Current Clinical Landscape and Global Potential of Bacteriophage Therapy
Abstract
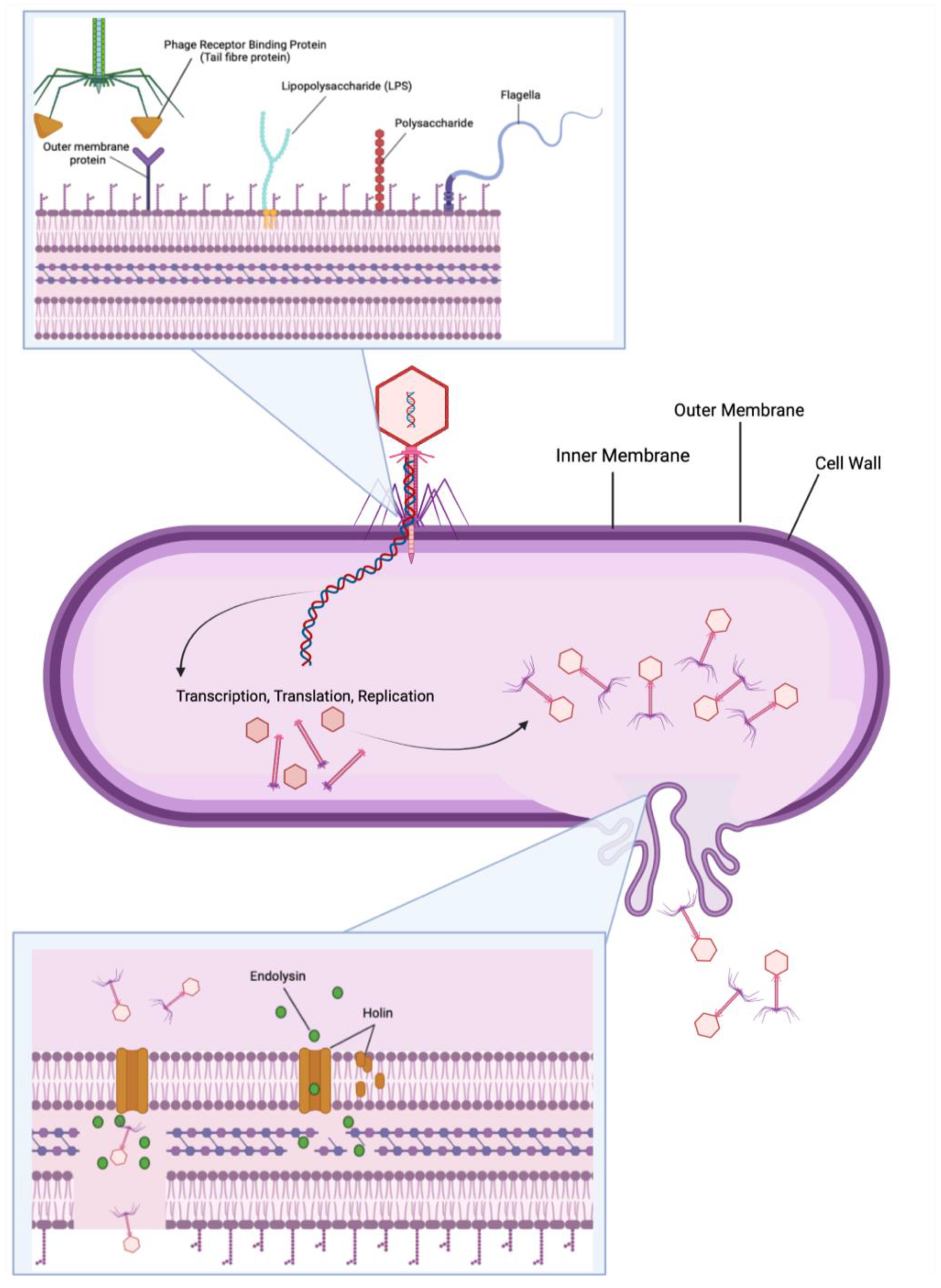
:1. Introduction
2. Bacterial Antimicrobial Resistance
2.1. Overview of AMR
2.2. Emergence and Progression of AMR
2.3. Epidemiology of AMR Infections
2.4. Economics of AMR
3. Antibiotics
4. Bacteriophages
4.1. Phage Overview
4.2. Classification, Taxonomy, and Genomic Diversity
4.3. Discovery and Isolation of Bacteriophages
5. Phage Therapy
5.1. Current Landscape
5.2. Phage Therapy Clinical Trials
5.2.1. Skin and Soft Tissue Infections (SSTI)
5.2.2. Lung Infections
5.2.3. Gastrointestinal (GI) Infections
5.2.4. Genitourinary (GU)/Urinary Tract Infection (UTI)
5.2.5. Prosthetic Joint Infection (PJI)
5.2.6. Bacteremia
5.2.7. Other Infections
5.3. Ethical and Regulatory Considerations
6. Potential and Limitations of Phage Therapy
6.1. Benefits and Potential
6.2. Limitations and Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riedel, S.; Hobden, J.A.; Miller, S.; Morse, S.A.; Mietzner, T.A.; Detrick, B.; Mitchell, T.G.; Sakanari, J.A.; Hotez, P.; Mejia, R. Microbial Genetics. In Jawetz, Melnick, & Adelberg’s Medical Microbiology; McGraw Hill: New York, NY, USA, 2019. [Google Scholar]
- Nair, A.; Ghugare, G.S.; Khairnar, K. An Appraisal of Bacteriophage Isolation Techniques from Environment. Microb. Ecol. 2022, 83, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, K.; Shen, Y.; Yuan, J.; Waheed, I.; Mao, C.; Zhou, X. Advances in the Development of Phage-Based Probes for Detection of Bio-Species. Biosensors 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- D’ Herelle, F. Sur un microbe invisible antagoniste des bacilles dysenterique. Comptes Rendus L’académie Sci. 1917, 165, 373–375. [Google Scholar]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- D’Herelle, F. Bacteriophage as a Treatment in Acute Medical and Surgical Infections. Bull. N. Y. Acad. Med. 1931, 7, 329. [Google Scholar]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef]
- Nagel, T.E.; Chan, B.K.; De Vos, D.; El-Shibiny, A.; Kang’ethe, E.K.; Makumi, A.; Pirnay, J.-P. The Developing World Urgently Needs Phages to Combat Pathogenic Bacteria. Front. Microbiol. 2016, 7, 882. [Google Scholar] [CrossRef]
- Frost, I.; Craig, J.; Joshi, J.; Faure, K.; Laxminarayan, R. Access Barriers to Antibiotics; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2019. [Google Scholar]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Medicin. Chem. 2014, 6, PMC-S14459. [Google Scholar] [CrossRef]
- Tommasi, R.; Brown, D.G.; Walkup, G.K.; Manchester, J.I.; Miller, A.A. ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 2015, 14, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 9 February 2023).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Surette, M.D.; Wright, G.D. Lessons from the Environmental Antibiotic Resistome. Annu. Rev. Microbiol. 2017, 71, 309–329. [Google Scholar] [CrossRef]
- Hicks, L.A.; Taylor, T.H.; Hunkler, R.J. U.S. Outpatient Antibiotic Prescribing, 2010. N. Engl. J. Med. 2013, 368, 1461–1462. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Spiegelman, G.B.; Yim, G. The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 2006, 9, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Makumi, A.; Mhone, A.L.; Odaba, J.; Guantai, L.; Svitek, N. Phages for Africa: The Potential Benefit and Challenges of Phage Therapy for the Livestock Sector in Sub-Saharan Africa. Antibiotics 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Reports 2020, 93, 231. [Google Scholar] [CrossRef] [PubMed]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 9 February 2023).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Lastinger, L.M.; Alvarez, C.R.; Kofman, A.; Konnor, R.Y.; Kuhar, D.T.; Nkwata, A.; Patel, P.R.; Pattabiraman, V.; Xu, S.Y.; Dudeck, M.A. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect. Control Hosp. Epidemiol. 2022, 43, 1–5. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report. 2022. Available online: https://www.cdc.gov/drugresistance/pdf/covid19-impact-report-508.pdf (accessed on 9 February 2023).
- Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; HM Government & Wellcome Trust: London, UK, 2014. [Google Scholar]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36. [Google Scholar] [CrossRef]
