Humoral and T Cell Immune Responses against SARS-CoV-2 after Primary and Homologous or Heterologous Booster Vaccinations and Breakthrough Infection: A Longitudinal Cohort Study in Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Plasma Isolation
2.3. Elecsys Anti-Spike and Anti-Nucleoprotein Immunoassay
2.4. Cells and Pseudoviruses
2.5. Pseudovirus Neutralization Assay
2.6. Interferon-γ Release Assay
2.7. Statistical Analysis
3. Results
3.1. Vaccinated Post-COVID-19 Subjects Demonstrated Higher Neutralizing Antibody with Longer Durability but Similar Decline in T Cell Responses Compared to Vaccinated Uninfected Subjects
3.2. The Homologous and Heterologous Booster Regime Induced Comparable Neutralizing Antibody and T Cell Responses
3.3. Low T Cell Responses upon Breakthrough Infection in the Homologous Booster Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.M.; Ciling, A.; Taha, T.Y.; Chen, I.P.; Khalid, M.M.; Sreekumar, B.; Chen, P.Y.; Kumar, G.R.; Suryawanshi, R.; Silva, I.; et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc. Natl. Acad. Sci. USA 2022, 119, e2200592119. [Google Scholar] [CrossRef]
- Muik, A.; Lui, B.G.; Wallisch, A.K.; Bacher, M.; Muhl, J.; Reinholz, J.; Ozhelvaci, O.; Beckmann, N.; Guimil Garcia, R.C.; Poran, A.; et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 2022, 375, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.X.; Durrant, L.G.; Chok, Y.L.; Lai, O.M. Susceptibility to SARS-CoV-2 omicron following ChAdOx1 nCoV-19 and BNT162b2 versus CoronaVac vaccination. iScience 2022, 25, 105379. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Naranbhai, V.; Nathan, A.; Kaseke, C.; Berrios, C.; Khatri, A.; Choi, S.; Getz, M.A.; Tano-Menka, R.; Ofoman, O.; Gayton, A.; et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell 2022, 185, 1041–1051.e6. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Schietzel, S.; Anderegg, M.; Limacher, A.; Born, A.; Horn, M.P.; Maurer, B.; Hirzel, C.; Sidler, D.; Moor, M.B. Humoral and cellular immune responses on SARS-CoV-2 vaccines in patients with anti-CD20 therapies: A systematic review and meta-analysis of 1342 patients. RMD Open 2022, 8, e002036. [Google Scholar] [CrossRef]
- Paniskaki, K.; Anft, M.; Meister, T.L.; Marheinecke, C.; Pfaender, S.; Skrzypczyk, S.; Seibert, F.S.; Thieme, C.J.; Konik, M.J.; Dolff, S.; et al. Immune response in moderate to critical breakthrough COVID-19 infection after mRNA vaccination. Front. Immunol. 2022, 13, 816220. [Google Scholar] [CrossRef]
- Yang, S.L.; Mat Ripen, A.; Leong, C.T.; Lee, J.V.; Yen, C.H.; Chand, A.K.; Koh, K.; Abdul Rahim, N.A.B.; Gokilavanan, V.; Mohamed, N.; et al. COVID-19 breakthrough infections and humoral immune response among BNT162b2 vaccinated healthcare workers in Malaysia. Emerg. Microbes Infect. 2022, 11, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.V.V.; Nguyet, L.A.; Dung, N.T.; Quang, V.M.; Truong, N.T.; Toan, L.M.; Hung, L.M.; Man, D.N.H.; Khoa, D.B.; Phong, N.T.; et al. Kinetics of neutralizing antibodies against Omicron variant in Vietnamese healthcare workers after primary immunization with ChAdOx1-S and booster immunization with BNT162b2. Am. J. Trop. Med. Hyg. 2023, 108, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Lim, S.M.; Jia, H.; Chen, M.W.; Ng, S.Y.; Gao, X.H.; Somani, J.; Sengupta, S.; Tay, D.M.Y.; Chua, P.W.L.; et al. Rapid evaluation of vaccine booster effectiveness against SARS-CoV-2 variants. Microbiol. Spectr. 2022, 10, e02257-22. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, H.; Ye, B.; Zhao, M.; Zhan, J.; Dong, S.; Guo, Y.; Zhao, Y.; Li, M.; Liu, S.; et al. One-year sustained cellular and humoral immunities in Coronavirus disease 2019 (COVID-19) convalescents. Clin. Infect. Dis. 2022, 75, e1072–e1081. [Google Scholar] [CrossRef]
- COVIDNOW. Vaccinations in Malaysia. Available online: https://covidnow.moh.gov.my/vaccinations (accessed on 31 December 2022).
