Genomic Analysis of a New Freshwater Cyanophage Lbo240-yong1 Suggests a New Taxonomic Family of Bacteriophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Cyanophages
2.2. Preparation of Cyanophage Suspensions
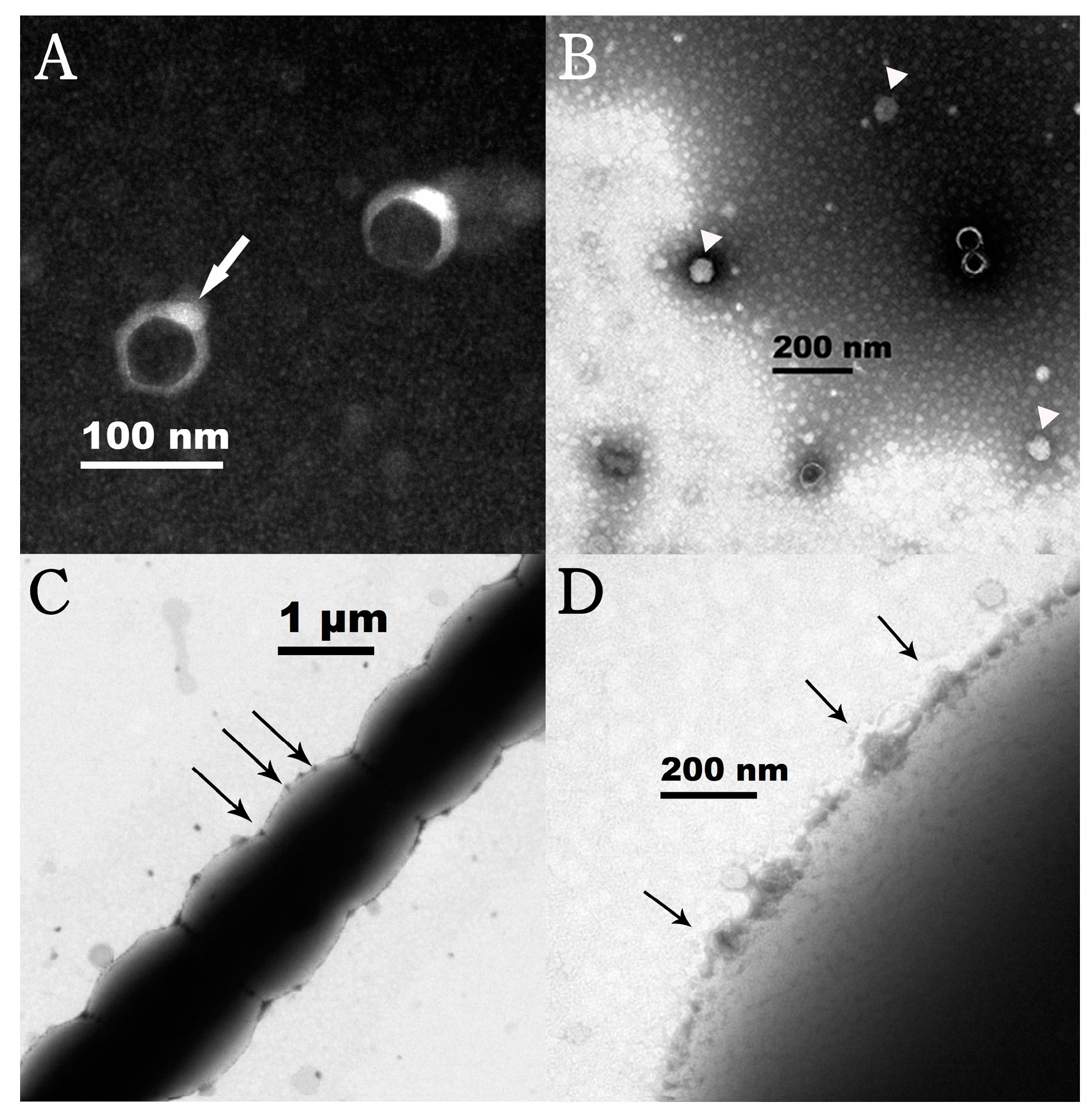
2.3. Electron Microscopy Observation
2.4. Host Range Experiments
2.5. DNA Isolation, Genome Sequencing
2.6. Genome Annotation
2.7. Taxonomic Analysis
3. Results
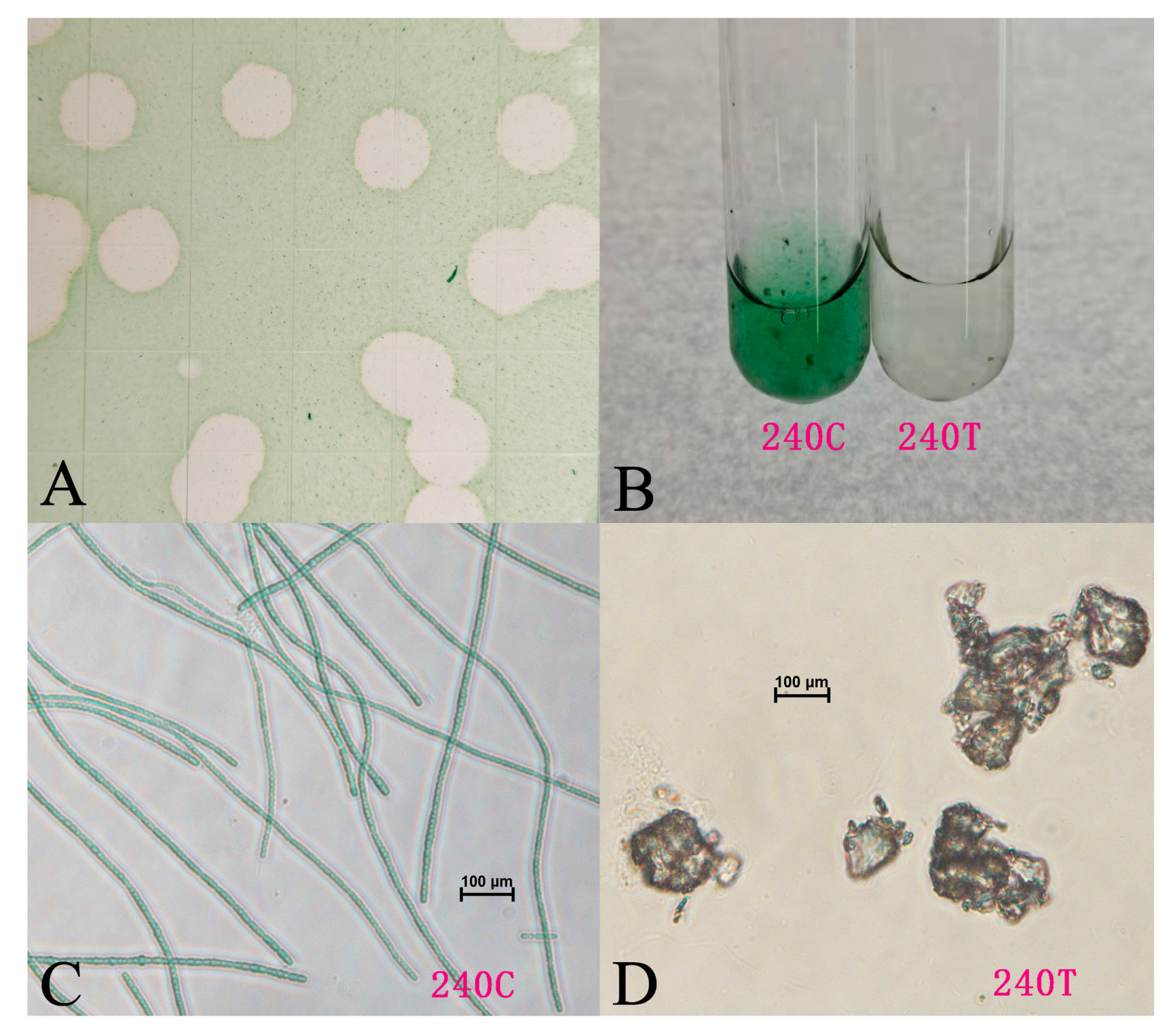
3.1. Cyanophage Isolation
3.2. General Features of Lbo240-yong1
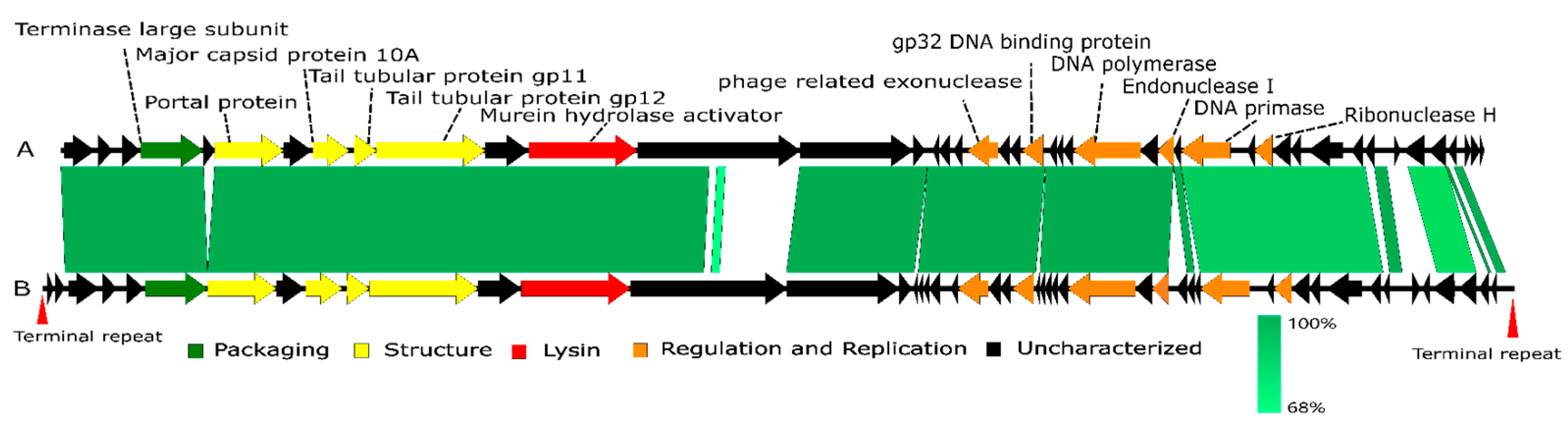
3.3. Genomic Analysis of Lbo240-yong1
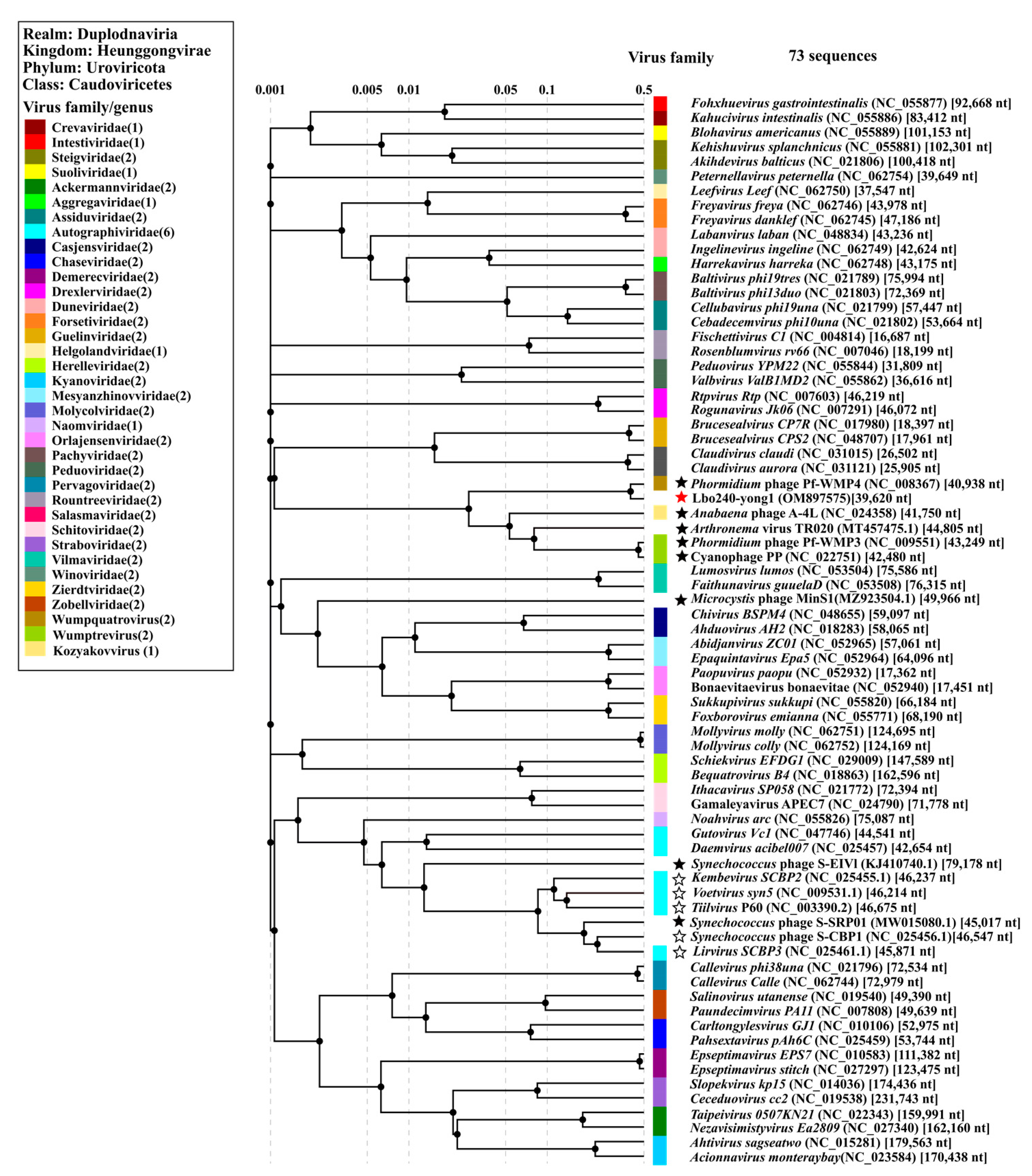
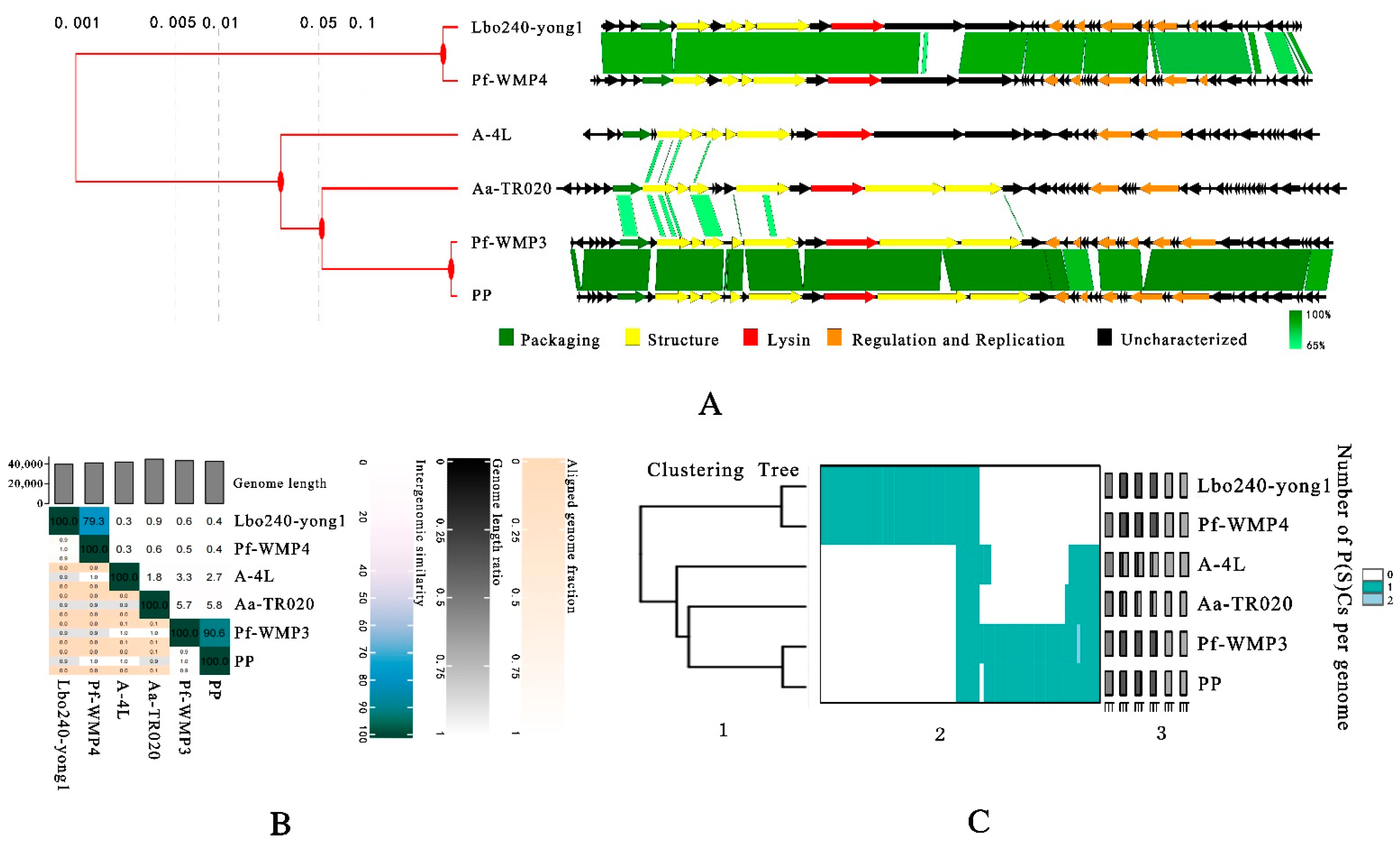
3.4. Taxonomic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trung, B.; Dao, T.S.; Faassen, E.; Lurling, M. Cyanobacterial Blooms and Microcystins in Southern Vietnam. Toxins 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.; Lee, J. Cyanobacterial blooms: A player in the freshwater environmental resistome with public health relevance? Environ. Res. 2023, 216, 114612. [Google Scholar] [CrossRef]
- Huo, D.; Gan, N.; Geng, R.; Cao, Q.; Song, L.; Yu, G.; Li, R. Cyanobacterial blooms in China: Diversity, distribution, and cyanotoxins. Harmful Algae 2021, 109, 102106. [Google Scholar] [CrossRef] [PubMed]
- Ferrao-Filho, A.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Zervou, S.K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef]
- Suttle, C.A. Marine viruses--major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Yang, F.; Jin, H.; Wang, X.Q.; Li, Q.; Zhang, J.T.; Cui, N.; Jiang, Y.L.; Chen, Y.; Wu, Q.F.; Zhou, C.Z.; et al. Genomic Analysis of Mic1 Reveals a Novel Freshwater Long-Tailed Cyanophage. Front. Microbiol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Yoshida-Takashima, Y.; Yoshida, M.; Ogata, H.; Nagasaki, K.; Hiroishi, S.; Yoshida, T. Cyanophage infection in the bloom-forming cyanobacteria Microcystis aeruginosa in surface freshwater. Microbes Environ. 2012, 27, 350–355. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, S.; Jiao, N.; Chen, F. Marine cyanophages demonstrate biogeographic patterns throughout the global ocean. Appl. Environ. Microb. 2015, 81, 441–452. [Google Scholar] [CrossRef]