- Forrester, J.D.; Cao, S.; Schaps, D.; Liou, R.; Patil, A.; Stave, C.; Sokolow, S.H.; Leo, G. De Influence of Socioeconomic and Environmental Determinants of Health on Human Infection and Colonization with Antibiotic-Resistant and Antibiotic-Associated Pathogens: A Scoping Review. Surg. Infect. 2022, 23, 209–225. [Google Scholar] [CrossRef]
- Eur. Med. Agency (EMA). The Bacterial Challenge—Time to React a Call to Narrow the Gap between Multidrug-Resistant Bacteria in the EU and Development of New Antibacterial Agents. Available online: https://www.ema.europa.eu/en/news/bacterial-challenge-time-react-call-narrow-gap-between-multidrug-resistant-bacteria-eu-development (accessed on 12 April 2023).
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef] [PubMed]
- Calbo, E.; Boix-Palop, L.; Garau, J. Clinical and economic impact of bacterial resistance: An approach to infection control and antimicrobial stewardship solutions. Curr. Opin. Infect. Dis. 2020, 33, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Jit, M.; Ng, D.H.L.; Luangasanatip, N.; Sandmann, F.; Atkins, K.E.; Robotham, J.V.; Pouwels, K.B. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: Rapid methodological review, conceptual framework and recommendations for future studies. BMC Med. 2020, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Stålsby Lundborg, C.; Sun, X.; Gu, S.; Dong, H. Clinical and Economic Burden of Carbapenem-Resistant Infection or Colonization Caused by Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii: A Multicenter Study in China. Antibiotics 2020, 9, 514. [Google Scholar] [CrossRef]
- Tabak, Y.P.; Merchant, S.; Ye, G.; Vankeepuram, L.; Gupta, V.; Kurtz, S.G.; Puzniak, L.A. Incremental clinical and economic burden of suspected respiratory infections due to multi-drug-resistant Pseudomonas aeruginosa in the United States. J. Hosp. Infect. 2019, 103, 134–141. [Google Scholar] [CrossRef]
- Coates, A.R.; Halls, G.; Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 2011, 163, 184–194. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- World Health Organization. Antibacterial Agents in Clinical and Preclinical Development an Overview and Analysis. 2020. Available online: https://www.who.int/publications/i/item/9789240021303 (accessed on 9 February 2023).
- Marshall, M.S. Observations on D’Herelle’s Bacteriophage. J. Infect. Dis. 1925, 37, 126160. [Google Scholar] [CrossRef]
- Fruciano, D.E.; Bourne, S. Phage as an Antimicrobial Agent: D’herelle’s Heretical Theories and Their Role in the Decline of Phage Prophylaxis in the West. Can. J. Infect. Dis. Med. Microbiol. 2007, 18, 19–26. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Devoto, A.E.; Santini, J.M.; Olm, M.R.; Anantharaman, K.; Munk, P.; Tung, J.; Archie, E.A.; Turnbaugh, P.J.; Seed, K.D.; Blekhman, R.; et al. Megaphages infect Prevotella and variants are widespread in gut microbiomes. Nat. Microbiol. 2019, 4, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, E.; Bonnin, R.A.; Rocha, E.P.C. Phage-Plasmids Spread Antibiotic Resistance Genes through Infection and Lysogenic Conversion. MBio 2022, 13, e01851-22. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H. Phage Classification and Characterization. In Bacteriophages. Methods in Molecular BiologyTM; Clokie, M.R., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 501. [Google Scholar]
- Tolstoy, I.; Kropinski, A.M.; Brister, J.R. Bacteriophage Taxonomy: An Evolving Discipline. In Bacteriophage Therapy: From Lab to Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2018; pp. 57–71. [Google Scholar]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and their genomes. Curr. Opin. Virol. 2011, 1, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of morphology-based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef]
- Danis-Wlodarczyk, K.; Dąbrowska, K.; Abedon, S.T. Phage Therapy: The Pharmacology of Antibacterial Viruses. Curr. Issues Mol. Biol. 2021, 40, 81–164. [Google Scholar] [CrossRef]
- GenBank, Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore (accessed on 12 April 2023).
- SEA-PHAGES HHMI Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science Program. Available online: https://seaphages.org (accessed on 9 February 2023).
