Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Myocarditis and Acute Coronary Syndrome after COVID-19 Vaccine
3. Hypertension and COVID-19 Vaccine
4. Thromboembolic Events after COVID-19 Vaccine
5. SARS-CoV-2 Vaccination-Induced Thrombotic Thrombocytopenia (VITT)
- (i).
- A COVID-19 vaccine administered between 4 and 42 days before the onset of symptoms;
- (ii).
- Venous or arterial thrombosis that may occur in uncommon sites such as cerebral and splanchnic vessels;
- (iii).
- Thrombocytopenia;
- (iv).
- A positive PF4 HIT ELISA result (non-rapid tests should be used);
- (v).
- Elevated D-dimer levels.
6. Incidence Rates of Acute Myocardial Infarction, Deep Vein Thrombosis, Pulmonary Embolism, Myocarditis and Pericarditis Following COVID-19 Vaccine
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bramwell, V.W.; Perrie, Y. The rational design of vaccines. Drug Discov. Today 2005, 10, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Schijns, V.; Majhen, D.; van der Ley, P.; Thakur, A.; Summerfield, A.; Berisio, R.; Nativi, C.; Fernandez-Tejada, A.; Alvarez-Dominguez, C.; Gizurarson, S.; et al. Rational vaccine design in times of emerging diseases: The critical choices of immunological correlates of protection, vaccine antigen and immunomodulation. Pharmaceutics 2021, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell. Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Shahzamani, K.; Mahmoudian, F.; Ahangarzadeh, S.; Ranjbar, M.M.; Beikmohammadi, L.; Bahrami, S.; Mohammadi, E.; Esfandyari, S.; Alibakhshi, A.; Javanmard, S.H. Vaccine design and delivery approaches for COVID-19. Int. Immunopharmacol. 2021, 100, 108086. [Google Scholar] [CrossRef]
- Bayat, M.; Asemani, Y.; Najafi, S. Essential considerations during vaccine design against COVID-19 and review of pioneering vaccine candidate platforms. Int. Immunopharmacol. 2021, 97, 107679. [Google Scholar] [CrossRef]
- Lozano-Rodriguez, R.; Valentin-Quiroga, J.; Avendano-Ortiz, J.; Martin-Quiros, A.; Pascual-Iglesias, A.; Terron-Arcos, V.; Montalban-Hernandez, K.; Casalvilla-Duenas, J.C.; Bergon-Gutierrez, M.; Alcami, J.; et al. Cellular and humoral functional responses after BNT162b2 mRNA vaccination differ longitudinally between naive and subjects recovered from COVID-19. Cell Rep. 2022, 38, 110235. [Google Scholar] [CrossRef]
- Lau, C.L.; Mayfield, H.J.; Sinclair, J.E.; Brawn, S.J.; Waller, M.; Enjeti, A.K.; Baird, A.; Short, K.R.; Mengersen, K.; Litt, J. Risk-benefit analysis of the AstraZeneca COVID-19 vaccine in Australia using a Bayesian network modelling framework. Vaccine 2021, 39, 7429–7440. [Google Scholar] [CrossRef]
- Lund, L.C.; Hallas, J.; Nielsen, H.; Koch, A.; Mogensen, S.H.; Brun, N.C.; Christiansen, C.F.; Thomsen, R.W.; Pottegard, A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect. Dis. 2021, 21, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattor, T.H.; Tjonnfjord, G.E.; et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Torjesen, I. COVID-19: Risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. Br. Med. J. 2021, 373, n1005. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. Br. Med. J. 2021, 374, n1931. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kru¨ger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Y.B.; Wang, Q.; Li, J.Y.; Zhou, Z.J.; Liao, C.H.; Ge, X.-Y. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020, 22, 221–225. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef]
- Devaux, C.A.; Camoin-Jau, L. An update on angiotensin-converting enzyme 2 structure/functions, polymorphism, and duplicitous nature in the pathophysiology of coronavirus disease 2019: Implications for vascular and coagulation disease associated with severe acute respiratory syndrome coronavirus infection. Front. Microbiol. 2022, 13, 1042200. [Google Scholar] [CrossRef]
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.-D.; Sacco, C.; Bertuzzi, A.; et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb. Res. 2020, 191, 9–14. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. COVID-19 vaccine and myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Shiravi, A.A.; Ardekani, A.; Sheikhbahaei, E.; Heshmat-Ghahdarijani, K. Cardiovascular complications of SARS-CoV-2 vaccines: An overview. Cardiol. Ther. 2022, 11, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.; Neil, M.; Fenton, N.; McLachlan, S.; Smalley, J.; Guetzkow, J.; Engler, J.; Russell, D.; Rose, J. Official Mortality Data for England Reveal Systematic Undercounting of Deaths Occurring within First Two Weeks of COVID-19 Vaccination; Preprint available online (not peer reviewed); Research Gate Net: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Oleszak, F.; Maryniak, A.; Botti, E.; Abrahim, C.; Salifu, M.O.; Youssef, M.; Henglein, V.L.; McFarlane, S.I. Myocarditis associated with COVID-19. Am. J. Med. Case Rep. 2020, 8, 498–502. [Google Scholar] [CrossRef]
