-Chi, Guan-Ming Ke, Pei-Yu Chu, and Liang-Yin Ke. 2023. "Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation" Viruses 15, no. 3: 798. https://doi.org/10.3390/v15030798
APA StyleCheng, C.-C., Ke, G.-M., Chu, P.-Y., & Ke, L.-Y. (2023). Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation. Viruses, 15(3), 798. https://doi.org/10.3390/v15030798