- Mavrich, T.N.; Hatfull, G.F. Bacteriophage evolution differs by host, lifestyle and genome. Nat. Microbiol. 2017, 2, 17112. [Google Scholar] [CrossRef]
- Dunstan, R.A.; Bamert, R.S.; Belousoff, M.J.; Short, F.L.; Barlow, C.K.; Pickard, D.J.; Wilksch, J.J.; Schittenhelm, R.B.; Strugnell, R.A.; Dougan, G.; et al. Mechanistic Insights into the Capsule-Targeting Depolymerase from a Klebsiella pneumoniae Bacteriophage. Microbiol. Spectr. 2021, 9, e01023-21. [Google Scholar] [CrossRef]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A. Basics of Antibody Phage Display Technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef]
- Boeckaerts, D.; Stock, M.; De Baets, B.; Briers, Y. Identification of Phage Receptor-Binding Protein Sequences with Hidden Markov Models and an Extreme Gradient Boosting Classifier. Viruses 2022, 14, 1329. [Google Scholar] [CrossRef] [PubMed]
- Boeckaerts, D.; Stock, M.; Criel, B.; Gerstmans, H.; De Baets, B.; Briers, Y. Predicting bacteriophage hosts based on sequences of annotated receptor-binding proteins. Sci. Rep. 2021, 11, 1467. [Google Scholar] [CrossRef] [PubMed]
- Tétart, F.; Repoila, F.; Monod, C.; Krisch, H.M. Bacteriophage T4 Host Range is Expanded by Duplications of a Small Domain of the Tail Fiber Adhesin. J. Mol. Biol. 1996, 258, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gingery, M.; Doulatov, S.R.; Liu, Y.; Hodes, A.; Baker, S.; Davis, P.; Simmonds, M.; Churcher, C.; Mungall, K.; et al. Genomic and Genetic Analysis of Bordetella Bacteriophages Encoding Reverse Transcriptase-Mediated Tropism-Switching Cassettes. J. Bacteriol. 2004, 186, 1503–1517. [Google Scholar] [CrossRef]
- de Jonge, P.A.; Nobrega, F.L.; Brouns, S.J.J.; Dutilh, B.E. Molecular and Evolutionary Determinants of Bacteriophage Host Range. Trends Microbiol. 2019, 27, 51–63. [Google Scholar] [CrossRef]
- Duffy, S.; Turner, P.E.; Burch, C.L. Pleiotropic Costs of Niche Expansion in the RNA Bacteriophage Φ6. Genetics 2006, 172, 751–757. [Google Scholar] [CrossRef]
- Poxleitner, M. Phage Discovery Guide. Available online: https://seaphagesphagediscoveryguide.helpdocsonline.com/home (accessed on 9 February 2023).
- Martins, W.M.B.S.; Toleman, M.A.; Gales, A.C. Clinical utilization of bacteriophages: A new perspective to combat the antimicrobial resistance in Brazil. Braz. J. Infect. Dis. 2020, 24, 239–246. [Google Scholar] [CrossRef]
- Semler, D.D.; Lynch, K.H.; Dennis, J.J. The Promise of Bacteriophage Therapy for Burkholderia cepacia Complex Respiratory Infections. Front. Cell. Infect. Microbiol. 2012, 1, 27. [Google Scholar] [CrossRef]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef]
- Newsroom Bacteriophage Therapy. Available online: https://health.ucsd.edu/news/topics/phage-therapy/pages/default.aspx (accessed on 9 February 2023).
- LaFee, S.; Buschman, H. Novel Phage Therapy Saves Patient with Multidrug-Resistant Bacterial Infection. UC San Diego Today. 2017. Available online: https://today.ucsd.edu/story/novel_phage_therapy_saves_patient_with_multidrug_resistant_bacterial_infect (accessed on 9 February 2023).
- U.S. Food and Drug Administration. National Institute of Allergy and Infectious Diseases Center for Biologics Evaluation and Research (CBER) Science and Regulation of Bacteriophage Therapy Workshop; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021.
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical Aspects of Phage Therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e02071-21. [Google Scholar] [CrossRef] [PubMed]
- Little, J.S.; Dedrick, R.M.; Freeman, K.G.; Cristinziano, M.; Smith, B.E.; Benson, C.A.; Jhaveri, T.A.; Baden, L.R.; Solomon, D.A.; Hatfull, G.F. Bacteriophage treatment of disseminated cutaneous Mycobacterium chelonae infection. Nat. Commun. 2022, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Chen, L.-K.; Zhu, T. Phage therapy for secondary bacterial infections with COVID-19. Curr. Opin. Virol. 2022, 52, 9–14. [Google Scholar] [CrossRef]
- Nicholls, P.; Aslam, S. Role of bacteriophage therapy for resistant infections in transplant recipients. Curr. Opin. Organ Transplant. 2022, 27, 546–553. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, S.; Ruth, M.M.; Mientjes, M.; de Sévaux, R.G.L.; van Ingen, J. A Dutch Case Report of Successful Treatment of Chronic Relapsing Urinary Tract Infection with Bacteriophages in a Renal Transplant Patient. Antimicrob. Agents Chemother. 2019, 64, e01281-19. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.; Fabijan, A.P.; Ho, J.; Lin, R.C.Y.; Ben Zakour, N.L.; Dugan, C.; Kliman, I.; Branston, S.; Morales, S.; Iredell, J.R. Bacteriophage Therapy of Ventilator-associated Pneumonia and Empyema Caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2019, 200, 1179–1181. [Google Scholar] [CrossRef]
- Abedon, S. Phage therapy dosing: The problem(s) with multiplicity of infection. Bacteriophage 2016, 6, e1220348. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J. Phage cocktail development for bacteriophage therapy: Toward improving spectrum of activity breadth and depth. Pharmaceuticals 2021, 14, 1019. [Google Scholar] [CrossRef]
- Gu Liu, C.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. MBio 2020, 11, e01462-20. [Google Scholar] [CrossRef]
- Torres-Barceló, C.; Arias-Sánchez, F.I.; Vasse, M.; Ramsayer, J.; Kaltz, O.; Hochberg, M.E. A Window of Opportunity to Control the Bacterial Pathogen Pseudomonas aeruginosa Combining Antibiotics and Phages. PLoS ONE 2014, 9, e106628. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E.; et al. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Mankovich, A.G.; Maciel, K.; Kavanaugh, M.; Kistler, E.; Muckle, E.; Weingart, C.L. Phage-antibiotic synergy reduces Burkholderia cenocepacia population. BMC Microbiol. 2023, 23, 2. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov (accessed on 28 February 2023).