- Wu, S.; Zou, G.; Lin, K.; Zhang, D. Effects of COVID-19 on the cardiovascular system and implications for management. J. Xiangya Med. 2021, 6, 7. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Husby, A.; Vinslov Hansen, J.; Fosbol, E.; Myrup Thiesson, E.; Madsen, M.; Thomsen, R.W.; Sorensen, H.T.; Andersen, M.; Wohlfahrt, J.; Gislason, G.; et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. Br. Med. J. 2021, 375, e068665. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Lai, F.T.T.; Li, X.; Peng, K.; Huang, L.; Ip, P.; Tong, X.; Ling Chui, C.S.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; et al. Carditis After COVID-19 Vaccination with a Messenger RNA Vaccine and an Inactivated Virus Vaccine: A Case–Control Study. Ann. Intern. Med. 2022, 175, 362–370. [Google Scholar] [CrossRef]
- Sun, C.L.F.; Jaffre, E.; Levi, R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Nat. Sci. Rep. 2022, 12, 6978. [Google Scholar] [CrossRef]
- Husby, A.; Lovdal Gulseth, H.; Hovi, P.; Vinslov Hansen, J.; Pihlström, N.; Gunnes, N.; Härkänen, T.; Dahl, J.; Karlstad, O.; Heliö, T.; et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNAvaccination in four Nordic countries: Population based cohort study. Br. Med. J. Med. 2023, 2, e000373. [Google Scholar] [CrossRef]
- Torjsesen, I. COVID-19: Pfizer-BioNTech vaccine is “likely” responsible for deaths of some elderly patients, Norwegian review finds. Br. Med. J. 2021, 373, n1372. [Google Scholar] [CrossRef] [PubMed]
- King, W.W.; Petersen, M.R.; Matar, R.M.; Budweg, J.B.; Cuervo Pardo, L.; Petersen, J.W. Myocarditis following mRNA vaccination against SARS-CoV-2, a case series. Am. Heart. J. Plus Cardiol. Res. Pract. 2021, 8, 100042. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Choi, J.I.; Hosseini, F.; Roberts, J.; Ramanathan, K.; Ong, K. Acute myocarditis following mRNA-1273 SARS-CoV-2 vaccination. CJC Open 2021, 3, 1410–1412. [Google Scholar] [CrossRef] [PubMed]
- Gundry, S.F. mRNA COVID Vaccines Dramatically Increase Endothelial Inflammatory Markers and ACS Risk as Measured by PULS Cardiac Test: A Warning. Circulation 2021, 144, A10712. Available online: www.ahajournals.org›abs›circ.144.suppl_1.10712 (accessed on 6 April 2023).
- Salzman, M.B.; Huang, C.W.; O’Brien, C.M.; Castillo, R.D. Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination. Emerg. Infect. Dis. 2021, 27, 1944–1948. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Herman, M.A.; Lipstich, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Buchhorn, R.; Meyer, C.; Schulze-Forster, K.; Junker, J.; Heidecke, H. Autoantibody Release in Children after Corona Virus mRNA Vaccination: A risk factor of Multisystem Inflammatory Syndrome? Vaccines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Meylan, S.; Livio, F.; Foerster, M.; Genoud, P.J.; Marguet, F.; Wuerzner, G.; CHUV COVID Vaccination Center. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension 2021, 77, e56–e57. [Google Scholar] [CrossRef]
- Zappa, M.; Verdecchia, P.; Spanevello, A.; Visca, D.; Angeli, F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur. J. Intern. Med. 2021, 90, 111–113. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Liu, J.; Dalamaga, M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metab. Open 2022, 13, 100159. [Google Scholar] [CrossRef]
- Bouhanick, B.; Montastruc, F.; Tessier, S.; Brusq, C.; Bongard, V.; Senard, J.M.; Montastruc, J.-L.; Herin, F. Hypertension and COVID-19 vaccines: Are there any differences between the different vaccines? A safety signal. Eur. J. Clin. Pharmacol. 2021, 77, 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, K.J. Suspected cardiovascular side effects of two COVID-19 vaccines. J. Biol. Today World 2021, 10, 001–006. [Google Scholar]
- Kaur, R.J.; Dutta, S.; Charan, J.; Bhardwaj, P.; Tandon, A.; Yadav, D.; Islam, S.; Haque, M. Cardiovascular adverse events reported from COVID-19 vaccines: A study based on WHO Database. Int. J. Gen. Med. 2021, 14, 3909–3927. [Google Scholar] [CrossRef] [PubMed]
- Khani, E.; Entezari-Maleki, T. Hypertensive crisis following COVID-19 vaccination. J. Clin. Pharmacol. 2022, 62, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. From RNA to Protein. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; Available online: https://www.ncbi.nlm.nih.gov/books/NBK26829/ (accessed on 6 April 2023).
