High-Risk Regions of African Swine Fever Infection in Mozambique
Abstract
:1. Introduction
2. Materials and Methods
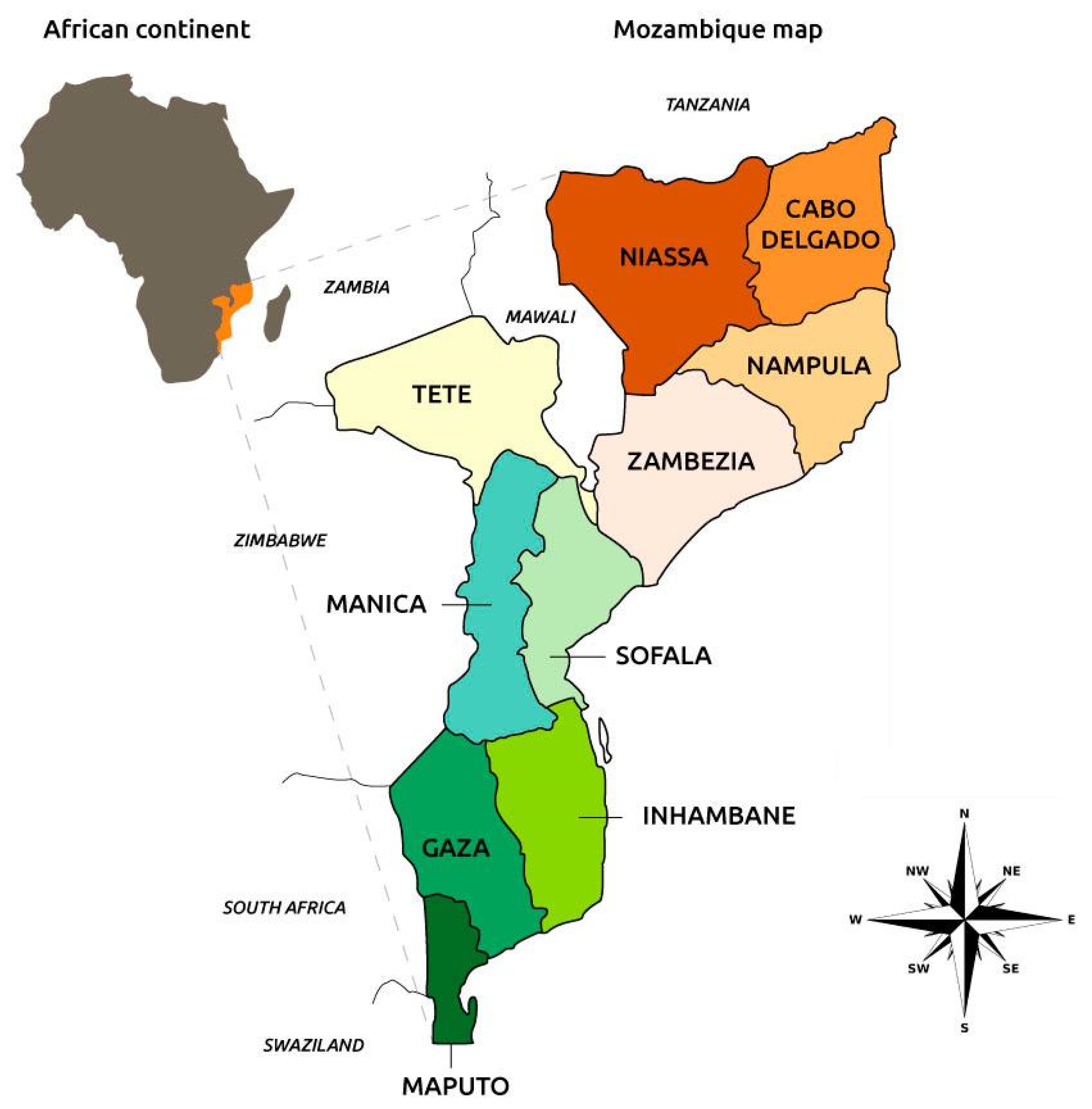
2.1. Study Area
2.2. Data Sources
2.3. Incidence Risk and Temporal Trend Analysis
2.4. Spatiotemporal Analysis
3. Results
3.1. Spatial Distribution of ASF
3.2. ASF Temporal Analysis
3.3. Spatiotemporal Analysis of ASF
3.4. Analysis of Temporal Trends
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W.; Jori, F.; Bastos, A.D. African swine fever virus eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef]
- Penrith, M.L.; Lopes Pereira, C.; Lopes da Silva, M.M.; Quembo, C.; Nhamusso, A.; Banze, J. African swine fever in Mozambique: Review, risk factors and considerations for control [Review]. Onderstepoort J. Vet. Res. 2007, 74, 149–160. [Google Scholar]
- Food and Agriculture Organization of the United Nations; World Organization for Animal Health; World Bank. Good Practices for Biosecurity in the Pig Sector—Issues and Options in Developing and Transition Countries; FAO Anim Production Health Paper; Food and Agriculture Organization: Rome, Italy, 2010; p. 169. Available online: https://www.fao.org/publications/card/en/c/4d7fd824-a334-5796-9249-4309a129170e/ (accessed on 6 October 2022).
- Denis, M. African swine fever: An epidemiological overview. Br. J. Virol. 2014, 1, 42–47. [Google Scholar]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; ICTV Report Consortium. ICTV virus taxonomy profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- Plowright, W.; Thomson, G.R.; Neser, J.A. African swine fever. In Infectious Diseases of Livestock, with Special Reference to Southern Africa; Coetzer, J.A.W., Thomson, G.R., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 1994; pp. 568–599. [Google Scholar]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef]
