Inhibition of Cytomegalovirus by Pentacta pygmaea Fucosylated Chondroitin Sulfate Depends on Its Molecular Weight
Abstract
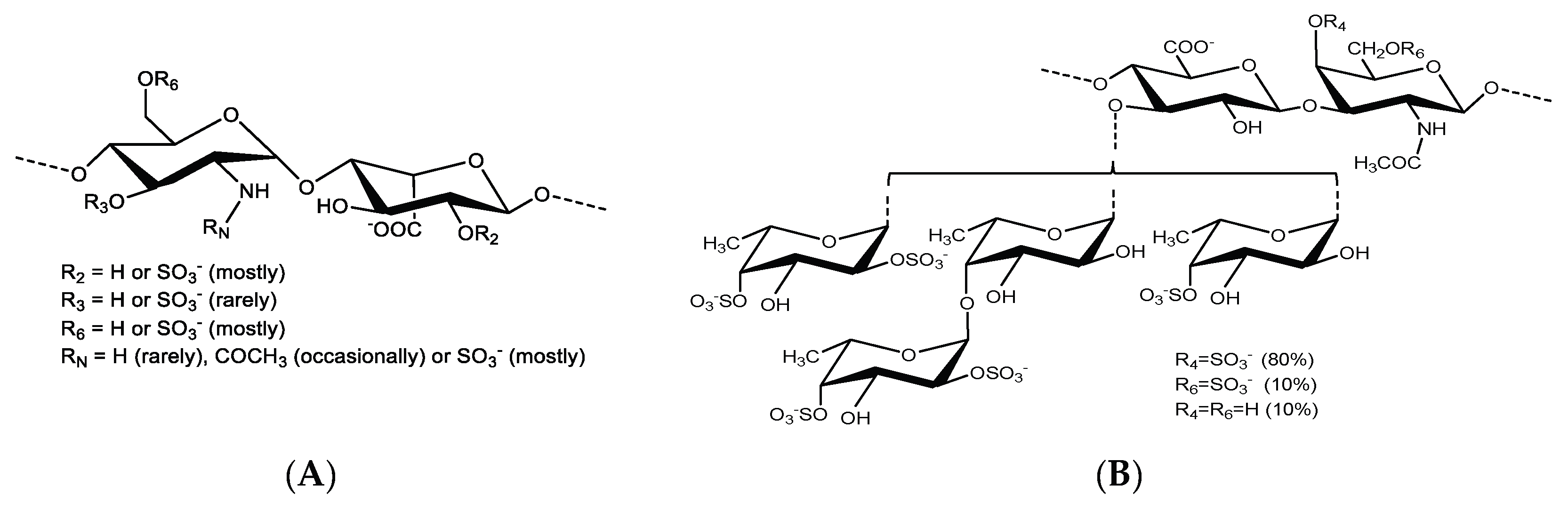
:1. Introduction
2. Materials and Methods
3. Results
3.1. Inhibition of HCMV by PpFucCS and Its Fractions
3.2. PpFucCS Specifically Inhibits HCMV Entry into Cells
3.3. Effect of PpFucCS and Its Oligosaccharides Treatment on Cell Viability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mocarski, E.S.J.; Shenk, T.; Pass, R.F. Fields Virology, 5th ed.; Wilkins, L.W., Ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2006. [Google Scholar]
- Torres-Madriz, G.; Boucher, H.W. Immunocompromised hosts: Perspectives in the treatment and prophylaxis of cytomegalovirus disease in solid-organ transplant recipients. Clin. Infect. Dis. 2008, 47, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J. Congenital Human Cytomegalovirus Infection and the Enigma of Maternal Immunity. J. Virol. 2017, 91, e02392-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Maschke, M.; Kastrup, O.; Diener, H.C. CNS manifestations of cytomegalovirus infections: Diagnosis and treatment. CNS Drugs 2002, 16, 303–315. [Google Scholar] [CrossRef]
- Mitra, D.; Hasan, M.H.; Bates, J.T.; Bidwell, G.L., 3rd; Tandon, R. Tegument Protein pp150 Sequence-Specific Peptide Blocks Cytomegalovirus Infection. Viruses 2021, 13, 2277. [Google Scholar] [CrossRef]
- Hodowanec, A.C.; Pikis, A.; Singer, M.E. The Development of Therapeutics for the Treatment and Prevention of CMV Disease in the Transplant Population: A Regulatory Perspective. J. Infect. Dis. 2020, 221, S109–S112. [Google Scholar] [CrossRef]
- Voelker, R. A Treatment Advance for Patients with Posttransplant CMV. JAMA 2022, 327, 27. [Google Scholar] [CrossRef]
- Lurain, N.S.; Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef] [Green Version]
- Chou, S. Approach to drug-resistant cytomegalovirus in transplant recipients. Curr. Opin. Infect. Dis. 2015, 28, 293–299. [Google Scholar] [CrossRef] [Green Version]
- El Chaer, F.; Shah, D.P.; Chemaly, R.F. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood 2016, 128, 2624–2636. [Google Scholar] [CrossRef] [Green Version]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95, e01987-20. [Google Scholar] [CrossRef]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulfate proteoglycans. Cold Spring Harb. Perspect. Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, D.; Spear, P.G. Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J. Clin. Investig. 2001, 108, 503–510. [Google Scholar] [CrossRef]
- Mitra, D.; Hasan, M.H.; Bates, J.T.; Bierdeman, M.A.; Ederer, D.R.; Parmar, R.C.; Fassero, L.A.; Liang, Q.; Qiu, H.; Tiwari, V.; et al. The degree of polymerization and sulfation patterns in heparan sulfate are critical determinants of cytomegalovirus entry into host cells. PLoS Pathog. 2021, 17, e1009803. [Google Scholar] [CrossRef]
- Stein, K.R.; Gardner, T.J.; Hernandez, R.E.; Kraus, T.A.; Duty, J.A.; Ubarretxena-Belandia, I.; Moran, T.M.; Tortorella, D. CD46 facilitates entry and dissemination of human cytomegalovirus. Nat. Commun. 2019, 10, 2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerna, G.; Kabanova, A.; Lilleri, D. Human Cytomegalovirus Cell Tropism and Host Cell Receptors. Vaccines 2019, 7, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, H.E.; Stevenson, P.G. Cytomegalovirus host entry and spread. J. Gen. Virol. 2019, 100, 545–553. [Google Scholar] [CrossRef]