- Cai, R.; Li, D.; Lin, W.; Qin, W.; Pan, L.; Wang, F.; Qian, M.; Liu, W.; Zhou, Q.; Zhou, C.; et al. Genome sequence of the novel freshwater Microcystis cyanophage Mwe-Yong1112-1. Arch. Virol. 2022, 167, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, J.; Li, N.; Hu, Z.; Deng, F. Characterization and genomic analysis of a plaque purified strain of cyanophage PP. Virol. Sin. 2013, 28, 272–279. [Google Scholar] [CrossRef]
- Zhang, S.; He, X.; Cao, L.; Tong, Y.; Zhao, B.; An, W. A Novel Wide-Range Freshwater Cyanophage MinS1 Infecting the Harmful Cyanobacterium Microcystis aeruginosa. Viruses 2022, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.A.; Haselkorn, R. LPP-1 infection of the blue-green alga Plectonema boryanum. I. Electron microscopy. J. Virol. 1970, 6, 820–833. [Google Scholar]
- Padan, E.; Ginzburg, D.; Shilo, M. The reproductive cycle of cyanophage LPP1-G in Plectonema boryanum and its dependence on photosynthetic and respiratory systems. Virology 1970, 40, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Shilo, M.; Oppenheim, A.B. Lysogeny of the blue-green alga Plectonema boryanum by LPP2-SPI cyanophage. Virology 1972, 47, 525–526. [Google Scholar] [CrossRef]
- Mendzhul, M.I.; Syrchin, S.A.; Rebentish, B.A.; Averkiev, A.A.; Busakhina, I.V. The resistance of the DNA of cyanophage LPP-3 to the action of different restriction endonucleases. Mikrobiol. Z. 1993, 55, 47–53. [Google Scholar]
- Barnet, Y.M.; Daft, M.J.; Stewart, W.D. The effect of suspended particulate material on cyanobacteria--cyanophage interactions in liquid culture. J. Appl. Bacteriol. 1984, 56, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rimon, A.; Oppenheim, A.B. Heat induction of the blue-green alga Plectonema boryanum lysogenic for the cyanophage SPlcts1. Virology 1975, 64, 454–463. [Google Scholar] [CrossRef]
- Safferman, R.S.; Morris, M.E.; Sherman, L.A.; Haselkorn, R. Serological and electron microscopic characterization of a new group of blue-green algal viruses (LPP-2). Virology 1969, 39, 775–780. [Google Scholar] [CrossRef]
