Differences in Longevity and Temperature-Driven Extrinsic Incubation Period Correlate with Varying Dengue Risk in the Arizona–Sonora Desert Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Sites
2.2. Climate and Dengue Case Data
2.3. Mosquito Sampling
2.4. Mosquito Parity and Transcription Assessment
2.5. Mosquito Blood Meal Analysis
2.6. Statistical Methods
3. Results
3.1. Mosquito Density and Parity
3.2. Dengue Incidence
3.3. Mosquito Blood Meal Analysis
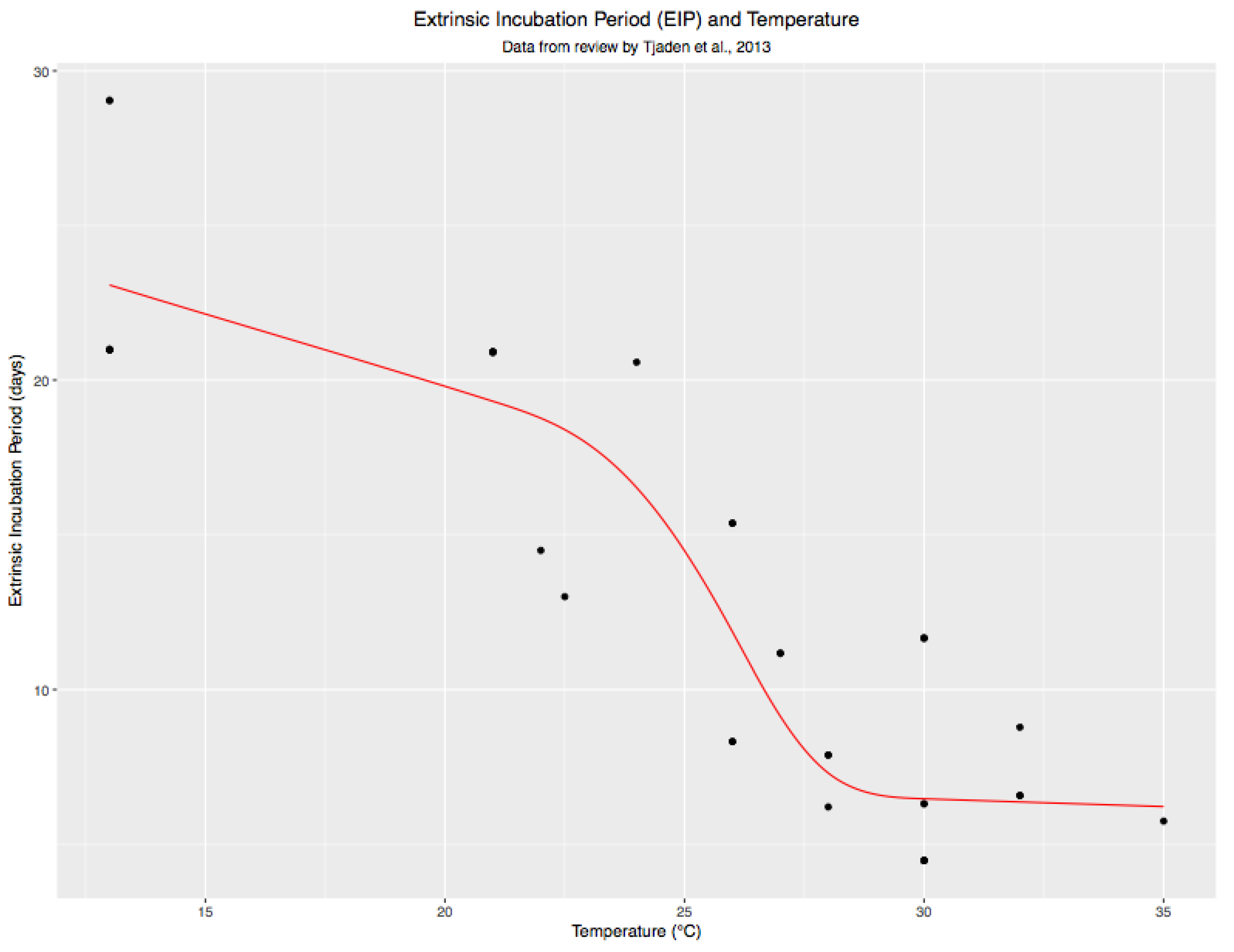
3.4. Variations in Average EIP by City and Time Period
3.5. Differences between City-Level Ae. Aegypti Age
3.6. Proportion of Mosquitoes That Survived Past the EIP
3.7. Estimated Potential Vectors Per Trap Night
3.8. Differences in Vector Potential (IRR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Soper, F.L. Dynamics of Aedes aegypti distribution and density. Seasonal fluctuations in the Americas. Bull World Health Organ 1967, 36, 536–538. [Google Scholar] [PubMed]
- Christophers, S.R. Aëdes aegypti (L.), the Yellow Fever Mosquito: Its Life History, Bionomics, and Structure; University Press: Cambridge, UK, 1960; p. 11. [Google Scholar]
- Lima, A.; Lovin, D.D.; Hickner, P.V.; Severson, D.W. Evidence for an Overwintering Population of Aedes aegypti in Capitol Hill Neighborhood, Washington, DC. Am. J. Trop. Med. Hyg. 2016, 94, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Liu-Helmersson, J.; Brannstrom, A.; Sewe, M.O.; Semenza, J.C.; Rocklov, J. Estimating Past, Present, and Future Trends in the Global Distribution and Abundance of the Arbovirus Vector Aedes aegypti under Climate Change Scenarios. Front. Public Health 2019, 7, 148. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.F.; Juarez, J.G.; Kraemer, M.U.G.; Messina, J.P.; Hamer, G.L. Global patterns of aegyptism without arbovirus. PLoS Negl. Trop. Dis. 2021, 15, e0009397. [Google Scholar] [CrossRef]
- Macdonald, G. Epidemiological basis of malaria control. Bull World Health Organ 1956, 15, 613–626. [Google Scholar]
- Brady, O.J.; Godfray, H.C.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; McKenzie, F.E.; Perkins, T.A.; Reiner, R.C., Jr.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef]
- Focks, D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. Special Program for Research and Training in Tropical Diseases (TDR); World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef]
