Dynamics of SARS-CoV-2 Antibody Responses up to 9 Months Post-Vaccination in Individuals with Previous SARS-CoV-2 Infection Receiving Inactivated Vaccines
Abstract
1. Introduction
2. Materials and Methods
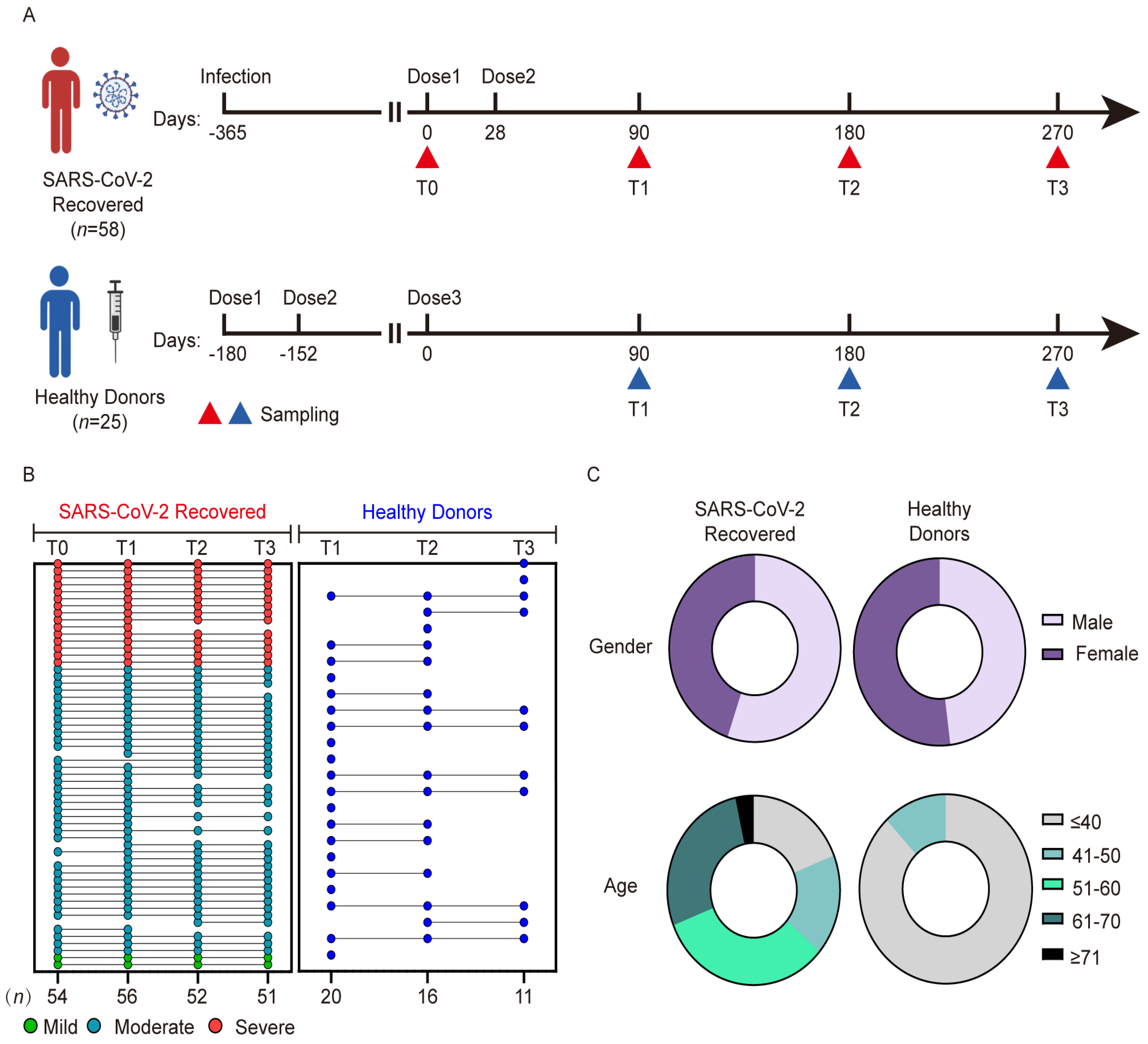
2.1. Human Subjects
2.2. Plasma Sample Collection
2.3. Antibody Measurement
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Characteristics of Participants with Previous SARS-CoV-2 Infection and Healthy Donors
3.2. Dynamic Profiles of Anti-SARS-CoV-2 Antibodies in Participants with Previous SARS-CoV-2 Infection and Healthy Donors after Vaccination
3.3. Correlation Analysis between Antibody Levels in Individuals with Previous SARS-CoV-2 Infection and Healthy Donors after Vaccination
3.4. Wild-Type NAb Duration after Vaccination Correlates with the Neutrophil-to-Lymphocyte Ratio
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 588, E6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 10 February 2023).