- Wilson, W.H.; Joint, I.R.; Carr, N.G.; Mann, N.H. Isolation and Molecular Characterization of Five Marine Cyanophages Propagated on Synechococcus sp. Strain WH7803. Appl. Environ. Microb. 1993, 59, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, D.; Sun, Z.; Tong, Y.; Yan, X.; Wang, C.; Zhang, X.; Pei, G. A novel freshwater cyanophage vB_MelS-Me-ZS1 infecting bloom-forming cyanobacterium Microcystis elabens. Mol. Biol. Rep. 2020, 47, 7979–7989. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Li, S.; An, X.; Pei, G.; Huang, Y.; Fan, H.; Mi, Z.; Zhang, Z.; Wang, W.; et al. A novel termini analysis theory using HTS data alone for the identification of Enterococcus phage EF4-like genome termini. BMC Genom. 2015, 16, 414. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007, 35, W52–W57. [Google Scholar] [CrossRef]
- Grissa, I.; Vergnaud, G.; Pourcel, C. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinform. 2007, 8, 172. [Google Scholar] [CrossRef]
- Roux, S.; Paez-Espino, D.; Chen, I.A.; Palaniappan, K.; Ratner, A.; Chu, K.; Reddy, T.; Nayfach, S.; Schulz, F.; Call, L.; et al. IMG/VR v3: An integrated ecological and evolutionary framework for interrogating genomes of uncultivated viruses. Nucleic Acids Res. 2021, 49, D764–D775. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods Mol Biol. 2019, 1962, 1–14. [Google Scholar]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.Z.; Rau, D.; Kubler, J.; Lozajic, M.; Gabler, F.; Soding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Figueras, M.J.; Beaz-Hidalgo, R.; Hossain, M.J.; Liles, M.R. Taxonomic affiliation of new genomes should be verified using average nucleotide identity and multilocus phylogenetic analysis. Genome Announc. 2014, 2, e00927-14. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Goker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Bao, Y.; Chetvernin, V.; Tatusova, T. Improvements to pairwise sequence comparison (PASC): A genome-based web tool for virus classification. Arch. Virol. 2014, 159, 3293–3304. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Zhu, B.; Tabor, S.; Raytcheva, D.A.; Hernandez, A.; King, J.A.; Richardson, C.C. The RNA polymerase of marine cyanophage Syn5. J. Biol. Chem. 2013, 288, 3545–3552. [Google Scholar] [CrossRef]
