Effective Treatment of Staphylococcus aureus Intramammary Infection in a Murine Model Using the Bacteriophage Cocktail StaphLyse™
Abstract
:1. Introduction
2. Materials and Methods
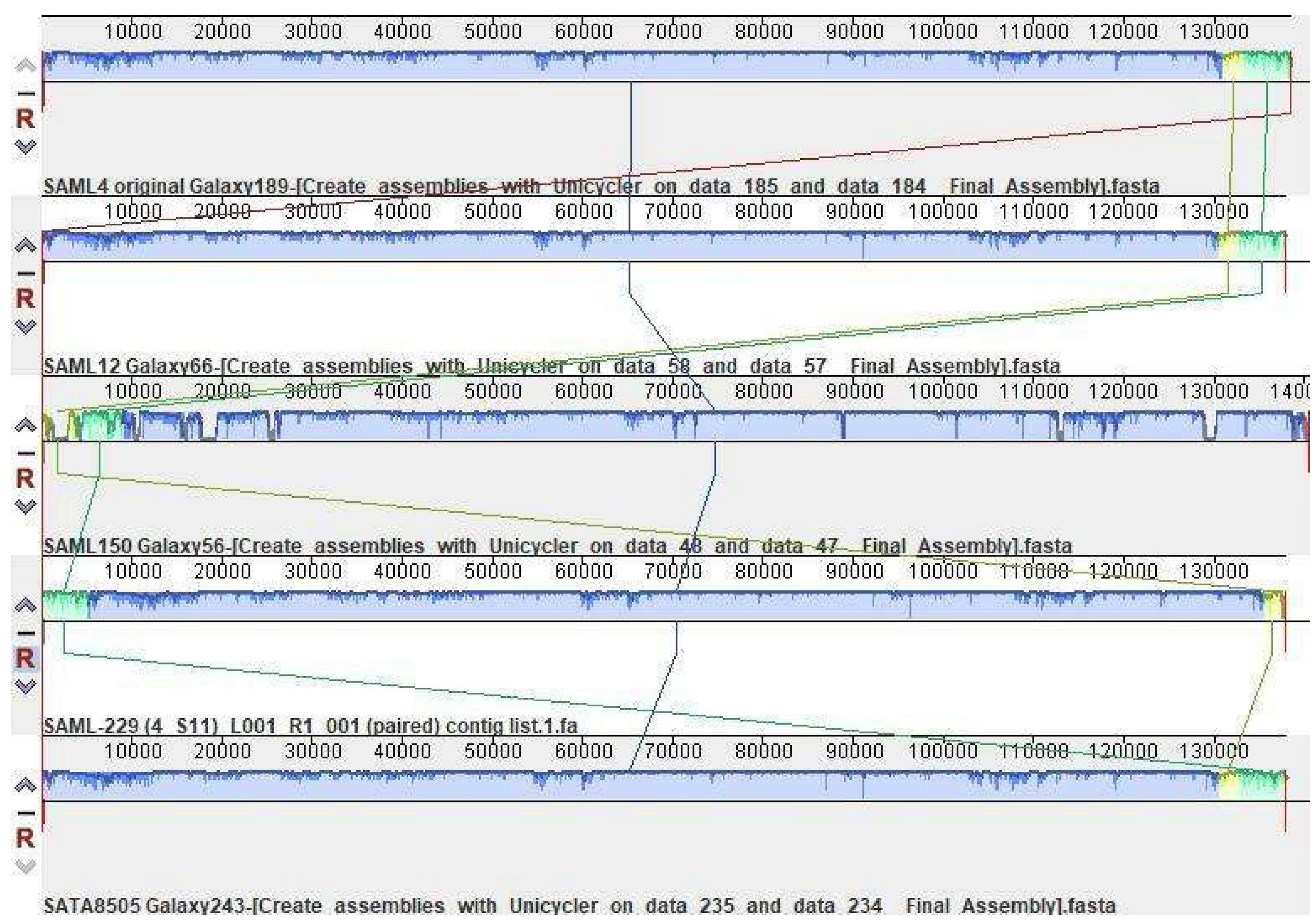
2.1. S. aureus Strains
2.2. StaphLyse™ Phage Cocktail
2.3. Lytic Range of the StaphLyse™
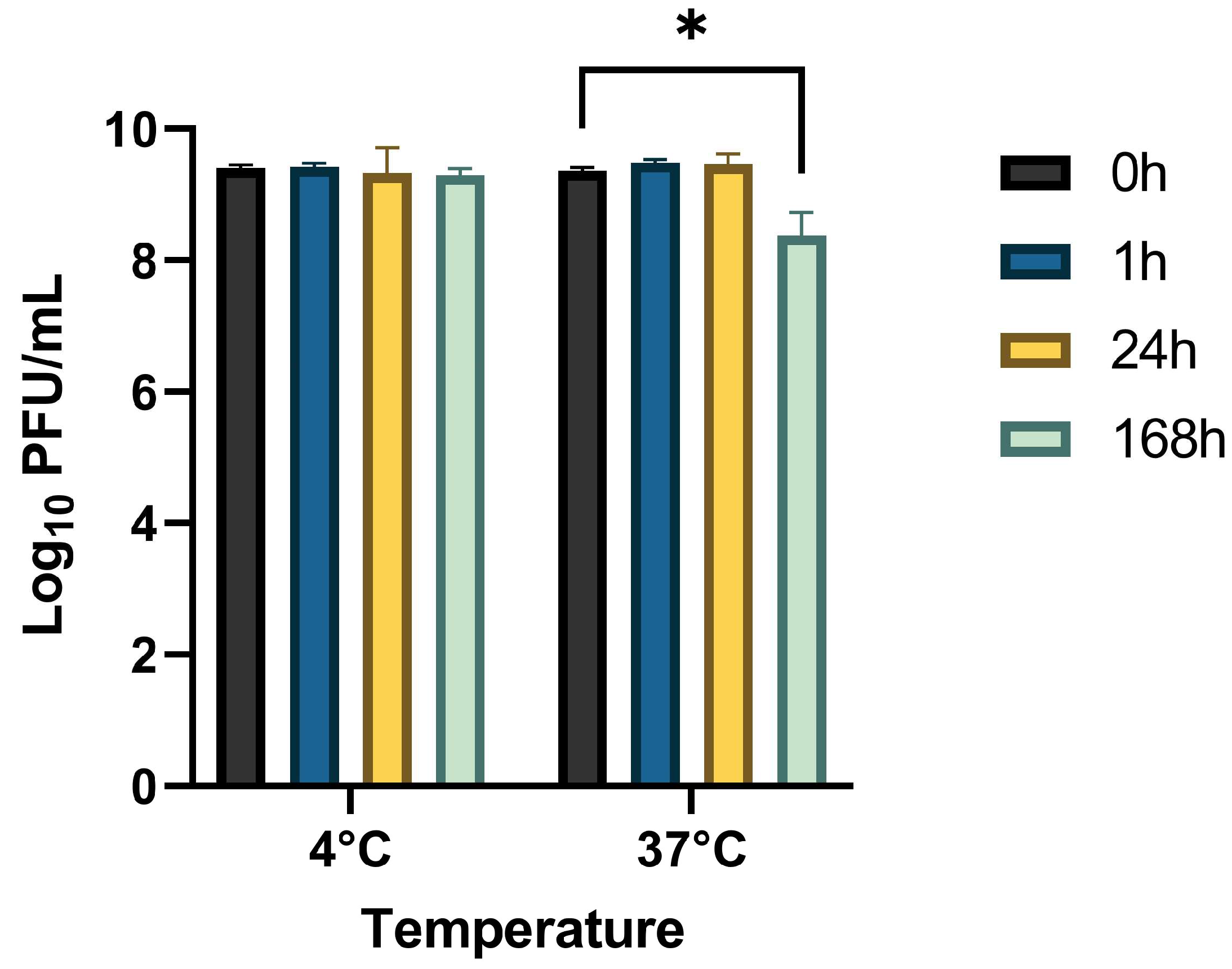
2.4. Stability Study
2.5. In Vitro Killing of S. aureus in Whole Milk
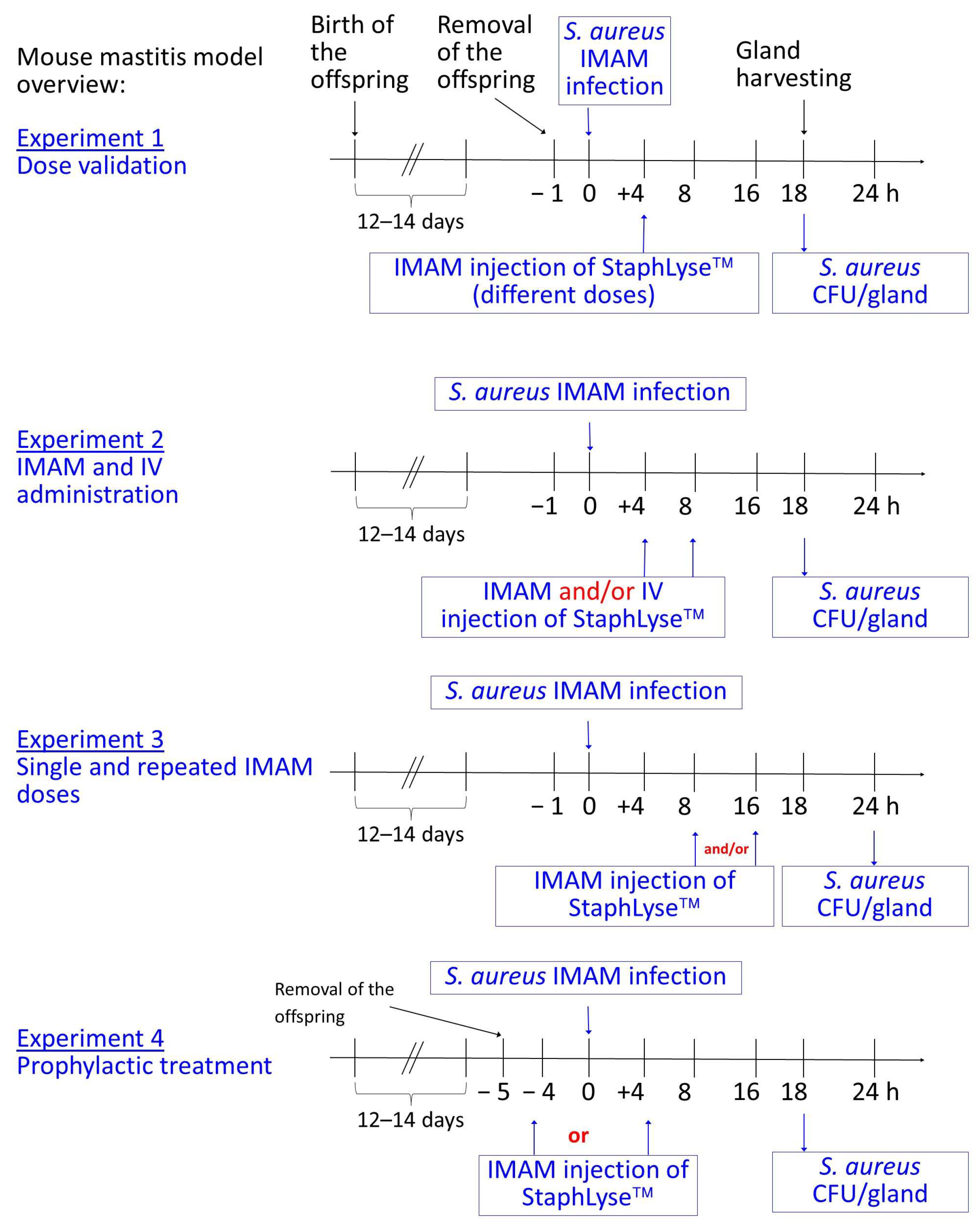
2.6. Mouse Mastitis Model
2.7. Statistical Analysis
3. Results
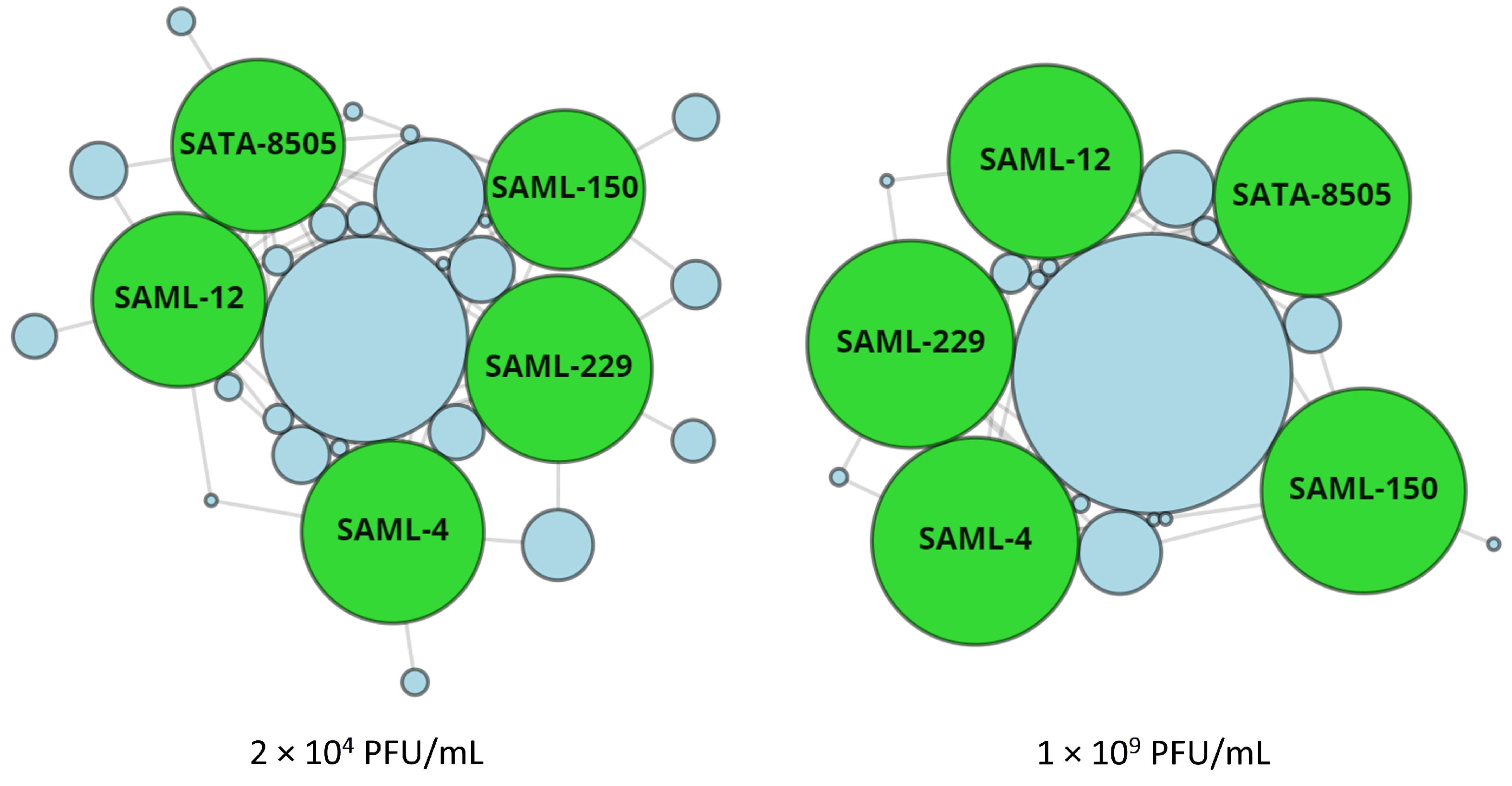
3.1. Lytic Range of the StaphLyse™ In Vitro
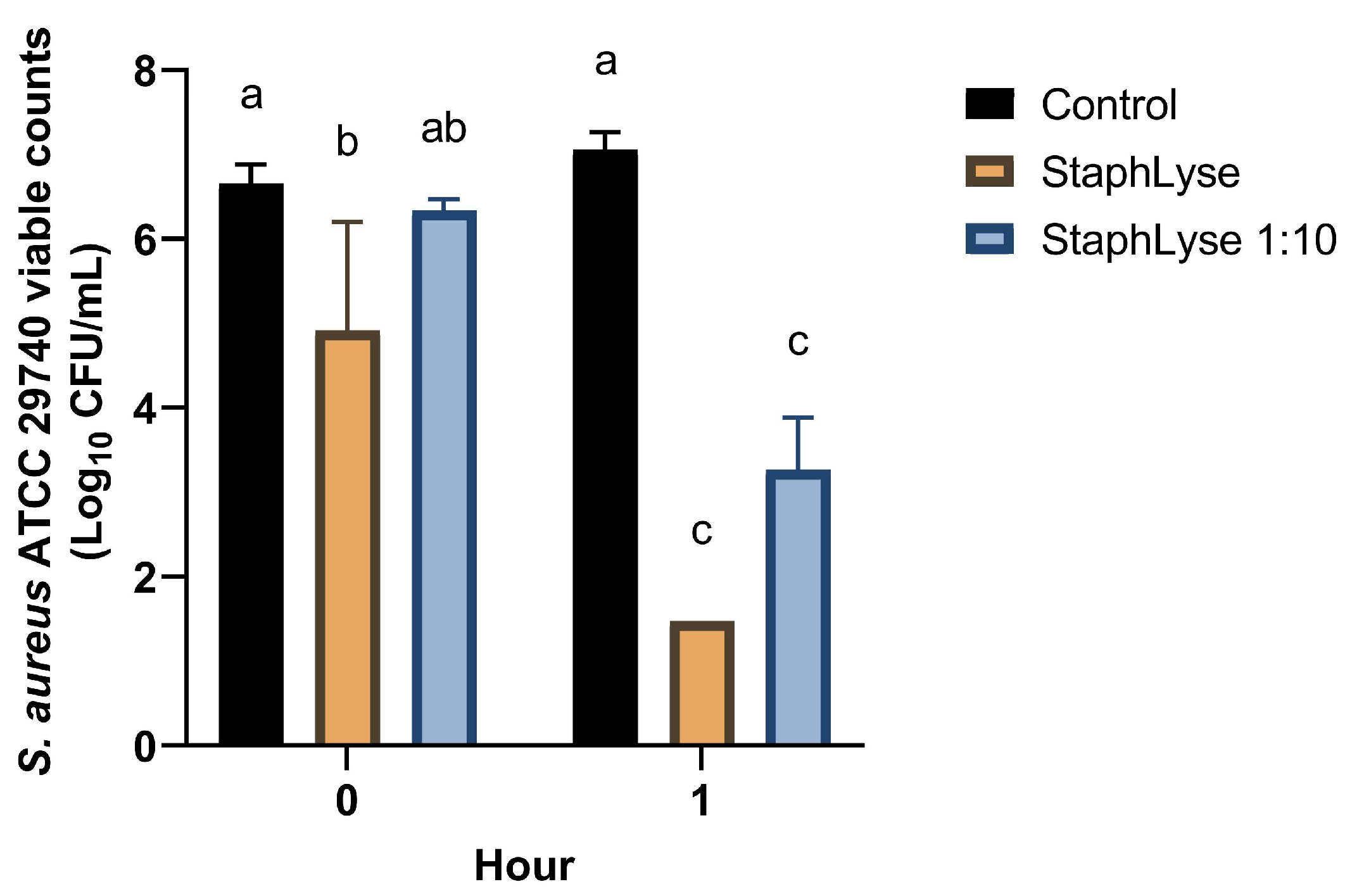
3.2. Stability of StaphLyse™ and In Vitro Killing of S. aureus in Whole Milk
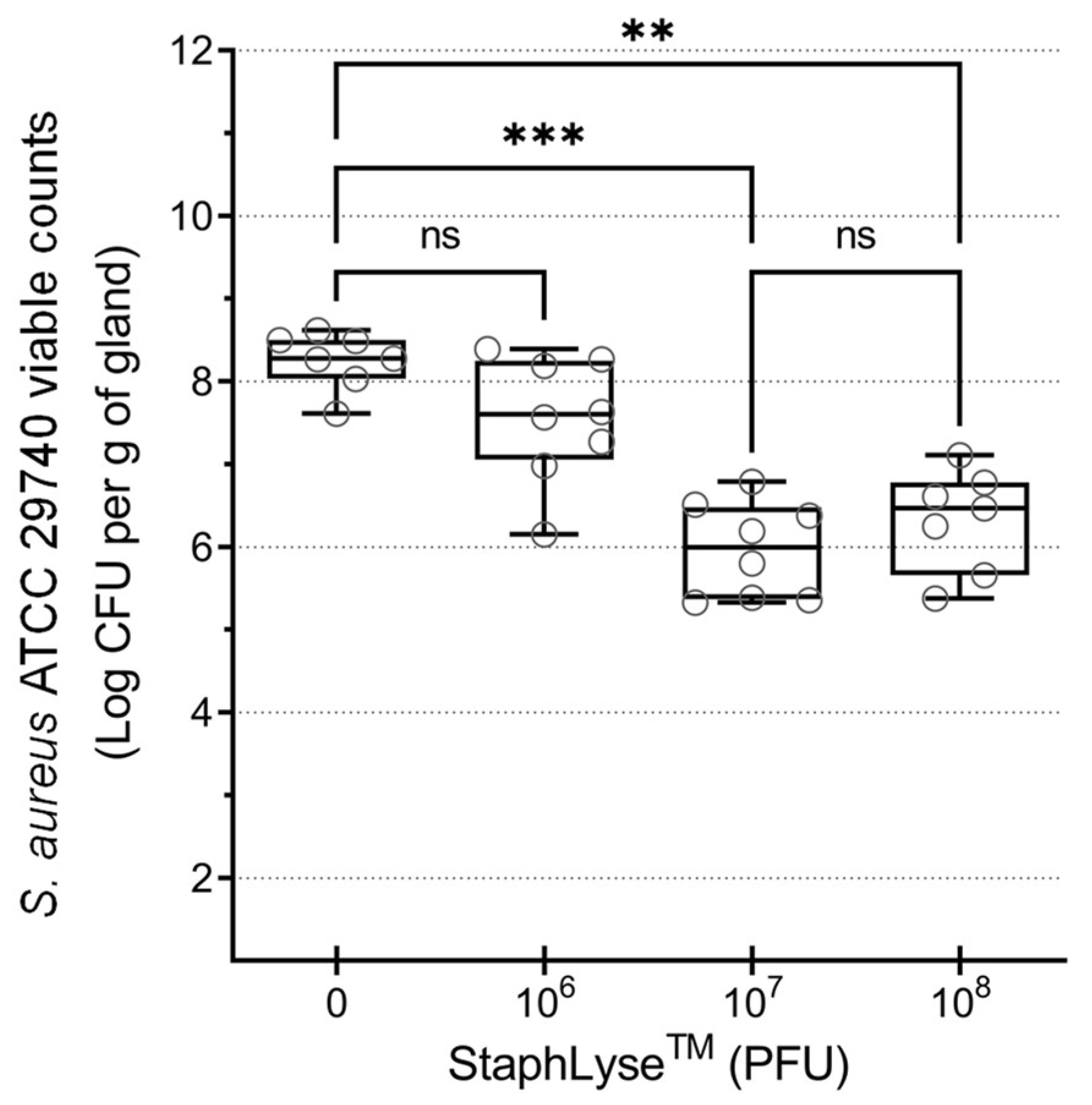
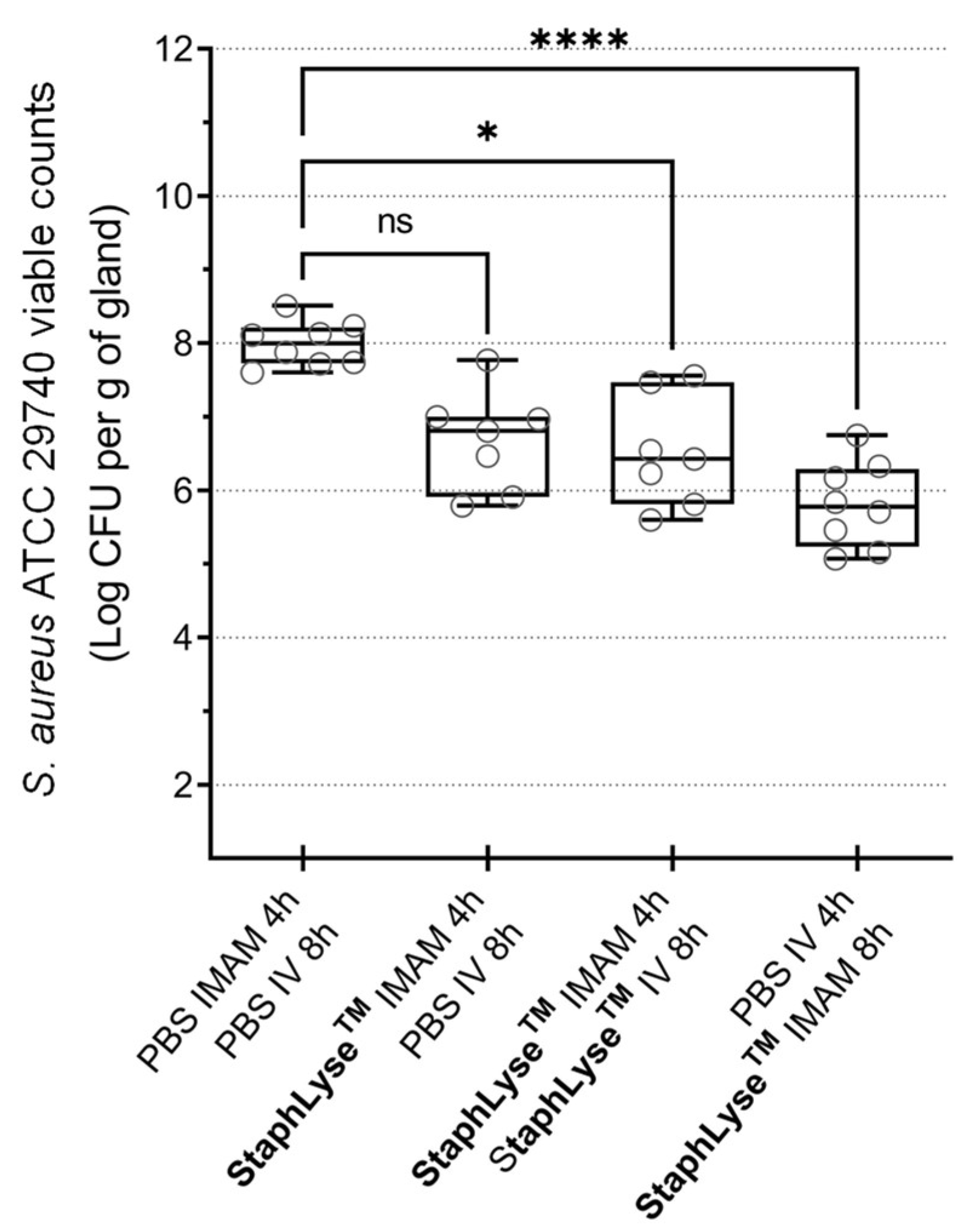
3.3. StaphLyse™ Efficacy in a Mouse Model to Treat S. aureus Intramammary Infection
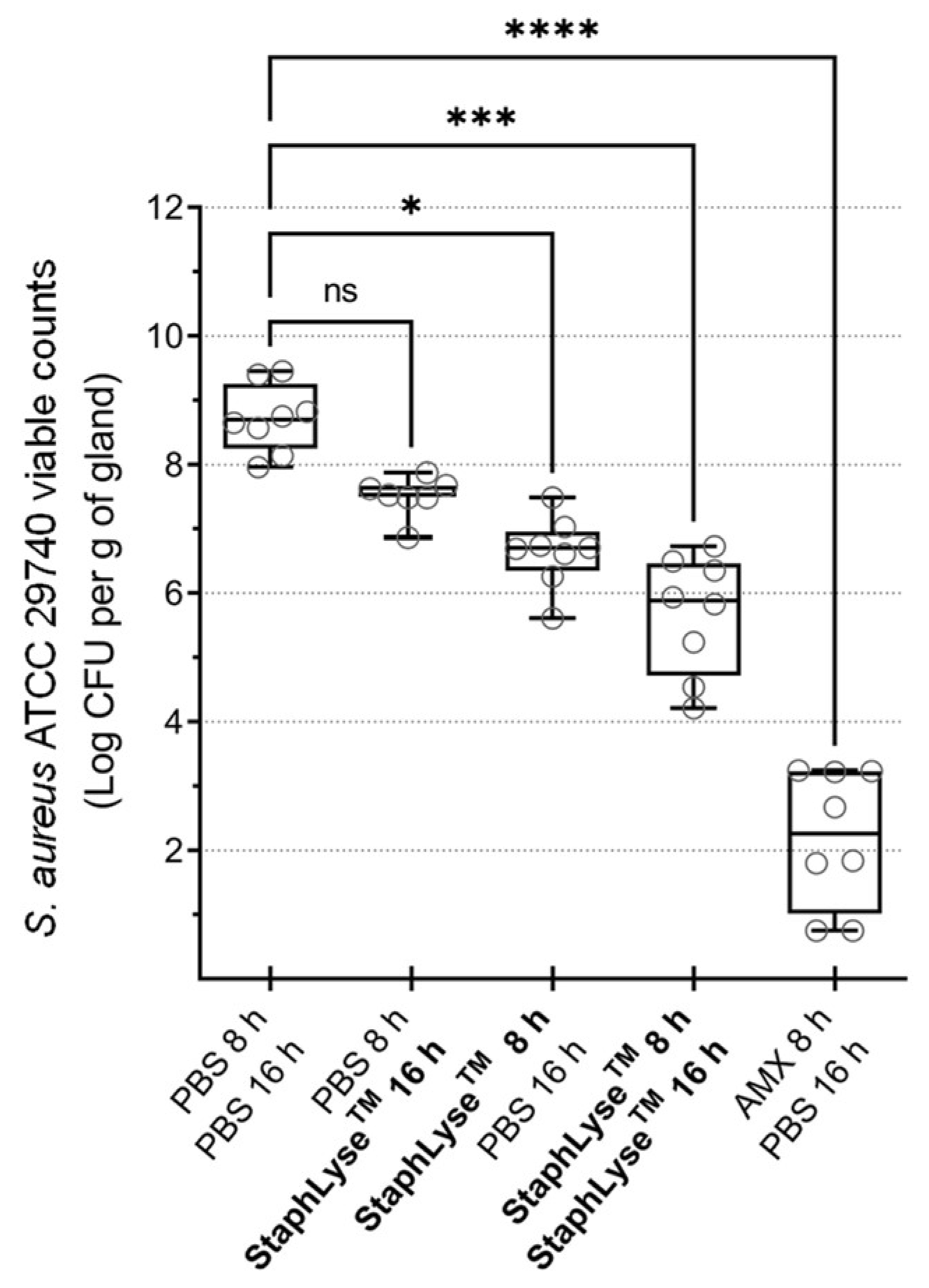
3.4. Prophylactic Treatment of S. aureus Intramammary Infection Using StaphLyse™
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huijps, K.; Hogeveen, H. Stochastic modeling to determine the economic effects of blanket, selective, and no dry cow therapy. J. Dairy Sci. 2007, 90, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Kabera, F.; Dufour, S.; Keefe, G.; Cameron, M.; Roy, J.P. Evaluation of quarter-based selective dry cow therapy using Petrifilm on-farm milk culture: A randomized controlled trial. J. Dairy Sci. 2020, 103, 7276–7287. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.P.; Murinda, S.E. Antimicrobial resistance of mastitis pathogens. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 165–185. [Google Scholar] [CrossRef]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-level mastitis-associated costs on Canadian dairy farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Krömker, V.; Leimbach, S. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 2017, 52, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyher, K.K.; Dufour, S.; Barkema, H.W.; Des Côteaux, L.; DeVries, T.J.; Dohoo, I.R.; Keefe, G.P.; Roy, J.P.; Scholl, D.T. The National Cohort of Dairy Farms—A data collection platform for mastitis research in Canada. J. Dairy Sci. 2011, 94, 1616–1626. [Google Scholar] [CrossRef] [Green Version]
- Wellnitz, O.; Bruckmaier, R.M. The innate immune response of the bovine mammary gland to bacterial infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Pichette-Jolette, S.; Millette, G.; Demontier, E.; Bran-Barrera, D.; Cyrenne, M.; Ster, C.; Haine, D.; Keefe, G.; Malouin, F.; Roy, J.P. Partial prediction of the duration and the clinical status of Staphylococcus aureus bovine intramammary infections based on the phenotypic and genotypic analysis of isolates. Vet. Microbiol. 2019, 228, 188–195. [Google Scholar] [CrossRef]
- Jacques, M.; Aragon, V.; Tremblay, Y.D. Biofilm formation in bacterial pathogens of veterinary importance. Anim. Health Res. Rev. Conf. Res. Work Anim. Dis. 2010, 11, 97–121. [Google Scholar] [CrossRef]
- Melchior, M.B.; Fink-Gremmels, J.; Gaastra, W. Extended antimicrobial susceptibility assay for Staphylococcus aureus isolates from bovine mastitis growing in biofilms. Vet. Microbiol. 