- Lai, A.; Bergna, A.; Della Ventura, C.; Menzo, S.; Bruzzone, B.; Sagradi, F.; Ceccherini-Silberstein, F.; Weisz, A.; Clementi, N.; Brindicci, G.; et al. Epidemiological and Clinical Features of SARS-CoV-2 Variants Circulating between April–December 2021 in Italy. Viruses 2022, 14, 2508. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, C.; Shi, Q.; Xiao, K.; Wang, L.; Liu, Z.; Li, Z.; Dong, X. Evolving trend change during the COVID-19 pandemic. Front. Public Health 2022, 10, 957265. [Google Scholar] [CrossRef]
- Rossler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef]
- Zhao, X.; Li, D.; Ruan, W.; Chen, Z.; Zhang, R.; Zheng, A.; Qiao, S.; Zheng, X.; Zhao, Y.; Dai, L.; et al. Effects of a Prolonged Booster Interval on Neutralization of Omicron Variant. N. Engl. J. Med. 2022, 386, 894–896. [Google Scholar] [CrossRef]
- Kim, J.; Seo, H.; Kim, H.W.; Kim, D.; Kwon, H.J.; Kim, Y.K. Effect of Previous COVID-19 Vaccination on Humoral Immunity 3 Months after SARS-CoV-2 Omicron Infection and Booster Effect of a Fourth COVID-19 Vaccination 2 Months after SARS-CoV-2 Omicron Infection. Viruses 2022, 14, 2458. [Google Scholar] [CrossRef]
- Ferguson, N.; Ghani, A.C.; Hinsley, W.; Volz, E.M. Report 50: Hospitalisation Risk for Omicron Cases in England. Available online: https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-50-severity-omicron/ (accessed on 13 February 2023).
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Yu, C.; Fengzhao, Z.; Hongmei, W.; Zeyuan, L.; Yu, L.; Yuhang, G.; Rufei, S.; Qingzhu, J.; Xiaorong, S.; Xia, W.; et al. The impact of vaccination on patients with COVID-19 during the wave of Omicron in Shanghai. Front. Public Health 2022, 10, 1054313. [Google Scholar] [CrossRef]
- Tangye, S.G.; Tarlinton, D.M. Memory B cells: Effectors of long-lived immune responses. Eur. J. Immunol. 2009, 39, 2065–2075. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Le Bert, N.; Tan, A.T. SARS-CoV-2-specific T cells in the changing landscape of the COVID-19 pandemic. Immunity 2022, 55, 1764–1778. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; De Martino, M.; Fabris, M.; Palese, A.; Visintini, E.; Graziano, E.; Gerussi, V.; Bontempo, G.; D’Aurizio, F.; Biasotto, A.; et al. The Fall in Antibody Response to SARS-CoV-2: A Longitudinal Study of Asymptomatic to Critically Ill Patients Up to 10 Months after Recovery. J. Clin. Microbiol. 2021, 59, e0113821. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Liu, H.Y.; Zhao, H.; Wang, G.Q.; Zhou, C.; Zheng, J.; Li, X.F.; Li, F.; Bai, C.Q.; Qin, C.F. Longitudinal dynamics of antibody responses in recovered COVID-19 patients. Signal Transduct. Target. Ther. 2021, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.T.; Lin, B.C.; Moore, C.; et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell 2022, 185, 1556–1571. [Google Scholar] [CrossRef]
- Kotagiri, P.; Mescia, F.; Rae, W.M.; Bergamaschi, L.; Tuong, Z.K.; Turner, L.; Hunter, K.; Gerber, P.P.; Hosmillo, M.; Cambridge Institute of Therapeutic Immunology and Infectious Disease-National Institute of Health Research (CITIID-NIHR) COVID BioResource Collaboration; et al. B cell receptor repertoire kinetics after SARS-CoV-2 infection and vaccination. Cell Rep. 2022, 38, 110393. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; Gonzalez, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Deng, H.J.; Hu, J.; Wei, X.Y.; Xue, J.J.; Li, T.T.; Fang, L.; Liu, B.Z.; Jin, A.S.; Xu, F.L.; et al. Humoral responses in naive or SARS-CoV-2 experienced individuals vaccinated with an inactivated vaccine. Cell Discov. 2021, 7, 68. [Google Scholar] [CrossRef]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-Garcia, J.; et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Chen, Y.; Zuiani, A.; Fischinger, S.; Mullur, J.; Atyeo, C.; Travers, M.; Lelis, F.J.N.; Pullen, K.M.; Martin, H.; Tong, P.; et al. Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell 2020, 183, 1496–1507. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.; Deng, C.; Li, M.; Li, L.; Liu, D.; Liu, M.; Ruan, X.; Mei, J.; Mo, R.; et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov. 2022, 8, 10. [Google Scholar] [CrossRef]