- Chen, F.; Lu, J. Genomic sequence and evolution of marine cyanophage P60: A new insight on lytic and lysogenic phages. Appl. Environ. Microb. 2002, 68, 2589–2594. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, S.; Jiao, N.; Chen, F. Comparative Genomic and Phylogenomic Analyses Reveal a Conserved Core Genome Shared by Estuarine and Oceanic Cyanopodoviruses. PLoS ONE 2015, 10, e142962. [Google Scholar] [CrossRef]
- Cristina, M. VirClust—A tool for hierarchical clustering, core gene detection and annotation of (prokaryotic) viruses. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Guo, J.; Bolduc, B.; Zayed, A.A.; Varsani, A.; Dominguez-Huerta, G.; Delmont, T.O.; Pratama, A.A.; Gazitua, M.C.; Vik, D.; Sullivan, M.B.; et al. VirSorter2: A multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome 2021, 9, 37. [Google Scholar] [CrossRef]
- Bin, J.H.; Bolduc, B.; Zablocki, O.; Kuhn, J.H.; Roux, S.; Adriaenssens, E.M.; Brister, J.R.; Kropinski, A.M.; Krupovic, M.; Lavigne, R.; et al. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by gene-sharing networks. Nat. Biotechnol. 2019, 37, 632–639. [Google Scholar]
- Liu, X.; Shi, M.; Kong, S.; Gao, Y.; An, C. Cyanophage Pf-WMP4, a T7-like phage infecting the freshwater cyanobacterium Phormidium foveolarum: Complete genome sequence and DNA translocation. Virology 2007, 366, 28–39. [Google Scholar] [CrossRef]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of morphology-based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kong, S.; Shi, M.; Fu, L.; Gao, Y.; An, C. Genomic analysis of freshwater cyanophage Pf-WMP3 Infecting cyanobacterium Phormidium foveolarum: The conserved elements for a phage. Microb. Ecol. 2008, 56, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Petrzik, K.; Lukavsky, J.; Koloniuk, I. Novel Virus on Filamentous Arthronema africanum Cyanobacterium. Microb. Ecol. 2021, 81, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.; Liao, X.Y.; Gao, X.C.; Xu, X.D.; Zhang, Q.Y. Unraveling the genome structure of cyanobacterial Podoviridae A-4L with long direct terminal repeats. Virus Res. 2015, 203, 4–9. [Google Scholar] [CrossRef]