- Vanarsdall, A.L.; Johnson, D.C. Human cytomegalovirus entry into cells. Curr. Opin. Virol. 2012, 2, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Compton, T.; Nowlin, D.M.; Cooper, N.R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 1993, 193, 834–841. [Google Scholar] [CrossRef]
- Borst, E.M.; Ständker, L.; Wagner, K.; Schulz, T.F.; Forssmann, W.G.; Messerle, M. A peptide inhibitor of cytomegalovirus infection from human hemofiltrate. Antimicrob. Agents Chemother. 2013, 57, 4751–4760. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, J.; Maus, E.; Zanotti, B.; Volin, M.V.; Tandon, R.; Shukla, D.; Tiwari, V. A role for 3-O-sulfated heparan sulfate in promoting human cytomegalovirus infection in human iris cells. J. Virol. 2015, 89, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.C.; Kamil, J.P. Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism. Viruses 2018, 10, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanarsdall, A.L.; Pritchard, S.R.; Wisner, T.W.; Liu, J.; Jardetzky, T.S.; Johnson, D.C. CD147 Promotes Entry of Pentamer-Expressing Human Cytomegalovirus into Epithelial and Endothelial Cells. mBio 2018, 9, e00781-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Collins-McMillen, D.; Buehler, J.C.; Goodrum, F.D.; Yurochko, A.D. Human Cytomegalovirus Requires Epidermal Growth Factor Receptor Signaling to Enter and Initiate the Early Steps in the Establishment of Latency in CD34+ Human Progenitor Cells. J. Virol. 2017, 91, e01206-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaofei, E.; Meraner, P.; Lu, P.; Perreira, J.M.; Aker, A.M.; McDougall, W.M.; Zhuge, R.; Chan, G.C.; Gerstein, R.M.; Caposio, P.; et al. OR14I1 is a receptor for the human cytomegalovirus pentameric complex and defines viral epithelial cell tropism. Proc. Natl. Acad. Sci. USA 2019, 116, 7043–7052. [Google Scholar] [CrossRef] [Green Version]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Samanta, P.; Sharma, P.; Zhang, F.; Mishra, S.K.; Kucheryavy, P.; Kim, S.B.; Aderibigbe, A.O.; Linhardt, R.J.; Tandon, R.; et al. Structural and kinetic analyses of holothurian sulfated glycans suggest potential treatment for SARS-CoV-2 infection. J. Biol. Chem. 2021, 297, 101207. [Google Scholar] [CrossRef]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Guide to anticoagulant therapy: Heparin: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 103, 2994–3018. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Sharma, P.; Eilts, F.; Zhang, F.; Linhardt, R.J.; Tandon, R.; Pomin, V.H. Anti-SARS-CoV-2 and anticoagulant properties of Pentacta pygmaea fucosylated chondroitin sulfate depend on high molecular weight structures. Glycobiology 2023, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Zoepfl, M.; Samanta, P.; Zhang, F.; Xia, K.; Thara, R.; Linhardt, R.J.; Doerksen, R.J.; McVoy, M.A.; Pomin, V.H. Fractionation of sulfated galactan from the red alga Botryocladia occidentalis separates its anticoagulant and anti-SARS-CoV-2 properties. J. Biol. Chem. 2022, 298, 101856. [Google Scholar] [CrossRef]
- Oh, J.H.; Martinez, A.D.; Cao, H.; George, G.W.; Cobb, J.S.; Sharma, P.; Fassero, L.A.; Arole, K.; Carr, M.A.; Lovell, K.M.; et al. Radio Frequency Heating of Washable Conductive Textiles for Bacteria and Virus Inactivation. ACS Appl. Mater. Interfaces 2022, 14, 43732–43740. [Google Scholar] [CrossRef]