- Moennig, V. Introduction to classical swine fever: Virus, disease and control policy. Vet. Microbiol. 2000, 73, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Bastos, A.D.S. Role of wild suids in the epidemiology of African swine fever. EcoHealth 2009, 6, 296–310. [Google Scholar] [CrossRef]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.L. History of “swine fever” in southern Africa. J. S. Afr. Vet. Assoc. 2013, 84, 1–6. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antiviral Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estatística (INE). Censo Agro-Pecuário Cap 2009–2010: Resultados Definitivos—Moçambique. Available online: www.ine.gov.mz/operacoes-estatisticas/censos/censo-agro-pecuario/cap-2009-2010/censo-agro-pecuario-2009-2010.pdf/view (accessed on 8 July 2022).
- Valdeira, M.L. Effect of ribavirin on the African swine fever virus replication. RPCV 2001, 96, 183–189. [Google Scholar]
- De Boer, C.J. Studies to determine neutralizing antibodies in sera from animals recovered from African swine fever and laboratory animals inoculated with African virus with adjuvants. Arch. Ges. Virusforsch. 1967, 20, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Netherton, C.L.; McCrossan, M.C.; Denyer, M.; Ponnambalam, S.; Armstrong, J.; Takamatsu, H.H.; Wileman, T.E. African swine fever virus causes microtubule-dependent dispersal of the trans-Golgi network and slows delivery of membrane protein to the plasma membrane. J. Virol. 2006, 80, 11385–11392. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine, 10th ed.; Sanders Elsevier: Madrid, Spain, 2007; pp. 2176–2177. [Google Scholar]
- de la Cruz, M.L.; Perez, A.; Bezos, J.; Pages, E.; Casal, C.; Carpintero, J.; Romero, B.; Dominguez, L.; Barker, C.M.; Diaz, R.; et al. Spatial dynamics of bovine tuberculosis in the Autonomous Community of Madrid, Spain (2010–2012). PLoS ONE 2014, 9, e115632. [Google Scholar] [CrossRef]
- MAEFP—Ministério da Administracao Estatal e Função Pública. Portal do Governo de Moçambique. Geografia de Moçambique. 2020. Available online: https://www.portaldogoverno.gov.mz/por/Mocambique/Populacao (accessed on 18 March 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Kulldorff, M.; SaTScan™: Software for the Spatial, Temporal, and Space-Time Scan Statistics. SaTScan Version 9.6.1. 2018. Available online: https://www.satscan.org/techdoc.html (accessed on 12 April 2022).