- Zhang, D.; He, Y.; Gin, K.Y. Novel Freshwater Cyanophages Provide New Insights into Evolutionary Relationships between Freshwater and Marine Cyanophages. Microbiol. Spectr. 2021, 9, e59321. [Google Scholar] [CrossRef]
- Ha, E.; Son, B.; Ryu, S. Clostridium perfringens Virulent Bacteriophage CPS2 and Its Thermostable Endolysin LysCPS2. Viruses 2018, 10, 251. [Google Scholar] [CrossRef]
- Volozhantsev, N.V.; Oakley, B.B.; Morales, C.A.; Verevkin, V.V.; Bannov, V.A.; Krasilnikova, V.M.; Popova, A.V.; Zhilenkov, E.L.; Garrish, J.K.; Schegg, K.M.; et al. Molecular characterization of podoviral bacteriophages virulent for Clostridium perfringens and their comparison with members of the Picovirinae. PLoS ONE 2012, 7, e38283. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, N.; Zheng, L.; Song, L. Host re-identification of cyanophage PP and its implications for host range and specificity. Virol. Sin. 2013, 28, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nagasaki, K.; Takashima, Y.; Shirai, Y.; Tomaru, Y.; Takao, Y.; Sakamoto, S.; Hiroishi, S.; Ogata, H. Ma-LMM01 infecting toxic Microcystis aeruginosa illuminates diverse cyanophage genome strategies. J. Bacteriol. 2008, 190, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.E.; Dupuis, M.E.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadan, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Melamed, S.; Ofir, G.; Leavitt, A.; Lopatina, A.; Keren, M.; Amitai, G.; Sorek, R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359, eaar4120. [Google Scholar] [CrossRef] [PubMed]

| Cyanophage | Accession Number | Genome Length | Host (Reference) | Morphology |
|---|---|---|---|---|
| PP | NC_022751 | 42,480 bp | L. boryanum, Phoridium foveolarum | Podovirus-like |
| Lbo240-yong1 | OM_897575 | 39,740 bp | L. boryanum | Podovirus-like |
| LPP-1 | - | - | L. boryanum [15] | - |
| LPP1-G | - | - | L. boryanum [16] | - |
| LPP2-SPI | - | - | L. boryanum [17] | - |
| LPP-3 | - | - | L. boryanum [18] | - |
| LPP-DUN1 | - | - | L. boryanum [19] | - |
| Cyanophage SPlcts1 | - | - | L. boryanum [20] | - |