2007, 125, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ster, C.; Lebeau, V.; Leclerc, J.; Fugere, A.; Veh, K.A.; Roy, J.P.; Malouin, F. In vitro antibiotic susceptibility and biofilm production of Staphylococcus aureus isolates recovered from bovine intramammary infections that persisted or not following extended therapies with cephapirin, pirlimycin or ceftiofur. Vet. Res. 2017, 48, 56. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, ARBA-0009-2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molineri, A.I.; Camussone, C.; Zbrun, M.V.; Suárez Archilla, G.; Cristiani, M.; Neder, V.; Calvinho, L.; Signorini, M. Antimicrobial resistance of Staphylococcus aureus isolated from bovine mastitis: Systematic review and meta-analysis. Prev. Vet. Med. 2021, 188, 105261. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ronholm, J. Staphylococcus aureus in agriculture: Lessons in evolution from a multispecies pathogen. Clin. Microbiol. Rev. 2021, 34, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A one health perspective on dairy production and dairy food safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31–39. [Google Scholar] [CrossRef]
- Wittebole, X.; De Roock, S.; Opal, S.M. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 2014, 5, 226–235. [Google Scholar] [CrossRef]
- Henein, A. What are the limitations on the wider therapeutic use of phage? Bacteriophage 2013, 3, e24872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speck, P.; Smithyman, A. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol. Lett. 2015, 363, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E.M.; Kuhl, S.J.; Abedon, S.T. Re-establishing a place for phage therapy in western medicine. Future Microbiol. 2015, 10, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Zduńczyk, S.; Janowski, T. Bacteriophages and associated endolysins in therapy and prevention of mastitis and metritis in cows: Current knowledge. Anim. Reprod. Sci. 2020, 218, 106504. [Google Scholar] [CrossRef] [PubMed]