- Scott, T.W.; Morrison, A.C. Vector dynamics and transmission of dengue virus: Implications for dengue surveillance and prevention strategies: Vector dynamics and dengue prevention. Curr. Top. Microbiol. Immunol. 2010, 338, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Sivagnaname, N.; Gunasekaran, K. Need for an efficient adult trap for the surveillance of dengue vectors. Indian J. Med. Res. 2012, 136, 739–749. [Google Scholar] [PubMed]
- CDC. Surveillance and Control of Aedes Aegypti and Aedes Albopictus in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Kuno, G. Factors influencing the transmission of dengue viruses. In Dengue and Dengue Hemorrhagic Fever; Gubler, D.J., Kuno, G., Eds.; CAB International: New York, NY, USA, 1997; pp. 61–88. [Google Scholar]
- Olson, M.F.; Ndeffo-Mbah, M.L.; Juarez, J.G.; Garcia-Luna, S.; Martin, E.; Borucki, M.K.; Frank, M.; Estrada-Franco, J.G.; Rodriguez-Perez, M.A.; Fernandez-Santos, N.A.; et al. High Rate of Non-Human Feeding by Aedes aegypti Reduces Zika Virus Transmission in South Texas. Viruses 2020, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, M. The effect of temperature on the growth rate and survival of the immature stages of Aedes aegypti (L.). Bull. Entomol. Res. 1958, 49, 157–163. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Comeau, G.; Monaghan, A.J.; Williamson, D.J.; Ernst, K.C. Effects of desiccation stress on adult female longevity in Aedes aegypti and Ae. albopictus (Diptera: Culicidae): Results of a systematic review and pooled survival analysis. Parasit. Vectors 2018, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Johansson, M.A. The incubation periods of Dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef]
- Carrington, L.B.; Armijos, M.V.; Lambrechts, L.; Scott, T.W. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl. Trop. Dis. 2013, 7, e2190. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Apfeldorf, K.M.; Dobson, S.; Devita, J.; Buczak, A.L.; Baugher, B.; Moniz, L.J.; Bagley, T.; Babin, S.M.; Guven, E.; et al. An open challenge to advance probabilistic forecasting for dengue epidemics. Proc. Natl. Acad. Sci. USA 2019, 116, 24268–24274. [Google Scholar] [CrossRef]