- Anand, R. Malaysia to start administering COVID-19 booster shots. The Straits Times, 19 September 2021. [Google Scholar]
- KKMNOW. The Latest Data on the National COVID-19 Immunisation Program. Available online: https://data.moh.gov.my/covid-vaccination (accessed on 31 January 2023).
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; Gonzalez, C.; Pizarro, A.; Acevedo, J.; Leo, K.; Paredes, F.; Bralic, T.; Vergara, V.; et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: A large-scale prospective cohort study. Lancet Glob. Health 2022, 10, e798–e806. [Google Scholar] [CrossRef]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-match COVID-19 vaccinations (heterologous boost): A review. Infect. Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Khairy: Pfizer Preferred COVID-19 Booster for All, AstraZeneca Alternative. Available online: https://codeblue.galencentre.org/2021/12/01/khairy-pfizer-preferred-covid-19-booster-for-all-astrazeneca-alternative/ (accessed on 5 October 2022).
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Vogel, E.; Kocher, K.; Priller, A.; Cheng, C.C.; Steininger, P.; Liao, B.H.; Korber, N.; Willmann, A.; Irrgang, P.; Held, J.; et al. Dynamics of humoral and cellular immune responses after homologous and heterologous SARS-CoV-2 vaccination with ChAdOx1 nCoV-19 and BNT162b2. EBioMedicine 2022, 85, 104294. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Franchini, M.; Joyner, M.J.; Casadevall, A.; Focosi, D. Analysis of anti-SARS-CoV-2 Omicron-neutralizing antibody titers in different vaccinated and unvaccinated convalescent plasma sources. Nat. Commun. 2022, 13, 6478. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Kronsteiner, B.; Longet, S.; Adele, S.; Deeks, A.S.; Liu, C.; Dejnirattisai, W.; Silva Reyes, L.; Meardon, N.; Faustini, S.; et al. Evolution of long-term hybrid immunity in healthcare workers after different COVID-19 vaccination regimens: A longitudinal observational cohort study. medRxiv 2022. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Muecksch, F.; Weisblum, Y.; Barnes, C.O.; Schmidt, F.; Schaefer-Babajew, D.; Lorenzi, J.C.C.; Flyak, A.I.; DeLaitsch, A.T.; Huey-Tubman, K.E.; Hou, S.; et al. Development of potency, breadth and resilience to viral escape mutations in SARS-CoV-2 neutralizing antibodies. bioRxiv 2021. [Google Scholar] [CrossRef]
