Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection
Abstract
1. The Epstein–Barr Virus and Multiple Sclerosis: Connected, but How?
2. PASC
3. COVID-19 and Inflammation
4. PASC and EBV Reactivation
5. Mechanisms for the Maintenance of Inflammation after EBV Reactivation, Acute COVID-19 Infection, or Multiple Sclerosis Onset
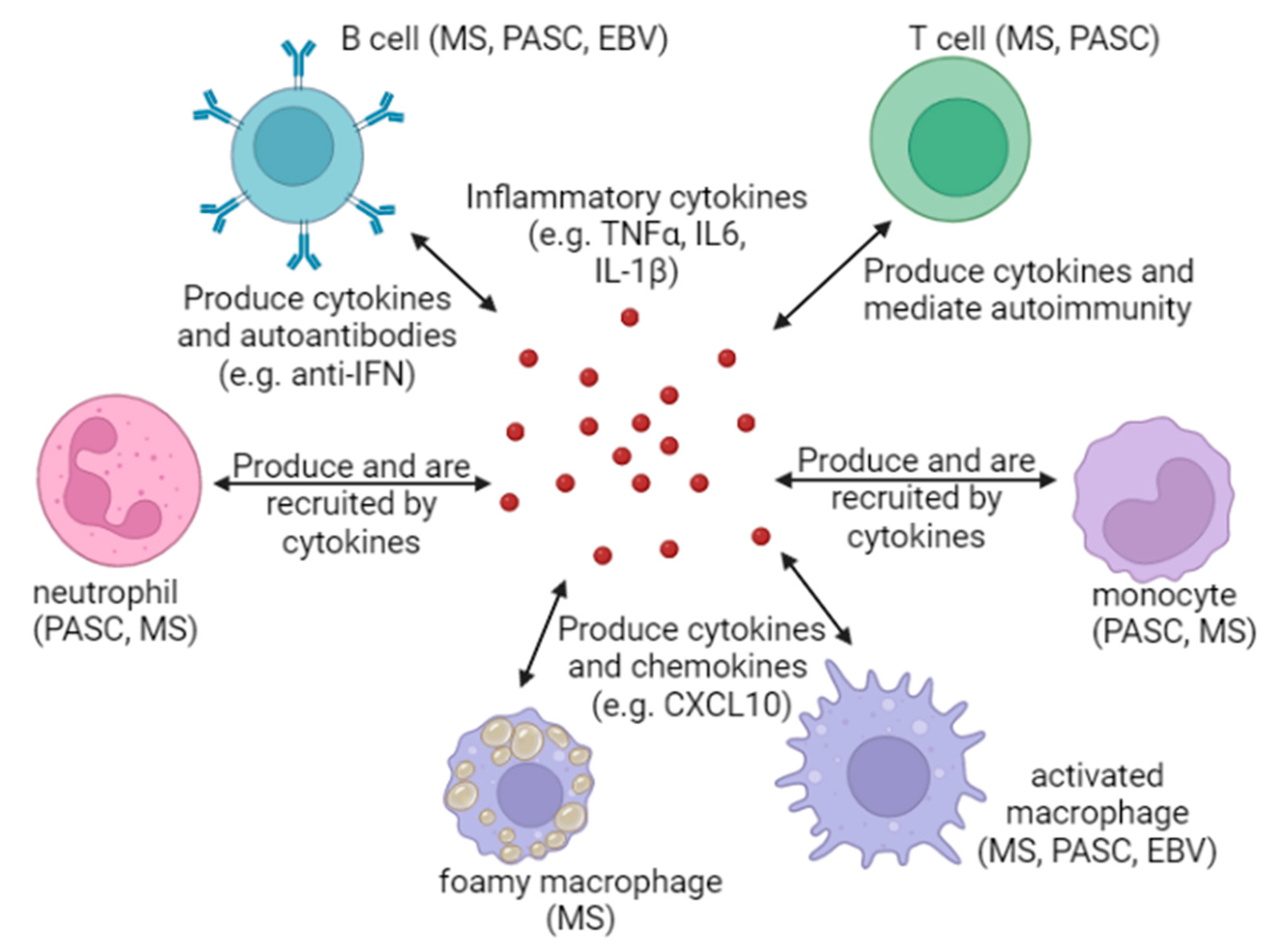
6. Inflammation: A Common Thread That Binds EBV, MS, and PASC
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kerr, J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J. Clin. Pathol. 2019, 72, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Rickinson, A.B. Epstein–Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Hacohen, Y.; Ciccarelli, O. New Evidence for Epstein-Barr Virus Infection as a Cause of Multiple Sclerosis. Neurology 2022, 98, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munch, M. Epstein-Barr Virus and Multiple Sclerosis. Epidemiology 2000, 11, 220–224. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.-P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein–Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies. Trends Mol. Med. 2020, 26, 296–310. [Google Scholar] [CrossRef]
- Levin, L.I.; Munger, K.L.; O’Reilly, E.J.; Falk, K.I.; Ascherio, A. Primary infection with the epstein-barr virus and risk of multiple sclerosis. Ann. Neurol. 2010, 67, 824–830. [Google Scholar] [CrossRef]
- Pakpoor, J.; Giovannoni, G.; Ramagopalan, S.V. Epstein–Barr virus and multiple sclerosis: Association or causation? Expert Rev. Neurother. 2013, 13, 287–297. [Google Scholar] [CrossRef]
- Simkins, T.J.; Duncan, G.J.; Bourdette, D. Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications. Curr. Neurol. Neurosci. Rep. 2021, 21, 26. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primer 2018, 4, 43. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Meier, U.-C.; Cipian, R.C.; Karimi, A.; Ramasamy, R.; Middeldorp, J.M. Cumulative Roles for Epstein-Barr Virus, Human Endogenous Retroviruses, and Human Herpes Virus-6 in Driving an Inflammatory Cascade Underlying MS Pathogenesis. Front. Immunol. 2021, 12, 757302. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.F.; Serafini, B.; Piras, E.; Severa, M.; Coccia, E.M.; Rosicarelli, B.; Ruggieri, S.; Gasperini, C.; Buttari, F.; Centonze, D.; et al. Increased CD8+ T Cell Response to Epstein-Barr Virus Lytic Antigens in the Active Phase of Multiple Sclerosis. PLoS Pathog. 2013, 9, e1003220. [Google Scholar] [CrossRef] [PubMed]
- Lunemann, J.D. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain 2006, 129, 1493–1506. [Google Scholar] [CrossRef]
- Mrad, M.F.; Saba, E.S.; Nakib, L.; Khoury, S.J. Exosomes From Subjects With Multiple Sclerosis Express EBV-Derived Proteins and Activate Monocyte-Derived Macrophages. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1004. [Google Scholar] [CrossRef] [PubMed]
- Soldan, S.S.; Su, C.; Lamontagne, R.J.; Grams, N.; Lu, F.; Zhang, Y.; Gesualdi, J.D.; Frase, D.M.; Tolvinski, L.E.; Martin, K.; et al. Epigenetic Plasticity Enables CNS-Trafficking of EBV-infected B Lymphocytes. PLOS Pathog. 2021, 17, e1009618. [Google Scholar] [CrossRef]