- Hussain-Alkhateeb, L.; Rivera Ramirez, T.; Kroeger, A.; Gozzer, E.; Runge-Ranzinger, S. Early warning systems (EWSs) for chikungunya, dengue, malaria, yellow fever, and Zika outbreaks: What is the evidence? A scoping review. PLoS Negl. Trop. Dis. 2021, 15, e0009686. [Google Scholar] [CrossRef]
- Baharom, M.; Ahmad, N.; Hod, R.; Abdul Manaf, M.R. Dengue Early Warning System as Outbreak Prediction Tool: A Systematic Review. Risk Manag. Healthc. Policy 2022, 15, 871–886. [Google Scholar] [CrossRef]
- Kamiya, T.; Greischar, M.A.; Wadhawan, K.; Gilbert, B.; Paaijmans, K.; Mideo, N. Temperature-dependent variation in the extrinsic incubation period elevates the risk of vector-borne disease emergence. Epidemics 2019, 30, 100382. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.K.; Jeffrey Gutierrez, E.H.; Ernst, K.; Walker, K.R.; Carriere, Y.; Torabi, M.; Riehle, M.A. Aging field collected Aedes aegypti to determine their capacity for dengue transmission in the southwestern United States. PLoS ONE 2012, 7, e46946. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.C.; Walker, K.R.; Reyes-Castro, P.; Joy, T.K.; Castro-Luque, A.L.; Diaz-Caravantes, R.E.; Gameros, M.; Haenchen, S.; Hayden, M.H.; Monaghan, A.; et al. Aedes aegypti (Diptera: Culicidae) Longevity and Differential Emergence of Dengue Fever in Two Cities in Sonora, Mexico. J. Med. Entomol. 2017, 54, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Watthanaworawit, W.; Turner, P.; Turner, C.L.; Tanganuchitcharnchai, A.; Jarman, R.G.; Blacksell, S.D.; Nosten, F.H. A prospective evaluation of diagnostic methodologies for the acute diagnosis of dengue virus infection on the Thailand-Myanmar border. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Long, S.A.; Russell, R.C.; Ritchie, S.A. Field efficacy of the BG-Sentinel compared with CDC Backpack Aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J. Am. Mosq. Control Assoc. 2006, 22, 296–300. [Google Scholar] [CrossRef]
- Joy, T.; Chen, M.; Arnbrister, J.; Williamson, D.; Li, S.; Nair, S.; Brophy, M.; Garcia, V.M.; Walker, K.; Ernst, K.; et al. Assessing Near-Infrared Spectroscopy (NIRS) for Evaluation of Aedes aegypti Population Age Structure. Insects 2022, 13, 360. [Google Scholar] [CrossRef]
- Harrell, F.E. Rms: Regression Modeling Strategies 4.5-0. Available online: https://github.com/harrelfe/rmsAccessed3/5/2023 (accessed on 23 February 2023).