- Lungren, M.P.; Donlan, R.M.; Kankotia, R.; Paxton, B.E.; Falk, I.; Christensen, D.; Kim, C.Y. Bacteriophage K antimicrobial-lock technique for treatment of Staphylococcus aureus central venous catheter-related infection: A leporine model efficacy analysis. J. Vasc. Interv. Radiol. 2014, 25, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Wills, Q.F.; Kerrigan, C.; Soothill, J.S. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob. Agents Chemother. 2005, 49, 1220–1221. [Google Scholar] [CrossRef] [Green Version]
- Takemura-Uchiyama, I.; Uchiyama, J.; Osanai, M.; Morimoto, N.; Asagiri, T.; Ujihara, T.; Daibata, M.; Sugiura, T.; Matsuzaki, S. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes Infect. 2014, 16, 512–517. [Google Scholar] [CrossRef]
- Sunagar, R.; Patil, S.A.; Chandrakanth, R.K. Bacteriophage therapy for Staphylococcus aureus bacteremia in streptozotocin-induced diabetic mice. Res. Microbiol. 2010, 161, 854–860. [Google Scholar] [CrossRef]
- Drilling, A.; Morales, S.; Boase, S.; Jervis-Bardy, J.; James, C.; Jardeleza, C.; Tan, N.C.W.; Cleland, E.; Speck, P.; Vreugde, S.; et al. Safety and efficacy of topical bacteriophage and ethylenediaminetetraacetic acid treatment of Staphylococcus aureus infection in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2014, 4, 176–186. [Google Scholar] [CrossRef]
- Breyne, K.; Honaker, R.W.; Hobbs, Z.; Richter, M.; Zaczek, M.; Spangler, T.; Steenbrugge, J.; Lu, R.; Kinkhabwala, A.; Marchon, B.; et al. Efficacy and safety of a bovine-associated Staphylococcus aureus phage cocktail in a murine model of mastitis. Front. Microbiol. 2017, 8, 2348. [Google Scholar] [CrossRef]