- Spender, L.C.; Cornish, G.H.; Rowland, B.; Kempkes, B.; Farrell, P.J. Direct and Indirect Regulation of Cytokine and Cell Cycle Proteins by EBNA-2 during Epstein-Barr Virus Infection. J. Virol. 2001, 75, 3537–3546. [Google Scholar] [CrossRef]
- Bhaduri-McIntosh, S.; McIntosh, M. Inflammasome, the Constitutive Heterochromatin Machinery, and Replication of an Oncogenic Herpesvirus. Viruses 2021, 13, 846. [Google Scholar] [CrossRef]
- Burton, E.M.; Goldbach-Mansky, R.; Bhaduri-McIntosh, S. A promiscuous inflammasome sparks replication of a common tumor virus. Proc. Natl. Acad. Sci. USA 2020, 117, 1722–1730. [Google Scholar] [CrossRef]
- Kahan, S.M.; Wherry, E.J.; Zajac, A.J. T cell exhaustion during persistent viral infections. Virology 2015, 479–480, 180–193. [Google Scholar] [CrossRef]
- Pender, M.P.; Csurhes, P.A.; Burrows, J.M.; Burrows, S.R. Defective T-cell control of Epstein–Barr virus infection in multiple sclerosis. Clin. Transl. Immunol. 2017, 6, e126. [Google Scholar] [CrossRef]
- Albert, L.J. Molecular Mimicry and Autoimmunity. N. Engl. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular Mimicry as a Mechanism of Autoimmune Disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jiménez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.-M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Hwang, S.-I.; Roy, D.B.; Lundgren, D.H.; Price, J.V.; Ousman, S.S.; Fernald, G.H.; Gerlitz, B.; Robinson, W.H.; Baranzini, S.E.; et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 2008, 451, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Wekerle, H. Multiple sclerosis sparked by virus-led autoimmunity. Nature 2022, 603, 230–232. [Google Scholar] [CrossRef]
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-acute sequelae of COVID-19: A metabolic perspective. eLife 2022, 11, e78200. [Google Scholar] [CrossRef]
- Calabria, M.; García-Sánchez, C.; Grunden, N.; Pons, C.; Arroyo, J.A.; Gómez-Anson, B.; García, M.d.C.E.; Belvís, R.; Morollón, N.; Vera Igual, J.; et al. Post-COVID-19 fatigue: The contribution of cognitive and neuropsychiatric symptoms. J. Neurol. 2022, 269, 3990–3999. [Google Scholar] [CrossRef]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: Prospective cohort study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef]
- Vallings, R. A case of chronic fatigue syndrome triggered by influenza H1N1 (swine influenza). J. Clin. Pathol. 2010, 63, 184–185. [Google Scholar] [CrossRef]
- Sneller, M.C.; Liang, C.J.; Marques, A.R.; Chung, J.Y.; Shanbhag, S.M.; Fontana, J.R.; Raza, H.; Okeke, O.; Dewar, R.L.; Higgins, B.P.; et al. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann. Intern. Med. 2022, 175, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Sans, H.M.; Forman, C.A.; Nylander, A.N.; Ho, H.; Lu, S.; Goldberg, S.A.; Hoh, R.; Tai, V.; Munter, S.E.; et al. Plasma Markers of Neurologic Injury and Inflammation in People With Self-Reported Neurologic Postacute Sequelae of SARS-CoV-2 Infection. Neurol.-Neuroimmunol. Neuroinflamm. 2022, 9, e200003. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef] [PubMed]