- Dunn, W.; Chou, C.; Li, H.; Hai, R.; Patterson, D.; Stolc, V.; Zhu, H.; Liu, F. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 2003, 100, 14223–14228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malenovská, H. The influence of stabilizers and rates of freezing on preserving of structurally different animal viruses during lyophilization and subsequent storage. J. Appl. Microbiol. 2014, 117, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Invitrogen. A Guide to Fluorescent Probes and Labeling Technologies. In Molecular Probes Handbook, 11th ed.; TF Scientific, Ed.; Invitrogen: Waltham, MA, USA, 2010; pp. 579–585. [Google Scholar]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Aquino, R.S.; Park, P.W. Glycosaminoglycans and infection. Front. Biosci. Landmark Ed. 2016, 21, 1260–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoepfl, M.; Dwivedi, R.; Taylor, M.C.; Pomin, V.H.; McVoy, M.A. Antiviral activities of four marine sulfated glycans against adenovirus and human cytomegalovirus. Antivir. Res. 2021, 190, 105077. [Google Scholar] [CrossRef]
- Yan, L.; Song, Y.; Xia, K.; He, P.; Zhang, F.; Chen, S.; Pouliot, R.; Weiss, D.J.; Tandon, R.; Bates, J.T.; et al. Heparan sulfates from bat and human lung and their binding to the spike protein of SARS-CoV-2 virus. Carbohydr. Polym. 2021, 260, 117797. [Google Scholar] [CrossRef]
- Badani, H.; Garry, R.F.; Wimley, W.C. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta 2014, 1838, 2180–2197. [Google Scholar] [CrossRef] [Green Version]
- De Paiva, R.E.F.; Marçal Neto, A.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What is holding back the development of antiviral metallodrugs? A literature overview and implications for SARS-CoV-2 therapeutics and future viral outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef]
- Hao, C.; Yu, G.; He, Y.; Xu, C.; Zhang, L.; Wang, W. Marine glycan-based antiviral agents in clinical or preclinical trials. Rev. Med. Virol. 2019, 29, e2043. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Mitra, D.; McCandless, M.G.; Sharma, P.; Tandon, R.; Zhang, F.; Linhardt, R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dong, X.; Chen, Y.H. Neutralizing antibodies mechanism of neutralization and protective activity against HIV-1. Immunol. Res. 2002, 25, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H.; Mulloy, B. Current structural biology of the heparin interactome. Curr. Opin. Struct. Biol. 2015, 34, 17–25. [Google Scholar] [CrossRef] [PubMed]



| Compounds | IC50 (µg/mL) | SEM (µg/mL) | Lower 95% Conf. Limit (µg/mL) | Upper 95% Conf. Limit (µg/mL) |
|---|---|---|---|---|
| PpFucCS | 3.6 | 0.3 | 3.0 | 4.2 |
| HdPpFucCS | 37.8 | 4.7 | 27.7 | 47.9 |
| Fraction1 | 15.7 | 5.4 | 4.2 | 27.3 |
| Fraction2 | 83.1 | 39.9 | 0 | 166.7 |
| Fraction3 | ND | ND | ND | ND |
| Fraction4 | ND | ND | ND | ND |
| UFH | 0.9 | 1.946 | 0 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Dwivedi, R.; Ray, P.; Shukla, J.; Pomin, V.H.; Tandon, R. Inhibition of Cytomegalovirus by Pentacta pygmaea Fucosylated Chondroitin Sulfate Depends on Its Molecular Weight. Viruses 2023, 15, 859. https://doi.org/10.3390/v15040859
Sharma P, Dwivedi R, Ray P, Shukla J, Pomin VH, Tandon R. Inhibition of Cytomegalovirus by Pentacta pygmaea Fucosylated Chondroitin Sulfate Depends on Its Molecular Weight. Viruses. 2023; 15(4):859. https://doi.org/10.3390/v15040859
Chicago/Turabian StyleSharma, Poonam, Rohini Dwivedi, Priya Ray, Jayanti Shukla, Vitor H. Pomin, and Ritesh Tandon. 2023. "Inhibition of Cytomegalovirus by Pentacta pygmaea Fucosylated Chondroitin Sulfate Depends on Its Molecular Weight" Viruses 15, no. 4: 859. https://doi.org/10.3390/v15040859