- Davis, E.E. Development of lactic acid-receptor sensitivity and host-seeking behaviour in newly emerged female Aedes aegypti mosquitoes. J. Insect Physiol. 1984, 30, 211–215. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. Package ‘mass’. Cran R 2013, 538, 113–120. [Google Scholar]
- Scott, T.W.; Morrison, A.C.; Takken, W.C. Aedes aegypti density and the risk of dengue virus transmission. Ecol. Asp. Appl. Genet. Modif. Mosq. 2003, 2, 187–206. [Google Scholar]
- Honorio, N.A.; Nogueira, R.M.; Codeco, C.T.; Carvalho, M.S.; Cruz, O.G.; Magalhaes Mde, A.; de Araujo, J.M.; de Araujo, E.S.; Gomes, M.Q.; Pinheiro, L.S.; et al. Spatial evaluation and modeling of Dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2009, 3, e545. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Figueroa, L.; Rigau-Perez, J.G.; Suarez, E.L.; Reiter, P. Risk factors for dengue infection during an outbreak in Yanes, Puerto Rico in 1991. Am. J. Trop. Med. Hyg. 1995, 52, 496–502. [Google Scholar] [CrossRef]
- Ong, J.; Aik, J.; Ng, L.C. Short Report: Adult Aedes abundance and risk of dengue transmission. PLoS Negl. Trop. Dis. 2021, 15, e0009475. [Google Scholar] [CrossRef] [PubMed]
- Norman, L.M.; Caldeira, F.; Callegary, J.; Gray, F.; O’Rourke, M.K.; Meranza, V.; Van Rijn, S. Socio-environmental health analysis in Nogales, Sonora, Mexico. Water Qual. Expo. Health 2012, 4, 79–91. [Google Scholar] [CrossRef]
- Robison, A.; Young, M.C.; Byas, A.D.; Ruckert, C.; Ebel, G.D. Comparison of Chikungunya Virus and Zika Virus Replication and Transmission Dynamics in Aedes aegypti Mosquitoes. Am. J. Trop. Med. Hyg. 2020, 103, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Brunkard, J.M.; Robles Lopez, J.L.; Ramirez, J.; Cifuentes, E.; Rothenberg, S.J.; Hunsperger, E.A.; Moore, C.G.; Brussolo, R.M.; Villarreal, N.A.; Haddad, B.M. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg. Infect. Dis. 2007, 13, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P.; Lathrop, S.; Bunning, M.; Biggerstaff, B.; Singer, D.; Tiwari, T.; Baber, L.; Amador, M.; Thirion, J.; Hayes, J.; et al. Texas lifestyle limits transmission of dengue virus. Emerg. Infect. Dis. 2003, 9, 86–89. [Google Scholar] [CrossRef]