- Ruenjaiman, V.; Sodsai, P.; Kueanjinda, P.; Bunrasmee, W.; Klinchanhom, S.; Reantragoon, R.; Tunvirachaisakul, C.; Manothummetha, K.; Mejun, N.; Liengswangwong, K.; et al. Impact of SARS-CoV-2 infection on the profiles and responses of innate immune cells after recovery. J. Microbiol. Immunol. Infect. 2022, 55, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, S.R.; Gan, P.-Y. Cytokines: Names and Numbers You Should Care About. Clin. J. Am. Soc. Nephrol. 2015, 10, 2243–2254. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, Z.; Abdel-Mohsen, M.; Chen, X.; Montaner, L.J. Tissue injury and leukocyte changes in post-acute sequelae of SARS-CoV-2: Review of 2833 post-acute patient outcomes per immune dysregulation and microbial translocation in long COVID. J. Leukoc. Biol. 2023, 113, 236–254. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 746021. [Google Scholar] [CrossRef] [PubMed]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; Girndt, M.; et al. Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19. J. Med. Virol. 2023, 95, e28364. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Thomas, R.; Shin, M.-G.; Neidleman, J.; Luo, X.; Hoh, R.; Anglin, K.; Huang, B.; Argueta, U.; et al. Long COVID manifests with T cell dysregulation, inflammation, and an uncoordinated adaptive immune response to SARS-CoV-2. bioRxiv 2023. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef] [PubMed]
- Swank, Z.; Senussi, Y.; Alter, G.; Walt, D.R. Persistent circulating SARS-CoV-2 spike is associated with post-acute COVID-19 sequelae. Medrxiv 2022. [Google Scholar] [CrossRef]
- Apostolou, E.; Rizwan, M.; Moustardas, P.; Sjögren, P.; Bertilson, B.C.; Bragée, B.; Polo, O.; Rosén, A. Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 2022, 13, 949787. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Solomon, J.J.; Heyman, B.; Ko, J.P.; Condos, R.; Lynch, D.A. CT of Post-Acute Lung Complications of COVID-19. Radiology 2021, 301, E383–E395. [Google Scholar] [CrossRef]
- Soares, M.N.; Eggelbusch, M.; Naddaf, E.; Gerrits, K.H.L.; Schaaf, M.; Borst, B.; Wiersinga, W.J.; Vugt, M.; Weijs, P.J.M.; Murray, A.J.; et al. Skeletal muscle alterations in patients with acute Covid-19 and post-acute sequelae of Covid-19. J. Cachexia Sarcopenia Muscle 2022, 13, 11–22. [Google Scholar] [CrossRef]
- Amin, S.; Aktar, S.; Rahman, M.; Chowdhury, M.M.H. NLRP3 inflammasome activation in COVID-19: An interlink between risk factors and disease severity. Microbes Infect. 2022, 24, 104913. [Google Scholar] [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Akinyemi, I.A.; Chitre, S.A.; Loeb, J.C.; Lednicky, J.A.; McIntosh, M.T.; Bhaduri-McIntosh, S. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology 2022, 568, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Blevins, H.M.; Xu, Y.; Biby, S.; Zhang, S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022, 14, 879021. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.-D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ambati, M.; Apicella, I.; Wang, S.; Narendran, S.; Leung, H.; Pereira, F.; Nagasaka, Y.; Huang, P.; Varshney, A.; Baker, K.L.; et al. Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2102975118. [Google Scholar] [CrossRef]
- Németh, Z.K.; Szűcs, A.; Vitrai, J.; Juhász, D.; Németh, J.P.; Holló, A. Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: A retrospective case-control study. Ideggyógy. Szle. 2021, 74, 389–396. [Google Scholar] [CrossRef]