- Geng, H.; Zou, W.; Zhang, M.; Xu, L.; Liu, F.; Li, X.; Wang, L.; Xu, Y. Evaluation of phage therapy in the treatment of Staphylococcus aureus-induced mastitis in mice. Folia Microbiol. 2020, 65, 339–351. [Google Scholar] [CrossRef]
- Iwano, H.; Inoue, Y.; Takasago, T.; Kobayashi, H.; Furusawa, T.; Taniguchi, K.; Fujiki, J.; Yokota, H.; Usui, M.; Tanji, Y.; et al. Bacteriophage ΦSA012 has a broad host range against Staphylococcus aureus and effective lytic capacity in a mouse mastitis model. Biology 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelcher, M.; Powell, A.M.; Becker, S.C.; Camp, M.J.; Donovan, D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 2012, 78, 2297–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, F.; Xiong, X.; Zhang, S.; Li, G.; Wang, R.; Zhang, L.; Wang, X.; Zhou, H.; Li, J.; Li, Y.; et al. Efficacy assessment of phage therapy in treating Staphylococcus aureus-induced mastitis in mice. Viruses 2022, 14, 620. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Z. Isolation and characterization of a virulent bacteriophage SPW specific for Staphylococcus aureus isolated from bovine mastitis of lactating dairy cattle. Mol. Biol. Rep. 2014, 41, 5829–5838. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Ross, R.P.; Flynn, J.; Meaney, W.J.; Fitzgerald, G.F.; Coffey, A. Isolation and characterization of two anti-staphylococcal bacteriophages specific for pathogenic Staphylococcus aureus associated with bovine infections. Lett. Appl. Microbiol. 2005, 41, 482–486. [Google Scholar] [CrossRef]