- Wang, K.; Long, Q.X.; Deng, H.J.; Hu, J.; Gao, Q.Z.; Zhang, G.J.; He, C.L.; Huang, L.Y.; Hu, J.L.; Chen, J.; et al. Longitudinal Dynamics of the Neutralizing Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e531–e539. [Google Scholar] [CrossRef]
- Wang, G.L.; Wang, Z.Y.; Duan, L.J.; Meng, Q.C.; Jiang, M.D.; Cao, J.; Yao, L.; Zhu, K.L.; Cao, W.C.; Ma, M.J. Susceptibility of Circulating SARS-CoV-2 Variants to Neutralization. N. Engl. J. Med. 2021, 384, 2354–2356. [Google Scholar] [CrossRef]
- Duan, L.J.; Jiang, W.G.; Wang, Z.Y.; Yao, L.; Zhu, K.L.; Meng, Q.C.; Wang, B.S.; Li, L.B.; Wang, G.L.; Ma, M.J. Neutralizing immunity against SARS-CoV-2 Omicron BA.1 by infection and vaccination. iScience 2022, 25, 104886. [Google Scholar] [CrossRef] [PubMed]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesch, M.; Andrell, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med 2021, 2, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Bryant, V.L.; Hodgkin, P.D. Life, death, and antibodies. Science 2017, 358, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Weisblum, Y.; Barnes, C.O.; Schmidt, F.; Schaefer-Babajew, D.; Wang, Z.; Lorenzi, J.C.C.; Flyak, A.I.; DeLaitsch, A.T.; Huey-Tubman, K.E.; et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 2021, 54, 1853–1868. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, S.; Tong, X.; Tao, Y.; Ni, J.; Pan, J.; Li, M.; Wan, Y.; Mao, M.; Xiong, Y.; et al. Dynamic SARS-CoV-2-specific B-cell and T-cell responses following immunization with an inactivated COVID-19 vaccine. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2022, 28, 410–418. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, 6529. [Google Scholar] [CrossRef]
- Kumar, N.P.; Banurekha, V.V.; Kumar, C.P.G.; Nancy, A.; Padmapriyadarsini, C.; Shankar, S.; Hanna, L.E.; Murhekar, M.; Devi, K.R.U.; Babu, S. Inactivated COVID-19 vaccines: Durability of Covaxin/BBV152 induced immunity against variants of concern. J. Travel Med. 2022, 29, 88. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Turner, J.S.; Zhou, J.Q.; Han, J.; Schmitz, A.J.; Rizk, A.A.; Alsoussi, W.B.; Lei, T.; Amor, M.; McIntire, K.M.; Meade, P.; et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 2020, 586, 127–132. [Google Scholar] [CrossRef]
- Edara, V.-V.; Manning, K.E.; Ellis, M.; Lai, L.; Moore, K.M.; Foster, S.L.; Floyd, K.; Davis-Gardner, M.E.; Mantus, G.; Nyhoff, L.E.; et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. Cell Rep. Med. 2021, 3, 100529. [Google Scholar] [CrossRef]
- Kong, W.-H.; Zhao, R.; Zhou, J.-B.; Wang, F.; Kong, D.-G.; Sun, J.-B.; Ruan, Q.-F.; Liu, M.-Q. Serologic Response to SARS-CoV-2 in COVID-19 Patients with Different Severity. Virol. Sin. 2020, 35, 752–757. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Elmer, A.; Kingston, N.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488. [Google Scholar] [CrossRef]
- Laing, E.D.; Epsi, N.J.; Richard, S.A.; Samuels, E.C.; Wang, W.; Vassell, R.; Ewing, D.F.; Herrup, R.; Sterling, S.L.; Lindholm, D.A.; et al. SARS-CoV-2 antibodies remain detectable 12 months after infection and antibody magnitude is associated with age and COVID-19 severity. medRxiv 2021. [Google Scholar] [CrossRef]