- Kulldorff, M. SaTScan™ User Guide for Version 9.7. 2021. Available online: https://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download (accessed on 5 April 2022).
- Liu, M.Y.; Li, Q.H.; Zhang, Y.J.; Ma, Y.; Liu, Y.; Feng, W.; Hou, C.B.; Amsalu, E.; Li, X.; Wang, W.; et al. Spatial and temporal clustering analysis of tuberculosis in the mainland of China at the prefecture level, 2005–2015. Infect. Dis. Poverty 2018, 7, 106. [Google Scholar] [CrossRef]
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.L.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African swine fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef]
- OIE—World Organisation for Animal Health. African Swine Fever. Infection with African Swine Fever Virus. OIE Terrestrial Manual. 2021, pp. 1–18. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.09.01_ASF.pdf (accessed on 11 June 2022).
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrín, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound. Emerg. Dis. 2017, 64, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African swine fever virus (ASFV): Biology, genomics and genotypes circulating in sub-Saharan Africa. Viruses 2021, 13, 2285. [Google Scholar] [CrossRef]
- Schwarz, D.G.G.; de Sousa Júnior, P.F.; Saraiva da Silva, L.; Polveiro, R.C.; de Oliveira, J.F.; Faria, M.P.O.; Marinho, G.L.O.C.; de Oliveira, R.P.; Moreira, M.A.S. Spatiotemporal distribution and temporal trends of brucellosis and tuberculosis in water buffalo (Bubalus bubalis) in Brazil. Prev. Vet. Med. 2021, 193, 105417. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W. Review of African swine fever: Transmission, spread and control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; Sitoe, C.; Afonso, S.; Banze, J.; Baptista, J.; Dias, G.; Rodrigues, F.; Atanásio, A.; Nhamusso, A.; Penrith, M.L.; et al. A pilot study of common health problems in smallholder pigs in Angónia and Boane districts, Mozambique. J. S. Afr. Vet. Assoc. 2011, 82, 166–169. [Google Scholar] [CrossRef]
- Lubisi, B.A.; Dwarka, R.M.; Meenowa, D.; Jaumally, R. An investigation into the first outbreak of African swine fever in the Republic of Mauritius. Transbound. Emerg. Dis. 2009, 56, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African swine fever virus isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef]
- MAPA—Ministério da Agricultura, Pecuária e Abastecimento. In Controle da Raiva dos Herbívoros: Manual Técnico, 2nd ed.; Ministério da Agricultura, Pecuária e Abastecimento Secretaria de Defesa Agropecuária: Brasília, Brazil, 2009; 124p. Available online: https://www.gov.br/agricultura/pt-br/assuntos/sanidade-animal-e-vegetal/saude-animal/programas-de-saude-animal/raiva-dos-herbivoros-e-eeb/MANUAL_RAIVAHERBVOROS2009.pdf (accessed on 18 April 2022).