| LPP-2 | - | - | L. boryanum [21] | - |
| MinS1 | MZ923504 | 49,966 bp | Microcystis aeruginosa, L. boryana et al. | Siphovirus-like |
| ORF | Size (aa) | Prediction Function | Best BLAST Hit a | Identity b (aa) | E-Value c |
|---|---|---|---|---|---|
| 1 | 282 | hypothetical protein | ref|YP_762673.1| PfWMP4_43 [cyanophage Pf-WMP4] | 95% (270/283) | 0.0 |
| 2 | 136 | hypothetical protein | no hit | ||
| 3 | 170 | hypothetical protein | ref|YP_762671.1| PfWMP4_41 [cyanophage Pf-WMP4] | 98% (167/170) | 5 × 10−118 |
| 4 | 581 | terminase large subunit | ref|YP_762670.1| PfWMP4_40 [cyanophage Pf-WMP4] | 95% (553/581) | 0.0 |
| 5 | 105 | hypothetical protein | ref|WP_224176119.1| Gp49 family protein [Calothrix sp. PCC 7716] | 53% (46/86) | 2 × 10−29 |
| 6 | 641 | portal protein | ref|YP_762669.1| PfWMP4_39 [cyanophage Pf-WMP4] | 98% (631/641) | 0.0 |
| 7 | 263 | hypothetical protein | ref|YP_762668.1| PfWMP4_38 [cyanophage Pf-WMP4] | 93% (244/263) | 1 × 10−120 |
| 8 | 341 | major capsid protein 10A | ref|YP_762667.1| PfWMP4_37 [cyanophage Pf-WMP4] | 98% (333/341) | 0.0 |
| 9 | 211 | tail tubular protein gp11 | ref|YP_762666.1| PfWMP4_36 [cyanophage Pf-WMP4] | 98% (206/211) | 2 × 10−151 |
| 10 | 1012 | tail tubular protein gp12 | ref|YP_762665.1| PfWMP4_35 [cyanophage Pf-WMP4] | 98% (994/1012) | 0.0 |
| 11 | 400 | hypothetical protein | ref|YP_762664.1| PfWMP4_34 [cyanophage Pf-WMP4] | 95% (380/400) | 0.0 |
| 12 | 1012 | murein hydrolase activator | ref|YP_762663.1| PfWMP4_33 [cyanophage Pf-WMP4] | 93%(943/1014) | 0.0 |
| 13 | 1519 | hypothetical protein | ref|YP_762662.1| PfWMP4_32 [cyanophage Pf-WMP4] | 74% (661/891) | 0.0 |
| 14 | 1047 | hypothetical protein | ref|YP_762661.1| PfWMP4_31 [cyanophage Pf-WMP4] | 97% (1016/1047) | 0.0 |
| 15 | 110 | hypothetical protein | ref|YP_762660.1| PfWMP4_30 [cyanophage Pf-WMP4] | 97% (107/110) | 8 × 10−70 |
| 16 | 57 | hypothetical protein | no hit | ||
| 17 | 103 | hypothetical protein | ref|YP_762658.1| PfWMP4_28 [cyanophage Pf-WMP4] | 86% (78/91) | 3 × 10−49 |
| 18 | 80 | hypothetical protein | ref|YP_762657.1| PfWMP4_27 [cyanophage Pf-WMP4] | 98% (58/59) | 2 × 10−33 |