- Andreano, E.; Paciello, I.; Pierleoni, G.; Piccini, G.; Abbiento, V.; Antonelli, G.; Pileri, P.; Manganaro, N.; Pantano, E.; Maccari, G.; et al. COVID-19 mRNA third dose induces a unique hybrid immunity-like antibody response. bioRxiv 2022. [Google Scholar] [CrossRef]
- Andreano, E.; Paciello, I.; Piccini, G.; Manganaro, N.; Pileri, P.; Hyseni, I.; Leonardi, M.; Pantano, E.; Abbiento, V.; Benincasa, L.; et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature 2021, 600, 530–535. [Google Scholar] [CrossRef]
- Bates, T.A.; McBride, S.K.; Leier, H.C.; Guzman, G.; Lyski, Z.L.; Schoen, D.; Winders, B.; Lee, J.Y.; Lee, D.X.; Messer, W.B.; et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 2022, 7, eabn8014. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-Garcia, J.; et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pohlmann, S.; et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2022, 75, 103761. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Gram, M.A.; Emborg, H.D.; Schelde, A.B.; Friis, N.U.; Nielsen, K.F.; Moustsen-Helms, I.R.; Legarth, R.; Lam, J.U.H.; Chaine, M.; Malik, A.Z.; et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022, 19, e1003992. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Jaganathan, S.; Stieber, F.; Rao, S.N.; Nikolayevskyy, V.; Manissero, D.; Allen, N.; Boyle, J.; Howard, J. Preliminary evaluation of QuantiFERON SARS-CoV-2 and QIAreach anti-SARS-CoV-2 total test in recently vaccinated individuals. Infect. Dis. Ther. 2021, 10, 2765–2776. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell. Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- Nikolova, M.; Todorova, Y.; Emilova, R.; Trifonova, I.; Gladnishka, T.; Petrova-Yancheva, N.; Chervenyakova, T.; Dragusheva, E.; Popov, G.; Christova, I. Induction of humoral and cellular immune responses to COVID-19 mRNA and vector vaccines: A prospective cohort study in Bulgarian healthcare workers. J. Med. Virol. 2022, 94, 2008–2018. [Google Scholar] [CrossRef]
- Maringer, Y.; Nelde, A.; Schroeder, S.M.; Schuhmacher, J.; Horber, S.; Peter, A.; Karbach, J.; Jager, E.; Walz, J.S. Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 2022, 7, eadd3899. [Google Scholar] [CrossRef]