- Oliveira, F.A.S.; Castro, R.J.S.; de Oliveira, J.F.; Barreto, F.M.; Farias, M.P.O.; Marinho, G.L.O.C.; Soares, M.J.D.S.; Silva-Júnior, A.; Schwarz, D.G.G. Geographical and temporal spread of equine rabies in Brazil. Acta Trop. 2022, 227, 106302. [Google Scholar] [CrossRef]
- Chilundo, A.G.; Mukaratirwa, S.; Pondja, A.; Afonso, S.; Alfredo, Z.; Chato, E.; Johansen, M.V. Smallholder pig farming education improved community knowledge and pig management in Angónia district, Mozambique. Trop. Anim. Health Prod. 2020, 52, 1447–1457. [Google Scholar] [CrossRef]
- Cadenas-Fernández, E.; Ito, S.; Aguilar-Vega, C.; Sánchez-Vizcaíno, J.M.; Bosch, J. The role of the wild boar spreading African swine fever virus in Asia: Another underestimated problem. Front. Vet. Sci. 2022, 27, 844209. [Google Scholar] [CrossRef]




| Regions/Provinces | Cases | RF (%) | IR (per 100,000) |
|---|---|---|---|
| North | 18,584 | 64.9 | 7825.4 |
| Niassa | 20 | 0.1 | 101.3 |
| Cabo Delgado | 11,667 | 40.8 | 17,301.1 |
| Nampula | 6897 | 24.1 | 4588.7 |
| Central | 5085 | 17.8 | 786.4 |
| Zambézia | 154 | 0.5 | 62.9 |
| Tete | 1141 | 4.0 | 513 |
| Manica | 1996 | 7.0 | 2730.4 |
| Sofala | 1794 | 6.3 | 1691 |
| South | 4955 | 17.3 | 1066.9 |
| Inhambane | 122 | 0.4 | 45.4 |
| Gaza | 832 | 2.9 | 596.7 |
| Maputo Province | 3357 | 11.7 | 8868.6 |
| Maputo City | 644 | 2.2 | 3539 |
| Province | APC | Lower CI | Upper CI | p-Value | Trend |
|---|---|---|---|---|---|
| Niassa | −22.3 * | −28.3 | −15.8 | <0.001 | Decreasing |
| Cabo Delgado | −31.2 * | −43.7 | −16.1 | 0.001 | Decreasing |
| Nampula | −32.1 * | −37.4 | −26.4 | <0.001 | Decreasing |
| Zambézia | −9.5 * | −17.4 | −0.7 | 0.035 | Decreasing |
| Tete | −17.7 * | −28.9 | −4.6 | 0.012 | Decreasing |
| Manica | −23.2 * | −28.8 | −17.3 | <0.001 | Decreasing |
| Sofala | −1.2 | −9.9 | 8.3 | 0.780 | Stationary |
| Inhambane | 9.4 | −17.2 | 44.4 | 0.508 | Stationary |
| Gaza | −15.9 * | −26.8 | −3.3 | 0.018 | Decreasing |
| Maputo Province | 1.9 | −6.2 | 10.6 | 0.647 | Stationary |
| Maputo City | −22.3 * | −28.3 | −15.8 | <0.001 | Decreasing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mataca, A.R.; Oliveira, F.A.S.; Lampeão, Â.A.; Mendonça, J.P.; Moreira, M.A.S.; Mota, R.A.; Porto, W.J.N.; Schwarz, D.G.G.; Silva-Júnior, A. High-Risk Regions of African Swine Fever Infection in Mozambique. Viruses 2023, 15, 1010. https://doi.org/10.3390/v15041010
Mataca AR, Oliveira FAS, Lampeão ÂA, Mendonça JP, Moreira MAS, Mota RA, Porto WJN, Schwarz DGG, Silva-Júnior A. High-Risk Regions of African Swine Fever Infection in Mozambique. Viruses. 2023; 15(4):1010. https://doi.org/10.3390/v15041010
Chicago/Turabian StyleMataca, Azido Ribeiro, Francisco Alyson Silva Oliveira, Ângelo André Lampeão, José Pereira Mendonça, Maria Aparecida Scatamburlo Moreira, Rinaldo Aparecido Mota, Wagnner José Nascimento Porto, David Germano Gonçalves Schwarz, and Abelardo Silva-Júnior. 2023. "High-Risk Regions of African Swine Fever Infection in Mozambique" Viruses 15, no. 4: 1010. https://doi.org/10.3390/v15041010
APA StyleMataca, A. R., Oliveira, F. A. S., Lampeão, Â. A., Mendonça, J. P., Moreira, M. A. S., Mota, R. A., Porto, W. J. N., Schwarz, D. G. G., & Silva-Júnior, A. (2023). High-Risk Regions of African Swine Fever Infection in Mozambique. Viruses, 15(4), 1010. https://doi.org/10.3390/v15041010