| 19 | 285 | phage-related exonuclease | ref|YP_762655.1| PfWMP4_25 [cyanophage Pf-WMP4] | 96% (274/285) | 0.0 |
| 20 | 108 | hypothetical protein | ref|YP_762654.1| PfWMP4_24 [cyanophage Pf-WMP4] | 98% (106/108) | 3 × 10−73 |
| 21 | 100 | hypothetical protein | ref|YP_762653.1| PfWMP4_23 [cyanophage Pf-WMP4] | 71% (71/100) | 2 × 10−43 |
| 22 | 198 | gp32 single-stranded DNA-binding protein | ref|YP_762652.1| PfWMP4_22 [cyanophage Pf-WMP4] | 92% (168/182) | 1 × 10−122 |
| 23 | 73 | hypothetical protein | no hit | ||
| 24 | 58 | hypothetical protein | no hit | ||
| 25 | 95 | hypothetical protein | ref|YP_762650.1| PfWMP4_20 [cyanophage Pf-WMP4] | 91% (86/95) | 1 × 10−56 |
| 26 | 630 | DNA polymerase theta | ref|YP_762649.1| DNA polymerase [cyanophage Pf-WMP4] | 94% (594/630) | 0.0 |
| 27 | 160 | hypothetical protein | ref|YP_762648.1| PfWMP4_18 [cyanophage Pf-WMP4] | 95% (152/160) | 4 × 10−109 |
| 28 | 152 | endonuclease I | ref|YP_762647.1| PfWMP4_17 [cyanophage Pf-WMP4] | 94% (142/151) | 1 × 10−89 |
| 29 | 67 | hypothetical protein | ref|YP_762644.1| PfWMP4_14 [cyanophage Pf-WMP4] | 88% (59/67) | 8 × 10−38 |
| 30 | 461 | DNA primase/helicase | ref|YP_762642.1| DNA primase/helicase [cyanophage Pf-WMP4] | 99% (457/461) | 0.0 |
| 31 | 65 | hypothetical protein | ref|YP_762641.1| PfWMP4_11 [cyanophage Pf-WMP4] | 85% (53/62) | 5 × 10−31 |
| 32 | 167 | ribonuclease H | ref|YP_762640.1| PfWMP4_10 [cyanophage Pf-WMP4] | 77% (129/167) | 5 × 10−94 |
| 33 | 170 | hypothetical protein | ref|YP_762639.1| PfWMP4_09 [cyanophage Pf-WMP4] | 94% (159/170) | 6 × 10−113 |
| 34 | 95 | hypothetical protein | ref|YP_762638.1| PfWMP4_08 [cyanophage Pf-WMP4] | 90% (85/94) | 7 × 10−58 |
| 35 | 323 | hypothetical protein | ref|YP_762637.1| PfWMP4_07 [cyanophage Pf-WMP4] | 66% (189/288) | 7 × 10−126 |
| 36 | 104 | hypothetical protein | ref|YP_762636.1| PfWMP4_06 [cyanophage Pf-WMP4] | 79% (82/104) | 2 × 10−50 |
| 37 | 87 | hypothetical protein | no hit | ||
| 38 | 59 | hypothetical protein | no hit | ||
| 39 | 203 | hypothetical protein | ref|YP_762634.1| PfWMP4_04 [cyanophage Pf-WMP4] | 51% (103/202) | 5 × 10−58 |