- Ruberto, I.; Yaglom, H.; Erhart, L.M.; Plante, L.; Weiss, J.; Golenko, C.; Casal, M.; McCotter, O.; Adams, L.; Ernst, K.; et al. Dengue Knowledge, Attitudes, and Practices Among Arizona Health Care Providers, 2014–2015. Vector Borne Zoonotic Dis. 2019, 19, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.C.; Brown, H.E. Estimating Aedes aegypti (Diptera: Culicidae) Flight Distance: Meta-Data Analysis. J. Med. Entomol. 2022, 59, 1164–1170. [Google Scholar] [CrossRef]
- Reyes-Castro, P.A.; Harris, R.B.; Brown, H.E.; Christopherson, G.L.; Ernst, K.C. Spatio-temporal and neighborhood characteristics of two dengue outbreaks in two arid cities of Mexico. Acta Trop. 2017, 167, 174–182. [Google Scholar] [CrossRef]
- Strickman, D. Longevity of Aedes aegypti (Diptera: Culicidae) compared in cages and field under ambient conditions in rural Thailand. Southeast Asian J. Trop. Med. Public Health 2006, 37, 456–462. [Google Scholar]
- Maciel-de-Freitas, R.; Eiras, A.E.; Lourenco-de-Oliveira, R. Calculating the survival rate and estimated population density of gravid Aedes aegypti (Diptera, Culicidae) in Rio de Janeiro, Brazil. Cad Saude Publica 2008, 24, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Hugo, L.E.; Jeffery, J.A.; Trewin, B.J.; Wockner, L.F.; Nguyen, T.Y.; Nguyen, H.L.; Nghia le, T.; Hine, E.; Ryan, P.A.; Kay, B.H. Adult survivorship of the dengue mosquito Aedes aegypti varies seasonally in central Vietnam. PLoS Negl. Trop. Dis. 2014, 8, e2669. [Google Scholar] [CrossRef] [PubMed]
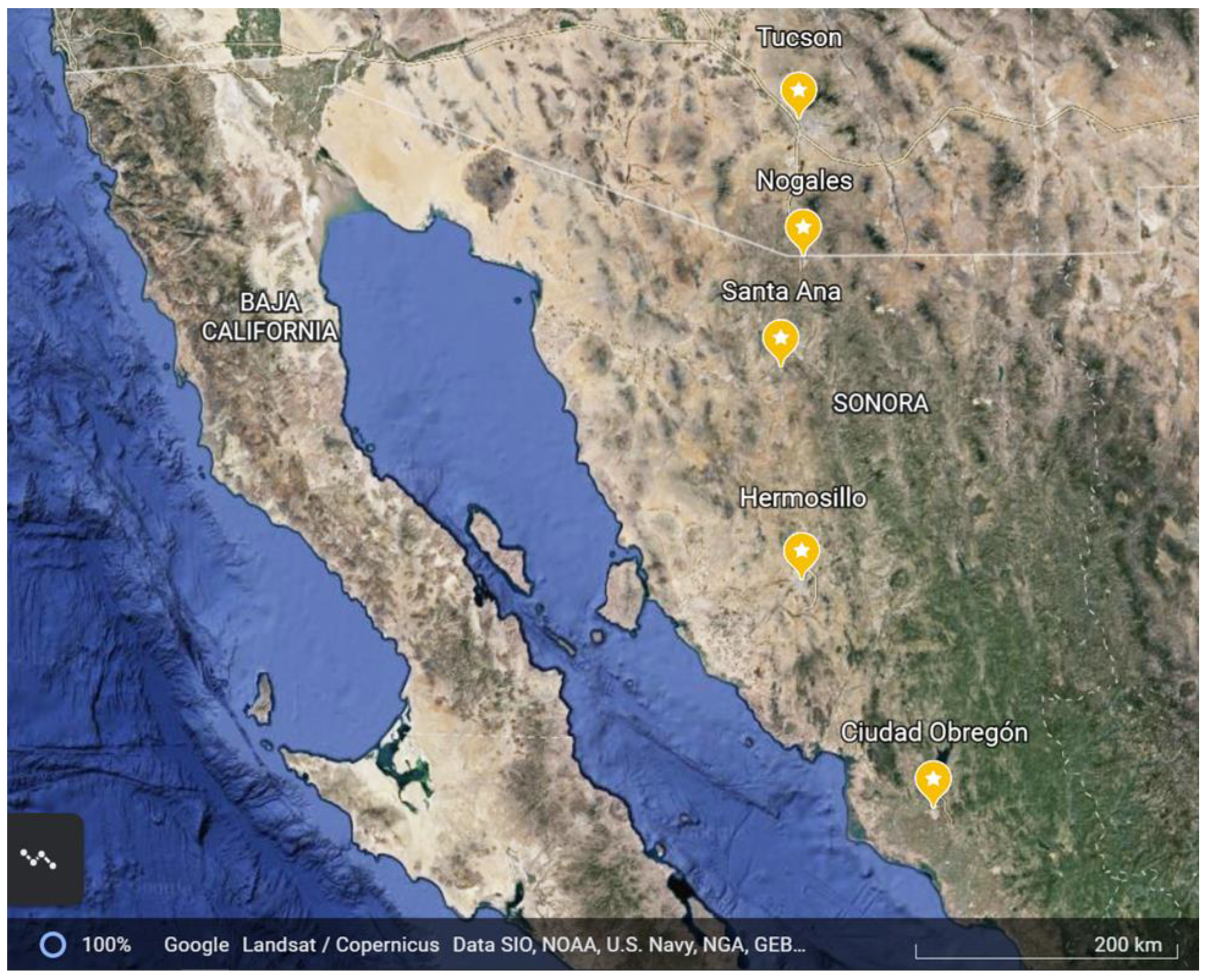




| City | Population 1 | Elevation (m Above Sea Level) | Average 2 Rainfall (mm) | Average High/Low July Temperature (°C) | Average High/Low August Temperature (°C) | Average High/Low September Temperature (°C) |
|---|---|---|---|---|---|---|
| Tucson, AZ | 520,561 | 807 | 294 | 38.3/24.2 | 33.3/23.9 | 35.0/21.7 |
| Nogales, AZ | 19,766 | 1167 | 460 | 34.4/19.4 | 36.1/16.7 | 31.1/11.1 |
| Heroica Heroica Nogales, SN | 212,533 | 1214 | 455 | 34.1/17.0 | 33.5/19.3 | 32.1/18.5 |
| Santa Ana, SN | 11,864 | 687 | 327 | 39.0/19.0 | 38.0/21.4 | 36.8/21.0 |
| Hermosillo, SN | 715,061 | 210 | 387 | 39.8/23.8 | 38.3/25.6 | 38.3/25.6 |
| Ciudad Obregon, SN | 298,625 | 40 | 223 | 38.3/23.3 | 38.5/25.5 | 38.4/25.2 |