- Kwiatek, M.; Parasion, S.; Mizak, L.; Gryko, R.; Bartoszcze, M.; Kocik, J. Characterization of a bacteriophage, isolated from a cow with mastitis, that is lytic against Staphylococcus aureus strains. Arch. Virol. 2012, 157, 225–234. [Google Scholar] [CrossRef]
- Han, J.E.; Kim, J.H.; Hwang, S.Y.; Choresca, C.H.; Shin, S.P.; Jun, J.W.; Chai, J.Y.; Park, Y.H.; Park, S.C. Isolation and characterization of a Myoviridae bacteriophage against Staphylococcus aureus isolated from dairy cows with mastitis. Res. Vet. Sci. 2013, 95, 758–763. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Boulanger, D.; Brouillette, E.; Jaspar, F.; Malouin, F.; Mainil, J.; Bureau, F.; Lekeux, P. Helenalin reduces Staphylococcus aureus infection in vitro and in vivo. Vet. Microbiol. 2007, 119, 330–338. [Google Scholar] [CrossRef]
- Brouillette, E.; Hyodo, M.; Hayakawa, Y.; Karaolis, D.K.R.; Malouin, F. 3′,5′-cyclic diguanylic acid reduces the virulence of biofilm-forming Staphylococcus aureus strains in a mouse model of mastitis infection. Antimicrob. Agents Chemother. 2005, 49, 3109–3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouillette, E.; Grondin, G.; Talbot, B.G.; Malouin, F. Inflammatory cell infiltration as an indicator of Staphylococcus aureus infection and therapeutic efficacy in experimental mouse mastitis. Vet. Immunol. Immunopathol. 2005, 104, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, E.; Grondin, G.; Lefebvre, C.; Talbot, B.G.; Malouin, F. Mouse mastitis model of infection for antimicrobial compound efficacy studies against intracellular and extracellular forms of Staphylococcus aureus. Vet. Microbiol. 2004, 101, 253–262. [Google Scholar] [CrossRef]