- Phagelux Inc. Phage Therapy for the Prevention and Treatment of Pressure Ulcers. Available online: https://clinicaltrials.gov/ct2/show/NCT04815798 (accessed on 28 February 2023).
- Phagelux Inc. Phage Therapy for the Prevention and Treatment of Wound Infections in Burned Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04323475 (accessed on 28 February 2023).
- Maruho Co., Ltd. A Study of Topical BX005-A in Subjects with Moderate to Severe Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/NCT05240300 (accessed on 28 February 2023).
- Pherecydes Pharma. Standard Treatment Associated with Phage Therapy Versus Placebo for Diabetic Foot Ulcers Infected by S. Aureus (PhagoPied). Available online: https://clinicaltrials.gov/ct2/show/NCT02664740 (accessed on 28 February 2023).
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Pherecydes Pharma. Evaluation of Phage Therapy for the Treatment of Escherichia Coli and Pseudomonas Aeruginosa Wound Infections in Burned Patients (PHAGOBURN). Available online: https://clinicaltrials.gov/ct2/show/NCT02116010 (accessed on 28 February 2023).
- VectorB2B. Bacteriophage Therapy TP-102 in Diabetic Foot Ulcers (REVERSE). Available online: https://clinicaltrials.gov/ct2/show/NCT04803708 (accessed on 28 February 2023).
- Nir-Paz, R.; Onallah, H.; Gellman, Y.N.; Haze, A.; Braunstein, R.; Hazan, R.; Leandro, C.; Barbosa, R.; Dordio, H.; Wagner, A.; et al. 1690. Assessing the safety of TP-102 bacteriophage treatment in the management of diabetic foot infections. Open Forum Infect. Dis. 2022, 9, ofac492-1320. [Google Scholar] [CrossRef]
- Wolcott, R. A Prospective, Randomized, Double-Blind Controlled Study of WPP-201 for the Safety and Efficacy of Treatment of Venous Leg Ulcers (WPP-201). Available online: https://clinicaltrials.gov/ct2/show/NCT00663091 (accessed on 28 February 2023).
- Armata Pharmaceuticals, Inc. Ascending Dose Study of the Safety of AB-SA01 When Topically Applied to Intact Skin of Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT02757755 (accessed on 28 February 2023).
- Koff, J.; Yale University. CYstic Fibrosis bacterioPHage Study at Yale (CYPHY). Available online: https://clinicaltrials.gov/ct2/show/NCT04684641 (accessed on 28 February 2023).
- BiomX, Inc. Nebulized Bacteriophage Therapy in Cystic Fibrosis Patients with Chronic Pseudomonas Aeruginosa Pulmonary Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT05010577 (accessed on 28 February 2023).
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase 1b/2 Trial of the Safety and Microbiological Activity of Bacteriophage Therapy in Cystic Fibrosis Subjects Colonized with Pseudomonas Aeruginosa. Available online: https://clinicaltrials.gov/ct2/show/NCT05453578 (accessed on 28 February 2023).
- Armata Pharmaceuticals, Inc. Study to Evaluate the Safety, Phage Kinetics, and Efficacy of Inhaled AP-PA02 in Subjects with Non-Cystic Fibrosis Bronchiectasis and Chronic Pulmonary Pseudomonas Aeruginosa Infection (Tailwind). Available online: https://clinicaltrials.gov/ct2/show/NCT05616221 (accessed on 28 February 2023).
- Cystic Fibrosis Foundation. Ph 1/2 Study Evaluating Safety and Tolerability of Inhaled AP-PA02 in Subjects with Chronic Pseudomonas Aeruginosa Lung Infections and Cystic Fibrosis (SWARM-Pa). Available online: https://clinicaltrials.gov/ct2/show/NCT04596319 (accessed on 28 February 2023).
- Mogayzel, P.J.; Naureckas, E.T.; Robinson, K.A.; Brady, C.; Guill, M.; Lahiri, T.; Lubsch, L.; Matsui, J.; Oermann, C.M.; Ratjen, F.; et al. Cystic Fibrosis Foundation Pulmonary Guideline. Pharmacologic Approaches to Prevention and Eradication of Initial Pseudomonas aeruginosa Infection. Ann. Am. Thorac. Soc. 2014, 11, 1640–1650. [Google Scholar] [CrossRef]
- University Hospital, Montpellier. Bacteriophage Effects on Pseudomonas Aeruginosa (MUCOPHAGES). Available online: https://clinicaltrials.gov/ct2/show/NCT01818206 (accessed on 12 April 2023).
- Adaptive Phage Therapeutics, Inc. Personalized Phage Treatment in COVID-19 Patients with Bacterial Co-Infections Microbials for Pneumonia or Bacteremia/Septicemia. Available online: https://clinicaltrials.gov/ct2/show/NCT04636554 (accessed on 28 February 2023).
- Kuzkov, V.V.; Northern State Medical University. The Effect of Supraglottic and Oropharyngeal Decontamination on the Incidence of Ventilator-Associated Pneumonia (SGDC-VAP). Available online: https://clinicaltrials.gov/ct2/show/NCT04325685 (accessed on 28 February 2023).
- Febvre, H.; Rao, S.; Gindin, M.; Goodwin, N.; Finer, E.; Vivanco, J.; Lu, S.; Manter, D.; Wallace, T.; Weir, T. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Weir, T.; Colorado State University. PHAGE Study: Bacteriophages as Novel Prebiotics. Available online: https://clinicaltrials.gov/ct2/show/NCT03269617 (accessed on 28 February 2023).
- Rappo, U.; Cohen, A.; Kario, E.; Bassan, M.; Puttagunta, S. A Phase 1, Randomized, Single-Blind, Placebo-Controlled Pharmacokinetic Study Evaluating Oral BX002-A Bacteriophage Therapy for Inflammatory Bowel Disease/Primary Sclerosing Cholangitis; American Association for the Study of Liver Diseases: Branford, CT, USA; Ness Ziona, Israel, 2021. [Google Scholar]
- BiomX, Inc. A Study to Evaluate the Safety, Tolerability, and Fecal Pharmacokinetics of BX002-A in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04737876 (accessed on 28 February 2023).