| 40 | 159 | hypothetical protein | ref|YP_762633.1| PfWMP4_03 [cyanophage Pf-WMP4] | 93% (148/159) | 4 × 10−91 |
| 41 | 79 | hypothetical protein | no hit | ||
| 42 | 56 | hypothetical protein | ref|YP_762675.1| PfWMP4_45 [cyanophage Pf-WMP4] | 100% (56/56) | 3 × 10−24 |
| 43 | 87 | hypothetical protein | ref|YP_762674.1| PfWMP4_44 [cyanophage Pf-WMP4] | 99% (86/87) | 2 × 10−54 |
| 44 | 40 | hypothetical protein | no hit |
| Cyanophage Name | Head Diameter | Tail Length | Accession Number | Genome Length | G + C Content | Host Range |
|---|---|---|---|---|---|---|
| Lbo240-yong1 | 50 ± 5 nm | 20 ± 5 nm | OM_897575 | 39,740 bp | 51.99% | L.boryana FACHB-240 |
| Pf-WMP4 | 55 nm | N | DQ875742.1 | 40,938 bp | 51.8% | P. foveolarum Gom |
| A-4L | 50 nm | N | KF356198 | 41,750 bp | 43.4% | Anabaena sp. and Nostoc sp. |
| Aa-TR020 | 50 nm | N | MT457475.1 | 44,805 bp | 46% | A. africanum 1980/01 |
| Pf-WMP3 | 55 nm | N | NC_009551.1 | 43,249 bp | 46.49% | P. tenue and P. foveolarum |
| PP | 52 nm | N | NC_022751 | 42,480 bp | 46.41% | L. boryanum and P. foveolarum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Li, D.; Lin, W.; Pan, L.; Qian, M.; Wang, F.; Cai, R.; Qu, C.; Tong, Y. Genomic Analysis of a New Freshwater Cyanophage Lbo240-yong1 Suggests a New Taxonomic Family of Bacteriophages. Viruses 2023, 15, 831. https://doi.org/10.3390/v15040831
Zhou Q, Li D, Lin W, Pan L, Qian M, Wang F, Cai R, Qu C, Tong Y. Genomic Analysis of a New Freshwater Cyanophage Lbo240-yong1 Suggests a New Taxonomic Family of Bacteriophages. Viruses. 2023; 15(4):831. https://doi.org/10.3390/v15040831
Chicago/Turabian StyleZhou, Qin, Dengfeng Li, Wei Lin, Linting Pan, Minhua Qian, Fei Wang, Ruqian Cai, Chenxin Qu, and Yigang Tong. 2023. "Genomic Analysis of a New Freshwater Cyanophage Lbo240-yong1 Suggests a New Taxonomic Family of Bacteriophages" Viruses 15, no. 4: 831. https://doi.org/10.3390/v15040831
APA StyleZhou, Q., Li, D., Lin, W., Pan, L., Qian, M., Wang, F., Cai, R., Qu, C., & Tong, Y. (2023). Genomic Analysis of a New Freshwater Cyanophage Lbo240-yong1 Suggests a New Taxonomic Family of Bacteriophages. Viruses, 15(4), 831. https://doi.org/10.3390/v15040831