- Weber, A.N.R. Targeting the NLRP3 Inflammasome via BTK. Front. Cell Dev. Biol. 2021, 9, 630479. [Google Scholar] [CrossRef]
- Xian, H.; Liu, Y.; Rundberg Nilsson, A.; Gatchalian, R.; Crother, T.R.; Tourtellotte, W.G.; Zhang, Y.; Aleman-Muench, G.R.; Lewis, G.; Chen, W.; et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 2021, 54, 1463–1477.e11. [Google Scholar] [CrossRef]
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.B.; et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019, 15, 556–559. [Google Scholar] [CrossRef]
- Perera, A.P.; Fernando, R.; Shinde, T.; Gundamaraju, R.; Southam, B.; Sohal, S.S.; Robertson, A.A.B.; Schroder, K.; Kunde, D.; Eri, R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xie, X.; Feng, X.-L.; Xu, L.; Han, J.-B.; Yu, D.; Zou, Q.-C.; Liu, Q.; Li, X.; Ma, G.; et al. Specific inhibition of the NLRP3 inflammasome suppresses immune overactivation and alleviates COVID-19 like pathology in mice. eBioMedicine 2022, 75, 103803. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein–Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Wood, J.; Jaycox, J.; Lu, P.; Dhodapkar, R.M.; Gehlhausen, J.R.; Tabachnikova, A.; Tabacof, L.; Malik, A.A.; Kamath, K.; et al. Distinguishing features of Long COVID identified through immune profiling; Infectious Diseases (except HIV/AIDS). Medrxiv 2022. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Cui, J.; Yan, W.; Xu, S.; Wang, Q.; Zhang, W.; Liu, W.; Ni, A. Anti-Epstein–Barr virus antibodies in Beijing during 2013–2017: What we have found in the different patients. PLoS ONE 2018, 13, e0193171. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Kongsomros, S.; Thanunchai, M.; Manopwisedjaroen, S.; Na-Ek, P.; Wang, S.-F.; Taechalertpaisarn, T.; Thitithanyanont, A. Trogocytosis with monocytes associated with increased α2,3 sialic acid expression on B cells during H5N1 influenza virus infection. PLoS ONE 2020, 15, e0239488. [Google Scholar] [CrossRef]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef]
- Verma, D.; Church, T.M.; Swaminathan, S. Epstein-Barr Virus Lytic Replication Induces ACE2 Expression and Enhances SARS-CoV-2 Pseudotyped Virus Entry in Epithelial Cells. J. Virol. 2021, 95, e00192-21. [Google Scholar] [CrossRef] [PubMed]
- Khani, E.; Entezari-Maleki, T. Fluvoxamine and long COVID-19; a new role for sigma-1 receptor (S1R) agonists. Mol. Psychiatry 2022, 27, 3562. [Google Scholar] [CrossRef] [PubMed]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Reed, A.; Desai, S.R.; Devaraj, A.; Faiez, T.S.; Laverty, S.; Kanwal, A.; Esneau, C.; Liu, M.K.C.; Kamal, F.; et al. A persistent neutrophil-associated immune signature characterizes post–COVID-19 pulmonary sequelae. Sci. Transl. Med. 2022, 14, eabo5795. [Google Scholar] [CrossRef]
- Ryan, F.J.; Hope, C.M.; Masavuli, M.G.; Lynn, M.A.; Mekonnen, Z.A.; Yeow, A.E.L.; Garcia-Valtanen, P.; Al-Delfi, Z.; Gummow, J.; Ferguson, C.; et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022, 20, 26. [Google Scholar] [CrossRef]
- De Bondt, M.; Hellings, N.; Opdenakker, G.; Struyf, S. Neutrophils: Underestimated Players in the Pathogenesis of Multiple Sclerosis (MS). Int. J. Mol. Sci. 2020, 21, 4558. [Google Scholar] [CrossRef]