- University of Maryland, Baltimore. Safety and Efficacy of the Bacteriophage Preparation, ShigActiveTM, in a Human Experimental Model of Shigellosis. Available online: https://clinicaltrials.gov/ct2/show/NCT05182749 (accessed on 28 February 2023).
- Jakobsen, G.R. PrePhage—Faecal Bacteriophage Transfer for Enhanced Gastrointestinal Tract Maturation in Preterm Infants. Available online: https://clinicaltrials.gov/ct2/show/NCT05272566 (accessed on 28 February 2023).
- Mount Sinai Hospital. Safety and Efficacy of EcoActive on Intestinal Adherent Invasive E. coli in Patients with Inactive Crohn’s Disease. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03808103 (accessed on 28 February 2023).
- Leitner, L.; Sybesma, W.; Chanishvili, N.; Goderdzishvili, M.; Chkhotua, A.; Ujmajuridze, A.; Schneider, M.P.; Sartori, A.; Mehnert, U.; Bachmann, L.M.; et al. Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomized, placebo-controlled, double-blind clinical trial. BMC Urol. 2017, 17, 90. [Google Scholar] [CrossRef]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef]
- Locus Biosciences. Safety, Tolerability, and PK of LBP-EC01 in Patients with Lower Urinary Tract Colonization Caused by E. coli. Available online: https://clinicaltrials.gov/ct2/show/NCT04191148 (accessed on 28 February 2023).
- Kim, P.; Sanchez, A.; Kime, J.; Ousterout, D. 1083. Phase 1b Results of Pharmacokinetics, Pharmacodynamics, and Safety for LBP-EC01, a CRISPR-Cas3 Enhanced Bacteriophage Cocktail Targeting Escherichia coli that Cause Urinary Tract Infections. Open Forum Infect. Dis. 2021, 8, S633. [Google Scholar] [CrossRef]
- Locus Biosciences. A Study of LBP-EC01 in the Treatment of Acute Uncomplicated UTI Caused by Multi-Drug Resistant E. coli (ELIMINATE Trial) (ELIMINATE). Available online: https://clinicaltrials.gov/ct2/show/NCT05488340 (accessed on 28 February 2023).
- United States Department of Defense. Bacteriophage Therapy in Patients with Urinary Tract Infections. Available online: https://clinicaltrials.gov/ct2/show/NCT04287478 (accessed on 28 February 2023).
- Gregory German, Unity Health Toronto. Phage Therapy for the Treatment of Urinary Tract Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT05537519 (accessed on 28 February 2023).
- Burton, J.; Lawson Health Research Institute. Effect of PreforPro® on Urinary and Vaginal Health. Available online: https://clinicaltrials.gov/ct2/show/NCT05590195 (accessed on 28 February 2023).
- Adaptive Phage Therapeutics, Inc. Bacteriophage Therapy in First Time Chronic Prosthetic Joint Infections (ACTIVE1). Available online: https://clinicaltrials.gov/ct2/show/NCT05269121 (accessed on 28 February 2023).
- Adaptive Phage Therapeutics, Inc. Bacteriophage Therapy in Patients with Prosthetic Joint Infections Who Previously Failed Surgery for PJI (ACTIVE2). Available online: https://clinicaltrials.gov/ct2/show/NCT05269134 (accessed on 28 February 2023).
- SNIPR Biome Aps. A Study Investigating the Safety, Recovery, and Pharmacodynamics of Multiple Oral Administrations of SNIPR001 in Healthy Subjects. Available online: https://clinicaltrials.gov/ct2/show/NCT05277350 (accessed on 28 February 2023).
- Armata Pharmaceuticals, Inc. Study Evaluating Safety, Tolerability, and Efficacy of Intravenous AP-SA02 in Subjects with S. Aureus Bacteremia (diSArm). Available online: https://clinicaltrials.gov/ct2/show/NCT05184764 (accessed on 28 February 2023).
- Tolkunovna, T.S.; MD, Tashkent Pediatric Medical Institute. Bacteriophage Therapy in Tonsillitis. Available online: https://clinicaltrials.gov/ct2/show/NCT04682964 (accessed on 28 February 2023).
- Adaptive Phage Therapeutics, Inc. Bacteriophage Therapy in Patients with Diabetic Foot Osteomyelitis. Available online: https://clinicaltrials.gov/ct2/show/NCT05177107 (accessed on 28 February 2023).