- Behrens, G.M.N.; Barros-Martins, J.; Cossmann, A.; Ramos, G.M.; Stankov, M.V.; Odak, I.; Dopfer-Jablonka, A.; Hetzel, L.; Kohler, M.; Patzer, G.; et al. BNT162b2-boosted immune responses six months after heterologous or homologous ChAdOx1nCoV-19/BNT162b2 vaccination against COVID-19. Nat. Commun. 2022, 13, 4872. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Cheung, P.P. Effectiveness of heterologous and homologous covid-19 vaccine regimens: Living systematic review with network meta-analysis. BMJ 2022, 377, e069989. [Google Scholar] [CrossRef] [PubMed]
- Almendro-Vazquez, P.; Chivite-Lacaba, M.; Utrero-Rico, A.; Gonzalez-Cuadrado, C.; Laguna-Goya, R.; Moreno-Batanero, M.; Sanchez-Paz, L.; Luczkowiak, J.; Labiod, N.; Folgueira, M.D.; et al. Cellular and humoral immune responses and breakthrough infections after three SARS-CoV-2 mRNA vaccine doses. Front. Immunol. 2022, 13, 981350. [Google Scholar] [CrossRef]
- Yang, S.L.; Teh, H.S.; Suah, J.L.; Husin, M.; Hwong, W.Y. SARS-CoV-2 in Malaysia: A surge of reinfection during the predominantly Omicron period. Lancet Reg. Health West Pac. 2022, 26, 100572. [Google Scholar] [CrossRef]
- Pusnik, J.; Monzon-Posadas, W.O.; Zorn, J.; Peters, K.; Baum, M.; Proksch, H.; Schluter, C.B.; Alter, G.; Menting, T.; Streeck, H. SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun. 2023, 14, 572. [Google Scholar] [CrossRef]
- Bertoletti, A.; Tan, A.T.; Le Bert, N. The T-cell response to SARS-CoV-2: Kinetic and quantitative aspects and the case for their protective role. Oxf. Open Immunol. 2021, 2, iqab006. [Google Scholar] [CrossRef]
- Faas, M.R.; Mak, W.A.; Markus, H.Y.; van der Zwan, E.M.; van der Vliet, M.; Koeleman, J.G.M.; Ong, D.S.Y. Dynamics of antibody and T cell immunity against SARS-CoV-2 variants of concern and the impact of booster vccinations in previously infected and infection positive individuals. Vaccines 2022, 10, 2132. [Google Scholar] [CrossRef]