- Brouillette, E.; Malouin, F. The pathogenesis and control of Staphylococcus aureus-induced mastitis: Study models in the mouse. Microbes Infect. 2005, 7, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Côté-Gravel, J.; Brouillette, E.; Obradović, N.; Ster, C.; Talbot, B.G.; Malouin, F. Characterization of a vraG mutant in a genetically stable Staphylococcus aureus small-colony variant and preliminary assessment for use as a live-attenuated vaccine against intrammamary infections. PLoS ONE 2016, 11, e0166621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulhbacher, J.; Brouillette, E.; Allard, M.; Fortier, L.C.; Malouin, F.; Lafontaine, D.A. Novel riboswitch ligand analogs as selective inhibitors of guanine-related metabolic pathways. PLoS Pathog. 2010, 6, e1000865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diarra, M.S.; Block, G.; Rempel, H.; Oomah, B.D.; Harrison, J.; McCallum, J.; Boulanger, S.; Brouillette, É.; Gattuso, M.; Malouin, F. In vitro and in vivo antibacterial activities of cranberry press cake extracts alone or in combination with β-lactams against Staphylococcus aureus. BMC Complement. Altern. Med. 2013, 13, 90. [Google Scholar] [CrossRef] [Green Version]
- Prasad, L.B.; Newbould, F.H. Inoculation of the bovine teat duct with Staph. aureus: The relationship of teat duct length, milk yield and milking rate to development of intramammary infection. Can. Vet. J. 1968, 9, 107–114. [Google Scholar]
- FDA. T.F.a.D.A. Bacteriophage Therapy: Scientific and Regulatory Issues Public Workshop. Available online: https://www.federalregister.gov/documents/2017/06/08/2017-11862/bacteriophage-therapy-scientific-and-regulatory-issues-public-workshop (accessed on 2 September 2021).