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Międzybrodzki, R.; Borysowski, J. (Eds.) Phage Therapy: A Practical Approach; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-26735-3. [Google Scholar]
- Fauconnier, A. Phage Therapy Regulation: From Night to Dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Lembke, A.; Papac, J.; Humphreys, K. Our Other Prescription Drug Problem. N. Engl. J. Med. 2018, 378, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Mackey, T.K.; Schoenfeld, V.J. Going “social” to access experimental and potentially life-saving treatment: An assessment of the policy and online patient advocacy environment for expanded access. BMC Med. 2016, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; De Vos, D.; Verbeken, G. Clinical application of bacteriophages in Europe. Microbiol. Aust. 2019, 40, 8. [Google Scholar] [CrossRef]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage Therapy in Poland—A Centennial Journey to the First Ethically Approved Treatment Facility in Europe. Front. Microbiol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Ceyssens, P.-J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Compassionate Use of Bacteriophage Therapy for Foot Ulcer Treatment as an Effective Step for Moving Toward Clinical Trials. In Bacteriophage Therapy: From Lab to Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2018; pp. 159–170. [Google Scholar]
- Verbeken, G.; Pirnay, J.-P. European regulatory aspects of phage therapy: Magistral phage preparations. Curr. Opin. Virol. 2022, 52, 24–29. [Google Scholar] [CrossRef]
- Abedon, S. Information phage therapy research should report. Pharmaceuticals 2017, 10, 43. [Google Scholar] [CrossRef]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef]
- Naureen, Z.; Malacarne, D.; Anpilogov, K.; Dautaj, A.; Camilleri, G.; Cecchin, S.; Bressan, S.; Casadei, A.; Albion, E.; Sorrentino, E.; et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Biomed. 2020, 91, e2020023. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Washington, H.A. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present; First Anchor Books: New York, NY, USA, 2006. [Google Scholar]
- Weigmann, K. The ethics of global clinical trials. EMBO Rep. 2015, 16, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.J.; Singh, S. Health, human rights, and the conduct of clinical research within oppressed populations. Global. Health 2007, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ré, R.; Rid, A.; Emanuel, E.; Wendler, D. The potential exploitation of research participants in high income countries who lack access to health care. Br. J. Clin. Pharmacol. 2016, 81, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Jamrozik, E.; Selgelid, M.J. Ethical issues surrounding controlled human infection challenge studies in endemic low-and middle-income countries. Bioethics 2020, 34, 797–808. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Merabishvili, M.; Vergauwen, B.; Lavigne, R.; Vaneechoutte, M. A comparative study of different strategies for removal of endotoxins from bacteriophage preparations. J. Microbiol. Methods 2017, 132, 153–159. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Johnson, A.J.; Slomka, M.; Fackler, J.; Horne, B.; Brownstein, M.J.; Henry, M.; Malagon, F.; Biswas, B. Salvage Bacteriophage Therapy for a Chronic MRSA Prosthetic Joint Infection. Antibiotics 2020, 9, 241. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and efficacy of phage therapy in difficult-to-treat infections: A systematic review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Rouveix, B. Antibiotic Safety Assessment. Int. J. Antimicrob. Agents 2003, 21, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Jeffres, M.N. The Whole Price of Vancomycin: Toxicities, Troughs, and Time. Drugs 2017, 77, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, K.E.; Stacey, H.J.; Harkin, G.; Hall, L.M.L.; Young, M.J.; Jones, J.D. Patient perceptions of phage therapy for diabetic foot infection. PLoS ONE 2020, 15, e0243947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic Engineering of Bacteriophages Against Infectious Diseases. Front. Microbiol. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Gibb, B.; Hyman, P.; Schneider, C. The Many Applications of Engineered Bacteriophages—An Overview. Pharmaceuticals 2021, 14, 634. [Google Scholar] [CrossRef]
- Rohde, C.; Resch, G.; Pirnay, J.-P.; Blasdel, B.; Debarbieux, L.; Gelman, D.; Górski, A.; Hazan, R.; Huys, I.; Kakabadze, E.; et al. Expert Opinion on Three Phage Therapy Related Topics: Bacterial Phage Resistance, Phage Training and Prophages in Bacterial Production Strains. Viruses 2018, 10, 178. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R.; Khalid, A.; Venturini, C.; Chard, R.; Morales, S.; et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef]
- Doub, J.B.; Shishido, A.; Srikumaran, U.; Haskoor, J.; Tran-Nguyen, P.; Lee, M.; Würstle, S.; Lee, A.; Kortright, K.; Chan, B.K. Salphage: Salvage bacteriophage therapy for a recalcitrant Klebsiella pneumoniae prosthetic shoulder infection—A case report. Acta Orthop. 2022, 93, 756–759. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, C.; Gonzales, F.; Buckley, M.; Biswas, B.; Henry, M.; Deschenes, M.V.; Horne, B.; Fackler, J.; Brownstein, M.J.; Schooley, R.T.; et al. Successful Treatment of Staphylococcus aureus Prosthetic Joint Infection with Bacteriophage Therapy. Viruses 2021, 13, 1182. [Google Scholar] [CrossRef]
- Kebriaei, R.; Lev, K.L.; Shah, R.M.; Stamper, K.C.; Holger, D.J.; Morrisette, T.; Kunz Coyne, A.J.; Lehman, S.M.; Rybak, M.J. Eradication of Biofilm-Mediated Methicillin-Resistant Staphylococcus aureus Infections In Vitro: Bacteriophage-Antibiotic Combination. Microbiol. Spectr. 2022, 10, e00411-22. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, J.W.; Moon, S.-Y.; Lee, Y.-K.; Ha, Y.-C.; Koo, K.-H. Current and Future Burden of Periprosthetic Joint Infection from National Claim Database. J. Korean Med. Sci. 2020, 35, e410. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.; Parracho, H.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and Biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Palazuelos-Munoz, S.; Balsells, E.M.; Nair, H.; Chit, A.; Kyaw, M.H. Cost of hospital management of Clostridium difficile infection in United States—A meta-analysis and modelling study. BMC Infect. Dis. 2016, 16, 447. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Colak, M.; Yilmaz, B.C.; Ersoz, G.; Kutateladze, M.; Gozlugol, M. Bacteriophage Therapy in Implant-Related Infections. J. Bone Jt. Surg. Am. 2013, 95, 117–125. [Google Scholar] [CrossRef]