- Molodtsov, I.A.; Kegeles, E.; Mitin, A.N.; Mityaeva, O.; Musatova, O.E.; Panova, A.E.; Pashenkov, M.V.; Peshkova, I.O.; Alsalloum, A.; Asaad, W.; et al. Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2)-specific T cells and antibodies in Coronavirus Disease 2019 (COVID-19) protection: A prospective study. Clin. Infect. Dis. 2022, 75, e1–e9. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.; Zens, K.D.; Ballouz, T.; Caduff, N.; Llanas-Cornejo, D.; Aschmann, H.E.; Domenghino, A.; Pellaton, C.; Perreau, M.; Fenwick, C.; et al. Heterogenous humoral and cellular immune responses with distinct trajectories post-SARS-CoV-2 infection in a population-based cohort. Nat. Commun. 2022, 13, 4855. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Phillips, E.; Adele, S.; Longet, S.; Malone, T.; Mason, C.; Stafford, L.; Jamsen, A.; Gardiner, S.; Deeks, A.; et al. Evaluation of QuantiFERON SARS-CoV-2 interferon-γ release assay following SARS-CoV-2 infection and vaccination. Clin. Exp. Immunol. 2022, uxad027. [Google Scholar] [CrossRef]
- Tomic, A.; Skelly, D.T.; Ogbe, A.; O’Connor, D.; Pace, M.; Adland, E.; Alexander, F.; Ali, M.; Allott, K.; Azim Ansari, M.; et al. Divergent trajectories of antiviral memory after SARS-CoV-2 infection. Nat. Commun. 2022, 13, 1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Galvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
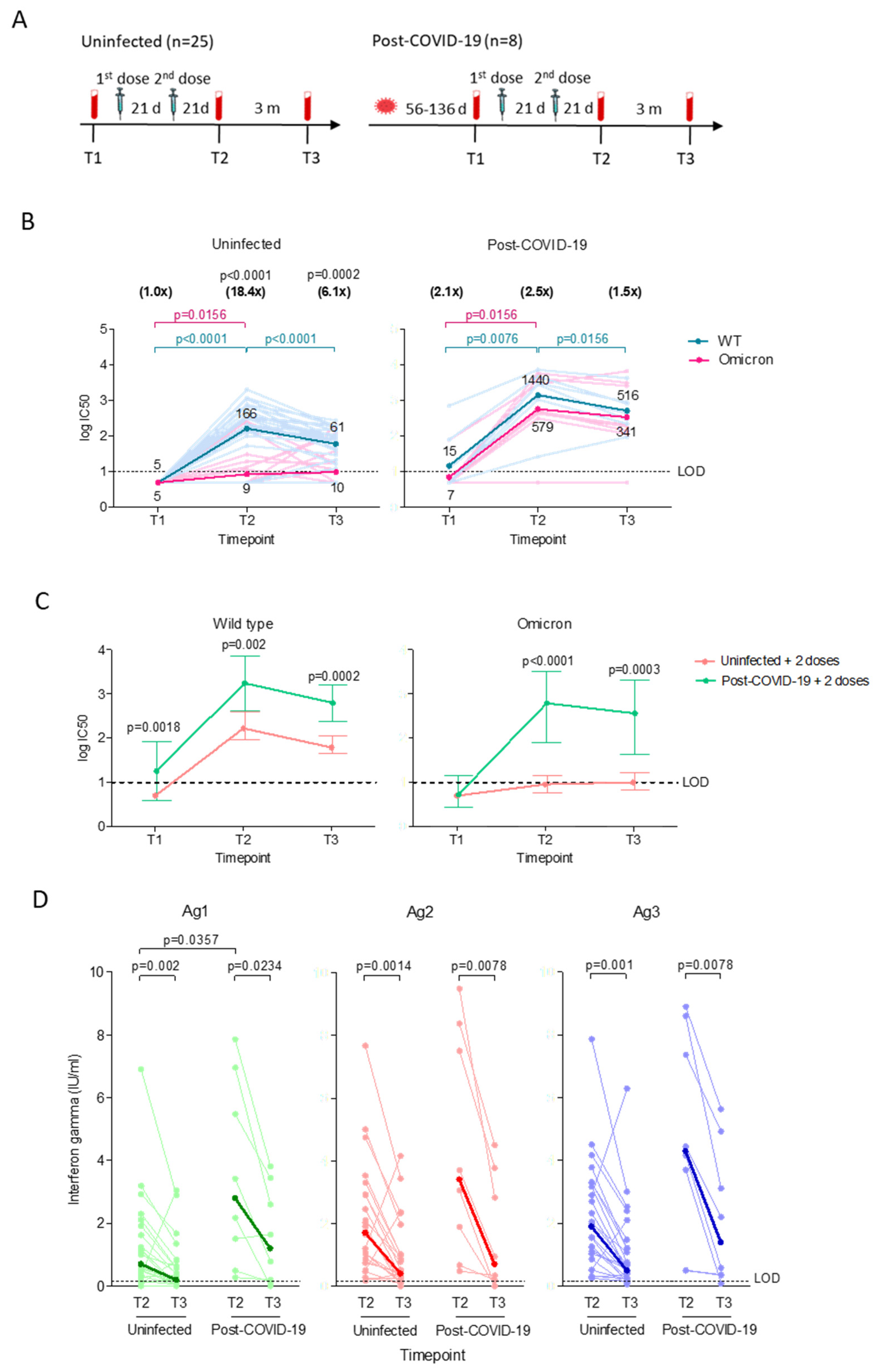
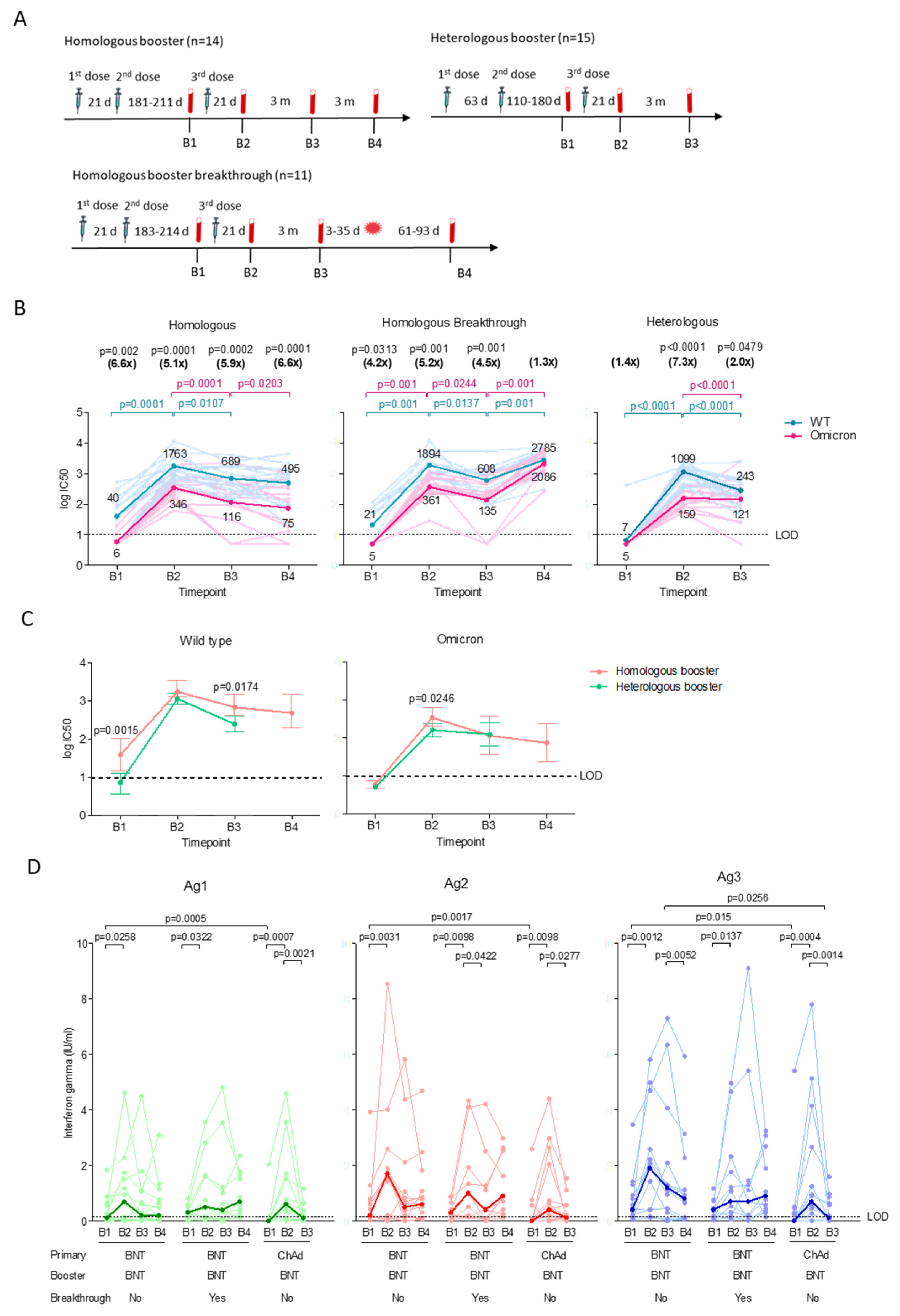
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.Y.L.; Pukhari, M.H.; Bador, M.K.; Sam, I.-C.; Chan, Y.F. Humoral and T Cell Immune Responses against SARS-CoV-2 after Primary and Homologous or Heterologous Booster Vaccinations and Breakthrough Infection: A Longitudinal Cohort Study in Malaysia. Viruses 2023, 15, 844. https://doi.org/10.3390/v15040844
Fu JYL, Pukhari MH, Bador MK, Sam I-C, Chan YF. Humoral and T Cell Immune Responses against SARS-CoV-2 after Primary and Homologous or Heterologous Booster Vaccinations and Breakthrough Infection: A Longitudinal Cohort Study in Malaysia. Viruses. 2023; 15(4):844. https://doi.org/10.3390/v15040844
Chicago/Turabian StyleFu, Jolene Yin Ling, Muhammad Harith Pukhari, Maria Kahar Bador, I-Ching Sam, and Yoke Fun Chan. 2023. "Humoral and T Cell Immune Responses against SARS-CoV-2 after Primary and Homologous or Heterologous Booster Vaccinations and Breakthrough Infection: A Longitudinal Cohort Study in Malaysia" Viruses 15, no. 4: 844. https://doi.org/10.3390/v15040844