| Cases per 100,000—Confirmed | Cases per 100,000—Suspected | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heroica Nogales, SN | Santa Ana, SN | Hermosillo, SN | Obregon, SN | Heroica Nogales, SN | Santa Ana, SN | Hermosillo, SN | Obregon, SN | |||
| 2013 July | 0.00 | 0.24 | 13 July | 0.00 | 1.22 | |||||
| 2013 August | 0.00 | 0.12 | 13 August | 0.00 | 1.22 | |||||
| 2013 September | 0.00 | 0.98 | 13 September | 0.00 | 2.93 | |||||
| 2013 October | 0.46 | 5.12 | 13 October | 0.91 | 14.63 | |||||
| 2014 July | 0.46 | 0.49 | 14 July | 0.91 | 1.34 | |||||
| 2014 August | 0.00 | 1.95 | 14 August | 0.00 | 2.80 | |||||
| 2014 September | 0.91 | 4.63 | 14 September | 1.37 | 18.54 | |||||
| 2014 October | 4.56 | 79.02 | 14 October | 4.56 | 195.61 | |||||
| 2015 July | 0.00 | 0 | 0.98 | 1.85 | 15 July | 0.00 | 0 | 1.59 | 2.46 | |
| 2015 August | 0.00 | 0 | 1.83 | 1.85 | 15 August | 0.46 | 0 | 6.22 | 9.23 | |
| 2015 September | 0.46 | 0 | 12.32 | 4.62 | 15 September | 0.91 | 0 | 43.05 | 9.23 | |
| 2015 October | 1.37 | 6.15 | 57.32 | 15.39 | 15 October | 2.28 | 6.15 | 120.73 | 22.16 | |
| Year | City | Total N | Age of Parous Mosquitoes, in Days, Median (Q1, Q3) | p-Value (Age) a | EIP + 2.63 Days, Median (Q1, Q3) | p-Value (EIP) b | Prob. Exceeds EIP, Median (Q1, Q3) | No. Potential Vectors/Trap/Day | IRR (95% CI) c |
|---|---|---|---|---|---|---|---|---|---|
| 2013 | Heroica Nogales, SN | 370 | 5.6 (4.0, 8.5) | Ref. | 15.6 (13.1, 22.0) | Ref. | 0.01 (0.00, 0.12) | 0.2 | Ref. |
| Tucson * | - | - | - | - | - | - | - | - | |
| Nogales, AZ | 182 | 6.5 (4.2, 12.6) | 0.11 | 16.8 (12.9, 21.1) | 0.86 | 0.03 (0.00, 0.27) | 0.4 | 2.1 (0.9, 4.9) | |
| Hermosillo | 599 | 6.9 (4.3, 12.8) | <0.001 | 9.4 (9.1, 10.0) | <0.001 | 0.24 (0.04, 0.75) | 0.8 | 4.0 (2.3, 7.2) | |
| 2014 | Heroica Nogales, SN | 586 | 7.8 (5.3, 11.8) | Ref. | 20.2 (15.4, 28.6) | Ref. | 0.01 (0.00, 0.17) | 0.25 | Ref. |
| Tucson | 1665 | 10.1 (5.6, 14.4) | <0.01 | 9.9 (9.4, 12.0) | <0.001 | 0.51 (0.11, 0.77) | 2.12 | 8.4 (5.0, 14.3) | |
| Nogales, AZ | 200 | 5.0 (3.9, 8.6) | <0.001 | 18.6 (15.4, 31.1) | 0.55 | 0.00 (0.00, 0.02) | 0.07 | 0.3 (0.1, 0.8) | |
| Hermosillo | 931 | 7.3 (4.7, 12.4) | 0.47 | 10.3 (10.0, 10.8) | <0.001 | 0.21 (0.03, 0.68) | 0.43 | 1.8 (1.1, 2.9) | |
| 2015 | Heroica Nogales, SN | 417 | 6.4 (4.4, 10.5) | Ref. | 12.9 (12.6, 19.6) | Ref. | 0.02 (0.00, 0.19) | 0.2 | Ref. |