- Valério, N.; Oliveira, C.; Jesus, V.; Branco, T.; Pereira, C.; Moreirinha, C.; Almeida, A. Effects of single and combined use of bacteriophages and antibiotics to inactivate Escherichia coli. Virus Res. 2017, 240, 8–17. [Google Scholar] [CrossRef]
- Kim, M.; Jo, Y.; Hwang, Y.J.; Hong, H.W.; Hong, S.S.; Park, K.; Myung, H. Phage-Antibiotic Synergy via Delayed Lysis. Appl. Environ. Microbiol. 2018, 84, e02085-18. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Fatima, R.; Hynes, A.P. Temperate phage-antibiotic synergy eradicates bacteria through depletion of lysogens. Cell Rep. 2021, 35, 109–172. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tétart, F.; Trojet, S.N.; Prère, M.-F.; Krisch, H.M. Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef] [PubMed]
- Miedzybrodzki, R.; Fortuna, W.; Weber-Dabrowska, B.; Górski, A. Phage therapy of staphylococcal infections (including MRSA) may be less expensive than antibiotic treatment. Postep. Hig. Med. Dosw. 2007, 61, 461–465. [Google Scholar]
- Nagel, T.; Musila, L.; Muthoni, M.; Nikolich, M.; Nakavuma, J.L.; Clokie, M.R. Phage banks as potential tools to rapidly and cost-effectively manage antimicrobial resistance in the developing world. Curr. Opin. Virol. 2022, 53, 101208. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert Rev. Anti-Infect. Ther. 2022, 20, 147–160. [Google Scholar] [CrossRef]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47. [Google Scholar] [CrossRef]
- Payne, R. Phage therapy: The peculiar kinetics of self-replicating pharmaceuticals. Clin. Pharmacol. Ther. 2000, 68, 225–230. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Bacteriophage Host Range and Bacterial Resistance. Adv. Appl. Microbiol. 2010, 70, 217–248. [Google Scholar]
- Caflisch, K.M.; Suh, G.A.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert Rev. Anti-Infect. Ther. 2019, 17, 1011–1041. [Google Scholar] [CrossRef]
- Yen, M.; Camilli, A. Mechanisms of the evolutionary arms race between Vibrio cholerae and Vibriophage clinical isolates. Int. Microbiol. 2017, 20, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Diallo, K.; Dublanchet, A. Benefits of Combined Phage–Antibiotic Therapy for the Control of Antibiotic-Resistant Bacteria: A Literature Review. Antibiotics 2022, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, L.; Li, X.; Tan, D.; Cong, C.; Xu, Y. Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Res. 2019, 272, 197734. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Kittler, S.; Klein, G.; Glünder, G. Impact of a Single Phage and a Phage Cocktail Application in Broilers on Reduction of Campylobacter jejuni and Development of Resistance. PLoS ONE 2013, 8, e78543. [Google Scholar] [CrossRef] [PubMed]
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing phage therapy through synthetic biology and genome engineering. Curr. Opin. Biotechnol. 2021, 68, 151–159. [Google Scholar] [CrossRef]
- Maimaiti, Z.; Li, Z.; Xu, C.; Chen, J.; Chai, W. Global trends and hotspots of phage therapy for bacterial infection: A bibliometric visualized analysis from 2001 to 2021. Front. Microbiol. 2003, 13, 1067803. [Google Scholar] [CrossRef]
- Strathdee, S.; Patterson, T.; Barker, T. The Perfect Predator: A Scientist’s Race to Save Her Husband from a Deadly Superbug: A Memoir; Hachette Books: New York, NY, USA, 2019. [Google Scholar]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R.; et al. Successful Treatment of Antibiotic-resistant, Poly-microbial Bone Infection with Bacteriophages and Antibiotics Combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef]
- Michodigni, N.; Nakayinga, R.; Makumi, A.; Kimani, J.; Mutai, I.; Dapuliga, C.; Getachew, B.; Agbankpè, J.; Nakavuma, J. The Africa Phage Forum: A New Collaborative Network for Bacteriophage Research in Africa. Commun. Prepr. 2022, 2022010345. [Google Scholar] [CrossRef]
- Willy, C.; Bugert, J.J.; Classen, A.Y.; Deng, L.; Düchting, A.; Gross, J.; Hammerl, J.A.; Korf, I.H.E.; Kühn, C.; Lieberknecht-Jouy, S.; et al. Phage Therapy in Germany—Update 2023. Viruses 2023, 15, 588. [Google Scholar] [CrossRef]
- Jones, J.D.; Trippett, C.; Suleman, M.; Clokie, M.R.J.; Clark, J.R. The Future of Clinical Phage Therapy in the United Kingdom. Viruses 2023, 15, 721. [Google Scholar] [CrossRef] [PubMed]
- Four Years on, the Global Observatory on Health R&D Continues to Identify Gaps and New Trends in the Health R&D Space. Available online: https://www.who.int/news/item/25-02-2021-four-years-on-the-global-observatory-on-health-r-d-continues-to-identify-gaps-and-new-trends-in-the-health-r-d-space (accessed on 12 April 2023).
- Garvey, M. Bacteriophages and the One Health Approach to Combat Multidrug Resistance: Is This the Way? Antibiotics 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, L.D.; Bisha, B. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 2011, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Sechter, I.; Touitou, E.; Donbrow, M. The influence of a non-ionic surfactant on rectal absorption of virus particles. Arch. Virol. 1989, 106, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Bochkareva, S.S.; Karaulov, A.V.; Aleshkin, A.V.; Novikova, L.I.; Kiseleva, I.A.; Rubal’skii, E.O.; Mekhtiev, E.R.; Styshnev, A.O.; Zul’karneev, E.R.; Anurova, M.N.; et al. Analysis of the Pharmacokinetics of Suppository Forms of Bacteriophages. Bull. Exp. Biol. Med. 2020, 168, 748–752. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phage Therapy in Livestock and Companion Animals. Antibiotics 2021, 10, 559. [Google Scholar] [CrossRef]