- Tang, J.; Grubbs, G.; Lee, Y.; Huang, C.; Ravichandran, S.; Forgacs, D.; Golding, H.; Ross, T.M.; Khurana, S. Antibody affinity maturation and cross-variant activity following SARS-CoV-2 mRNA vaccination: Impact of prior exposure and sex. EBioMedicine 2021, 74, 103748. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Liu, Q.; Mei, H.; Wang, Y.; Cui, G.; Zhao, S. Serological reactivity of inactivated SARS-CoV-2 vaccine based on an S-RBD neutralizing antibody assay. Int. J. Infect. Dis. 2022, 117, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2022, 23, 189–199. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, 2223. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Liu, S.; Xu, M.; Hu, Y.; Lv, K.; Wang, Y.; Ma, Y.; Zhai, Y.; Yue, X.; Liu, L.; et al. Comparative global B cell receptor repertoire difference induced by SARS-CoV-2 infection or vaccination via single-cell V(D)J sequencing. Emerg. Microbes Infect. 2022, 11, 2007–2020. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020, 11, 6044. [Google Scholar] [CrossRef] [PubMed]



| Healthy Donors Cohort | SARS-CoV-2 Recovered Cohort | |
|---|---|---|
| Total, n | 25 | 58 |
| Sex | ||
| Female, n (%) | 12 (48.0) | 26 (44.8) |
| Male, n (%) | 13 (52.0) | 32 (55.2) |
| Age (years), Median (range) | 30 (26, 50) | 55.5 (17, 73) |
| ≤60, n (%) | 25 (100.0) | 40 (69.0) |
| >61, n (%) | 0 (0.0) | 18 (50.0) |
| Race/Ethnicity | ||
| Asian, n (%) | 25 (100.0) | 58 (100.0) |
| Disease severity | ||
| Mild, n (%) | 2 (3.4) | |
| Moderate, n (%) | 41 (70.7) | |
| Severe, n (%) | 15 (25.9) | |
| Any comorbidity index | ||
| Hypertension, n (%) | NA 1 | 7 (12.1) |
| Diabetes, n (%) | NA | 3 (5.2) |
| Gout, n (%) | NA | 2 (3.4) |
| Hospital days, Median (range) | 15 (5, 36) | |
| Vaccine type | ||
| CoronaVac, n (%) | 25 (100.0) | 16 (27.6) |
| BBIBP-CorV, n (%) | 0 (0.0) | 42 (72.4) |
| Time between infection and vaccine, (days), Median (range) | 388 (335, 437) |
| Patients | Sustainer Group | Decayer Group | X2 | p | |
|---|---|---|---|---|---|
| Age | |||||
| ≤60 | 34 | 13 | 21 | 1.574 | 0.2097 |
| >60 | 15 | 3 | 12 | ||
| Hospital days | |||||
| ≤14 | 19 | 7 | 12 | 0.2476 | 0.6187 |
| >14 | 30 | 9 | 21 | ||
| Sex | |||||
| Male | 28 | 10 | 18 | 0.2784 | 0.5977 |
| Female | 21 | 6 | 15 | ||
| Disease severity | |||||
| Mild and Moderate | 37 | 12 | 25 | 0.003344 | 0.9539 |
| Severe | 12 | 4 | 8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, L.; Guo, N.; Yao, Y.-P.; Zhang, C.; Xu, R.; Jiao, Y.-M.; Li, Y.-Q.; Song, Y.-R.; Wang, F.-S.; et al. Dynamics of SARS-CoV-2 Antibody Responses up to 9 Months Post-Vaccination in Individuals with Previous SARS-CoV-2 Infection Receiving Inactivated Vaccines. Viruses 2023, 15, 917. https://doi.org/10.3390/v15040917
Wang J, Huang L, Guo N, Yao Y-P, Zhang C, Xu R, Jiao Y-M, Li Y-Q, Song Y-R, Wang F-S, et al. Dynamics of SARS-CoV-2 Antibody Responses up to 9 Months Post-Vaccination in Individuals with Previous SARS-CoV-2 Infection Receiving Inactivated Vaccines. Viruses. 2023; 15(4):917. https://doi.org/10.3390/v15040917
Chicago/Turabian StyleWang, Jing, Lei Huang, Nan Guo, Ya-Ping Yao, Chao Zhang, Ruonan Xu, Yan-Mei Jiao, Ya-Qun Li, Yao-Ru Song, Fu-Sheng Wang, and et al. 2023. "Dynamics of SARS-CoV-2 Antibody Responses up to 9 Months Post-Vaccination in Individuals with Previous SARS-CoV-2 Infection Receiving Inactivated Vaccines" Viruses 15, no. 4: 917. https://doi.org/10.3390/v15040917
APA StyleWang, J., Huang, L., Guo, N., Yao, Y.-P., Zhang, C., Xu, R., Jiao, Y.-M., Li, Y.-Q., Song, Y.-R., Wang, F.-S., & Fan, X. (2023). Dynamics of SARS-CoV-2 Antibody Responses up to 9 Months Post-Vaccination in Individuals with Previous SARS-CoV-2 Infection Receiving Inactivated Vaccines. Viruses, 15(4), 917. https://doi.org/10.3390/v15040917