| Tucson | 1344 | 5.9 (4.1, 9.7) | 0.15 | 9.7 (9.3, 10.6) | <0.001 | 0.13 (0.03, 0.49) | 1.1 | 5.1 (3.3, 8.0) | |
| Nogales, AZ | 220 | 4.6 (3.8, 6.8) | <0.001 | 12.7 (12.1, 20.1) | 0.1 | 0.00 (0.00, 0.05) | 0.1 | 0.4 (0.2, 1.1) | |
| Santa Ana | 500 | 5.0 (4.2, 7.7) | 0.01 | 10.1 (9.8, 11.1) | <0.001 | 0.06 (0.02, 0.24) | 0.4 | 1.5 (0.9, 2.6) | |
| Hermosillo | 469 | 5.8 (4.2, 9.7) | 0.34 | 9.7 (9.1, 10.7) | <0.001 | 0.13 (0.02, 0.50) | 0.4 | 1.9 (1.2, 3.1) | |
| Obregon | 674 | 5.3 (4.2, 8.4) | 0.01 | 9.6 (9.3, 9.9) | <0.001 | 0.08 (0.03, 0.36) | 0.6 | 2.7 (1.7, 4.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, K.C.; Walker, K.R.; Castro-Luque, A.L.; Schmidt, C.; Joy, T.K.; Brophy, M.; Reyes-Castro, P.; Díaz-Caravantes, R.E.; Encinas, V.O.; Aguilera, A.; et al. Differences in Longevity and Temperature-Driven Extrinsic Incubation Period Correlate with Varying Dengue Risk in the Arizona–Sonora Desert Region. Viruses 2023, 15, 851. https://doi.org/10.3390/v15040851
Ernst KC, Walker KR, Castro-Luque AL, Schmidt C, Joy TK, Brophy M, Reyes-Castro P, Díaz-Caravantes RE, Encinas VO, Aguilera A, et al. Differences in Longevity and Temperature-Driven Extrinsic Incubation Period Correlate with Varying Dengue Risk in the Arizona–Sonora Desert Region. Viruses. 2023; 15(4):851. https://doi.org/10.3390/v15040851
Chicago/Turabian StyleErnst, Kacey C., Kathleen R. Walker, A Lucia Castro-Luque, Chris Schmidt, Teresa K. Joy, Maureen Brophy, Pablo Reyes-Castro, Rolando Enrique Díaz-Caravantes, Veronica Ortiz Encinas, Alfonso Aguilera, and et al. 2023. "Differences in Longevity and Temperature-Driven Extrinsic Incubation Period Correlate with Varying Dengue Risk in the Arizona–Sonora Desert Region" Viruses 15, no. 4: 851. https://doi.org/10.3390/v15040851
APA StyleErnst, K. C., Walker, K. R., Castro-Luque, A. L., Schmidt, C., Joy, T. K., Brophy, M., Reyes-Castro, P., Díaz-Caravantes, R. E., Encinas, V. O., Aguilera, A., Gameros, M., Cuevas Ruiz, R. E., Hayden, M. H., Alvarez, G., Monaghan, A., Williamson, D., Arnbrister, J., Gutiérrez, E. J., Carrière, Y., & Riehle, M. A. (2023). Differences in Longevity and Temperature-Driven Extrinsic Incubation Period Correlate with Varying Dengue Risk in the Arizona–Sonora Desert Region. Viruses, 15(4), 851. https://doi.org/10.3390/v15040851