- Pyzik, E.; Radzki, R.P.; Urban-Chmiel, R. Experimental Phage Therapies in Companion Animals with A Historical Review. Curr. Rev. Clin. Exp. Pharmacol. 2021, 16, 17–29. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Międzybrodzki, R.; Drulis-Kawa, Z.; Cater, K.; Knežević, P.; Winogradow, C.; Amaro, K.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Rękas, J.; et al. Bacteriophages and antibiotic interactions in clinical practice: What we have learned so far. J. Biomed. Sci. 2022, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Guan, J.; Oromí-Bosch, A.; Mendoza, S.D.; Karambelkar, S.; Berry, J.D.; Bondy-Denomy, J. Bacteriophage genome engineering with CRISPR–Cas13a. Nat. Microbiol. 2022, 7, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.A.; Hessler, T.; Cress, B.F.; Lahiri, A.; Mutalik, V.K.; Barrangou, R.; Banfield, J.; Doudna, J.A. Broad-spectrum CRISPR-Cas13a enables efficient phage genome editing. Nat. Microbiol. 2022, 7, 1967–1979. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.S. Phage therapy—Constraints and possibilities. Ups. J. Med. Sci. 2014, 119, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, J.J. What Can Phages Tell Us about Host-Pathogen Coevolution? Int. J. Evol. Biol. 2012, 2012, 396165. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef]
- Mageeney, C.M.; Sinha, A.; Mosesso, R.A.; Medlin, D.L.; Lau, B.Y.; Rokes, A.B.; Lane, T.W.; Branda, S.S.; Williams, K.P. Computational Basis for On-Demand Production of Diversified Therapeutic Phage Cocktails. mSystems 2020, 5, e00659-20. [Google Scholar] [CrossRef]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett. 2007, 29, 995–1003. [Google Scholar] [CrossRef]
- Xu, Z.; Ding, Z.; Zhang, Y.; Liu, X.; Wang, Q.; Shao, S.; Liu, Q. Shelf-life prediction and storage stability of Aeromonas bacteriophage vB_AsM_ZHF. Virus Res. 2023, 323, 198997. [Google Scholar] [CrossRef]



| Study Title/Identifier | Condition or Disease | Microorganisms | Phase | Status | Sponsor/Collaborator |
|---|---|---|---|---|---|
| Cystic Fibrosis bacterioPHage Study at Yale (CYPHY) | Cystic Fibrosis | P. aeruginosa | 1/2 | Active, not recruiting | Yale New Haven Hospital New Haven, CT, USA |
| Bacteriophage Therapy in Tonsillitis | Acute Tonsillitis | Staphylococcus spp. Enterococcus spp. Streptococcus spp. Enteropathogenic E. coli | 3 | Active, not recruiting | Tashkent Pediatric Medical Institute Tashkent, Uzbekistan |
| Bacteriophage Therapy in Patients with Urinary Tract Infections | Urinary Tract Infection Bacterial | E. coli K. pneumoniae | 1/2 | Active, not recruiting | Adaptive Phage Therapeutics, Inc. (Washington, DC, USA) |
| Bacteriophages To Treat Liver Disease Eliminating Harmful Bacteria (BATTLE) | Alcoholic Hepatitis | E. faecalis | - | Recruiting | Copenhagen University Hospital Hvidovre Hvidovre, Denmark |
| A Phase 1b/2 Trial of the Safety and Microbiological Activity of Bacteriophage Therapy in Cystic Fibrosis Subjects Colonized with Pseudomonas Aeruginosa | Bacterial Disease Carrier; Cystic Fibrosis | P. aeruginosa | 1/2 | Recruiting | National Institute of Allergy and Infectious Diseases (NIAID) |
| Nebulized Bacteriophage Therapy in Cystic Fibrosis Patients with Chronic Pseudomonas Aeruginosa Pulmonary Infection | Chronic Pseudomonas Aeruginosa Infection; Cystic Fibrosis | P. aeruginosa | 1/2 | Recruiting | BiomX, Inc. (Ness Ziona, Israel) |
| Bacteriophage Therapy in Patients with Diabetic Foot Osteomyelitis | Osteomyelitis; Diabetic Foot Osteomyelitis | S. aureus | 2 | Recruiting | Adaptive Phage Therapeutics, Inc. (Washington, DC, USA) |
| Phage Safety Cohort Study (PHA-SA-CO) | Prosthetic Joint Infection; Severe Infection | * | * | Recruiting | Hospices Civils de Lyon (Lyon, France) |
| Phage Safety Retrospective Cohort Study (PHASACO-retro) | Bone and Joint Infection; Prosthetic Joint Infection | * | * | Recruiting | Hospices Civils de Lyon (Lyon, France) |
| Study to Evaluate the Safety, Phage Kinetics, and Efficacy of Inhaled AP-PA02 in Subjects with Non-Cystic Fibrosis Bronchiectasis and Chronic Pulmonary Pseudomonas Aeruginosa Infection (Tailwind) | Non-cystic Fibrosis Bronchiectasis; Pseudomonas Aeruginosa; Lung Infection | P. aeruginosa | 2 | Recruiting | Armata Pharmaceuticals, Inc. (Marina Del Rey, CA, USA) |
| Phage Therapy in Prosthetic Joint Infection Due to Staphylococcus Aureus Treated With DAIR. (PhagoDAIRI) | Infection of Total Hip Joint Prosthesis; Infection of Total Knee Joint Prosthesis | S. aureus | 2 | Recruiting | Pherecydes Pharma (Romainville, Paris) |
| Ph 1/2 Study Evaluating Safety and Tolerability of Inhaled AP-PA02 in Subjects with Chronic Pseudomonas Aeruginosa Lung Infections and Cystic Fibrosis (SWARM-Pa) | Cystic Fibrosis; Pseudomonas Aeruginosa; Pseudomonas; Lung Infection; Lung Infection Pseudomonal | P. aeruginosa | 1/2 | Recruiting | Armata Pharmaceuticals, Inc. (Marina Del Rey, CA, USA) |
| Safety and Efficacy of EcoActive on Intestinal Adherent Invasive E. Coli in Patients with Inactive Crohn’s Disease | Crohn’s Disease | Adherent invasive E. coli (AIEC) | 1/2 | Recruiting | Intralytix, Inc. (Columbia, MD, USA) |
| Study Evaluating Safety, Tolerability, and Efficacy of Intravenous AP-SA02 in Subjects with S. Aureus Bacteremia (diSArm) | SA Bacteremia (SAB) | S. aureus | 1/2 | Recruiting | Armata Pharmaceuticals, Inc. (Marina Del Rey, CA, USA) |
| A Study of LBP-EC01 in the Treatment of Acute Uncomplicated UTI Caused by Multi-drug-resistant E. Coli | Urinary Tract Infections | E. coli | Recruiting | Locus Biosciences (Morrisville, NC, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020. https://doi.org/10.3390/v15041020
Hitchcock NM, Devequi Gomes Nunes D, Shiach J, Valeria Saraiva Hodel K, Dantas Viana Barbosa J, Alencar Pereira Rodrigues L, Coler BS, Botelho Pereira Soares M, Badaró R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses. 2023; 15(4):1020. https://doi.org/10.3390/v15041020
Chicago/Turabian StyleHitchcock, Nicole Marie, Danielle Devequi Gomes Nunes, Job Shiach, Katharine Valeria Saraiva Hodel, Josiane Dantas Viana Barbosa, Leticia Alencar Pereira Rodrigues, Brahm Seymour Coler, Milena Botelho Pereira Soares, and Roberto Badaró. 2023. "Current Clinical Landscape and Global Potential of Bacteriophage Therapy" Viruses 15, no. 4: 1020. https://doi.org/10.3390/v15041020
APA StyleHitchcock, N. M., Devequi Gomes Nunes, D., Shiach, J., Valeria Saraiva Hodel, K., Dantas Viana Barbosa, J., Alencar Pereira Rodrigues, L., Coler, B. S., Botelho Pereira Soares, M., & Badaró, R. (2023). Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses, 15(4), 1020. https://doi.org/10.3390/v15041020