- Darling, A.E.; Mau, B.; Perna, N.T. Progressivemauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.H. Methods of study bacterial viruses. In Bacteriophages; Interscience Publishers, Ltd.: London, UK, 1959; pp. 443–522. [Google Scholar]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef]
- Sugden, R.; Kelly, R.; Davies, S. Combatting antimicrobial resistance globally. Nat. Microbiol. 2016, 1, 16187. [Google Scholar] [CrossRef] [PubMed]
- Lhermie, G.; Tauer, L.W.; Gröhn, Y.T. The farm cost of decreasing antimicrobial use in dairy production. PLoS ONE 2018, 13, e0194832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Danis-Wlodarczyk, K.; Dąbrowska, K.; Abedon, S.T. Phage therapy: The pharmacology of antibacterial viruses. Curr. Issues Mol. Biol. 2020, 40, 81–163. [Google Scholar] [CrossRef]
- Lin, Y.; Xia, L.; Turner, J.D.; Zhao, X. Morphologic observation of neutrophil diapedesis across bovine mammary gland epithelium in vitro. Am. J. Vet. Res. 1995, 56, 203–207. [Google Scholar]
- Burton, J.L.; Erskine, R.J. Immunity and mastitis: Some new ideas for an old disease. Vet. Clin. North Am. Food Anim. Pract. 2003, 19, 1–45. [Google Scholar] [CrossRef]
- Harmon, R.J. Physiology of mastitis and factors affecting somatic cell counts. J. Dairy Sci. 1994, 77, 2103–2112. [Google Scholar] [CrossRef]
- Gill, J.J.; Pacan, J.C.; Carson, M.E.; Leslie, K.E.; Griffiths, M.W.; Sabour, P.M. Efficacy and pharmacokinetics of bacteriophage therapy in treatment of subclinical Staphylococcus aureus mastitis in lactating dairy cattle. Antimicrob. Agents Chemother. 2006, 50, 2912–2918. [Google Scholar] [CrossRef] [Green Version]
- O’Flaherty, S.; Coffey, A.; Meaney, W.J.; Fitzgerald, G.F.; Ross, R.P. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 2005, 41, 274–279. [Google Scholar] [CrossRef] [PubMed]

| Monophage and Cocktail | GenBank Number | Lysis of Staphylococcus aureus Strains 1 | |||||
|---|---|---|---|---|---|---|---|
| 2 × 104 PFU/mL | 1 × 109 PFU/mL | ||||||
| n | % | Unique Kill 2 | n | % | Unique Kill 2 | ||
| SAML-4 | OP352121 | 552 | 77.9 | 5 | 700 | 98.7 | 0 |
| SAML-12 | OP352122 | 501 | 70.7 | 14 | 608 | 85.8 3 | 0 |
| SAML-150 | OP352123 | 423 | 59.7 | 15 | 683 | 96.3 | 1 |
| SAML-229 | OP352124 | 580 | 81.8 | 12 | 700 | 98.7 | 0 |
| SATA-8505 | OQ594774 | 495 | 69.78 | 4 | 631 | 89.0 3 | 0 |
| StaphLyse™ | - | 657 | 92.7 | - | 709 | 100 | - |
| Phenotype 1 | Number of Strains Tested | Lysis by StaphLyse™ | |||
|---|---|---|---|---|---|
| 2 × 104 PFU/mL | 1 × 109 PFU/mL | ||||
| n | % | n | % | ||
| MSSA 2 | 89 | 76 | 84.4 | 89 | 100 |
| MRSA | 396 | 376 | 94.9 | 396 | 100 |
| VISA 3 | 2 | 1 | 50.0 | 2 | 100 |
| Others | 222 | 204 | 91.9 | 222 | 100 |
| Total | 709 | 657 | 92.7 | 709 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brouillette, E.; Millette, G.; Chamberland, S.; Roy, J.-P.; Ster, C.; Kiros, T.; Hickey, S.; Hittle, L.; Woolston, J.; Malouin, F. Effective Treatment of Staphylococcus aureus Intramammary Infection in a Murine Model Using the Bacteriophage Cocktail StaphLyse™. Viruses 2023, 15, 887. https://doi.org/10.3390/v15040887
Brouillette E, Millette G, Chamberland S, Roy J-P, Ster C, Kiros T, Hickey S, Hittle L, Woolston J, Malouin F. Effective Treatment of Staphylococcus aureus Intramammary Infection in a Murine Model Using the Bacteriophage Cocktail StaphLyse™. Viruses. 2023; 15(4):887. https://doi.org/10.3390/v15040887
Chicago/Turabian StyleBrouillette, Eric, Guillaume Millette, Suzanne Chamberland, Jean-Pierre Roy, Céline Ster, Tadele Kiros, Stephanie Hickey, Lauren Hittle, Joelle Woolston, and François Malouin. 2023. "Effective Treatment of Staphylococcus aureus Intramammary Infection in a Murine Model Using the Bacteriophage Cocktail StaphLyse™" Viruses 15, no. 4: 887. https://doi.org/10.3390/v15040887