- Hertwig, L.; Pache, F.; Romero-Suarez, S.; Stürner, K.H.; Borisow, N.; Behrens, J.; Bellmann-Strobl, J.; Seeger, B.; Asselborn, N.; Ruprecht, K.; et al. Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica. Mult. Scler. J. 2016, 22, 160–173. [Google Scholar] [CrossRef]
- Haidar, M.; Loix, M.; Vanherle, S.; Dierckx, T.; Vangansewinkel, T.; Gervois, P.; Wolfs, E.; Lambrichts, I.; Bogie, J.F.J.; Hendriks, J.J.A. Targeting lipophagy in macrophages improves repair in multiple sclerosis. Autophagy 2022, 18, 2697–2710. [Google Scholar] [CrossRef]
- Vazirinejad, R.; Ahmadi, Z.; Kazemi Arababadi, M.; Hassanshahi, G.; Kennedy, D. The Biological Functions, Structure and Sources of CXCL10 and Its Outstanding Part in the Pathophysiology of Multiple Sclerosis. Neuroimmunomodulation 2014, 21, 322–330. [Google Scholar] [CrossRef]
- De Laere, M.; Berneman, Z.N.; Cools, N. To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis. J. Neuropathol. Exp. Neurol. 2018, 77, 178–192. [Google Scholar] [CrossRef]
- Littlefield, K.M.; Watson, R.O.; Schneider, J.M.; Neff, C.P.; Yamada, E.; Zhang, M.; Campbell, T.B.; Falta, M.T.; Jolley, S.E.; Fontenot, A.P.; et al. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLOS Pathog. 2022, 18, e1010359. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.H.; Smith, F.L.; Baumgarth, N. B Cell Activation and Response Regulation During Viral Infections. Viral Immunol. 2020, 33, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I Interferon Inhibits Interleukin-1 Production and Inflammasome Activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Bolze, A.; Jouanguy, E.; Zhang, S.-Y.; Cobat, A.; Notarangelo, L.D.; Su, H.C.; Abel, L.; Casanova, J.-L. Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med 2020, 1, 14–20. [Google Scholar] [CrossRef]
- Musella, A.; Fresegna, D.; Rizzo, F.R.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bruno, A.; Dolcetti, E.; Buttari, F.; et al. ‘Prototypical’ pro-inflammatory cytokine (IL-1) in multiple sclerosis: Role in pathogenesis and therapeutic targeting. Expert Opin. Ther. Targets 2020, 24, 37–46. [Google Scholar] [CrossRef]
- Hooftman, A.; Zotta, A.; O’Neill, L.A.J. Therapeutic opportunities targeting the NLRP3 inflammasome. In Inflammasome Biology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 555–563. [Google Scholar] [CrossRef]
- Pang, L.; Liu, H.; Quan, H.; Sui, H.; Jia, Y. Development of novel oridonin analogs as specifically targeted NLRP3 inflammasome inhibitors for the treatment of dextran sulfate sodium-induced colitis. Eur. J. Med. Chem. 2023, 245, 114919. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, B.A.; Bhaduri-McIntosh, S. Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses 2023, 15, 949. https://doi.org/10.3390/v15040949
Rousseau BA, Bhaduri-McIntosh S. Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses. 2023; 15(4):949. https://doi.org/10.3390/v15040949
Chicago/Turabian StyleRousseau, Beth A., and Sumita Bhaduri-McIntosh. 2023. "Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection" Viruses 15, no. 4: 949. https://doi.org/10.3390/v15040949
APA StyleRousseau, B. A., & Bhaduri-McIntosh, S. (2023). Inflammation and Epstein–Barr Virus at the Crossroads of Multiple Sclerosis and Post-Acute Sequelae of COVID-19 Infection. Viruses, 15(4), 949. https://doi.org/10.3390/v15040949