- Shamir, M.; Bar-on, Y.; Phillips, R.; Milo, R. SnapShot: Timescales in cell biology. Cell 2016, 164, 1302. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; Reboldi, G.; Visca, D.; Verdecchia, P. SARS-CoV-2 vaccines: Lights and shadows. Eur. J. Intern. Med. 2021, 88, 1–8. [Google Scholar] [CrossRef]
- Tran, V.N.; Nguyen, H.A.; Le, T.T.A.; Truong, T.T.; Nguyen, P.T.; Nguyen, T.T.H. Factors influencing adverse events following immunization with AZD1222 in Vietnamese adults during first half of 2021. Vaccine 2021, 39, 6485–6491. [Google Scholar] [CrossRef]
- Bouhanick, B.; Brusq, C.; Bongard, V.; Tessier, S.; Montastruc, J.L.; Senard JMMontastruc, F.; Herin, F. Blood pressure measurements after mRNA-SARS-CoV-2 tozinameran vaccination: A retrospective analysis in a university hospital in France. J. Hum. Hypertens. 2022, 36, 580–581. [Google Scholar] [CrossRef]
- Simonini, M.; Scarale, M.G.; Tunesi, F.; Manunta, P.; Lanzani, C. COVID-19 vaccines effect on blood pressure. Eur. J. Intern. Med. 2022, 105, 109–110. [Google Scholar] [CrossRef]
- Syrigos, N.; Kollias, A.; Grapsa, D.; Fyta, E.; Kyriakoulis, K.G.; Vathotis, J.; Kotteas, E.; Syrigou, E. Significant Increase in Blood Pressure Following BNT162b2 mRNA COVID-19 Vaccination among Healthcare Workers: A Rare Event. Vaccines 2022, 10, 745. [Google Scholar] [CrossRef]
- Ch’ng, C.C.; Ong, L.M.; Wong, K.M. Changes in blood pressure after Messenger RNA COVID-19 vaccination. Med. J. Malays. 2022, 77, 768–770. [Google Scholar]
- Angeli, F.; Reboldi, G.; Trapasso, M.; Santilli, G.; Zappa, M.; Verdecchia, P. Blood pressure increase following COVID-19 vaccination: A systematic overview and meta- analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Yue, Q.Y.; Chocron, R.; Sanchez, O.; Lillo-Le Louet, A. Vaccination against COVID-19: Insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur. Respir. J. 2021, 58, 2100956. [Google Scholar] [CrossRef]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Belleke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-Garcia, J.; et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Korwarz, E.; Krutske, L.; Külp, M.; Streb, P.; Larghero, P.; Reis, J.; Bracharz, S.; Engler, T.; Kochanek, S.; Marschalek, R. Vaccine-induced COVID-19 mimicry syndrome. eLife 2022, 11, e74974. [Google Scholar] [CrossRef] [PubMed]
- Welsh, K.J.; Baumblatt, J.; Chege, W.; Goud, R.; Nair, N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2021, 39, 3329–3332. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 vaccine-related thrombosis: A systematic review and exploratory analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Greinacher, A. Heparin-Induced Thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef]
- Islam, A.; Sheraz Bashir, M.; Joyce, K.; Rashid, H.; Laher, I.; Elshazly, S. An Update on COVID-19 Vaccine induced thrombotic thrombocytopenia syndrome and some management recommendations. Molecules 2021, 26, 5004. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Tamborska, A.; Singh, B.; Craven, B.; Marigold, R.; Arthur-Farraj, P.; Yeo, J.M.; Zhang, L.; Hassan-Smith, G.; Jones, M.; et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: A multicentre cohort study. Lancet 2021, 398, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Elalamy, I.; Gerotziafas, G.; Alamanowitch, S.; Laroche, J.P.; van Dreden, P.; Ageno, W.; Beyer-Westendorf, J.; Cohen, A.T.; Jimenez, D.; Brenner, B.; et al. SARS-CoV-2 vaccine and thrombosis: An expert consensus on vaccine-induced immune thrombotic thrombocytopenia. Thromb. Haemost. 2021, 121, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Afshar, Z.M.; Barary, M.; Babazadeh, A.; Hosseinzadeh, R.; Alijanpour, A.; Miri, S.R.; Sio, T.T.; Sullman, M.J.M.; Carson-Chahhoud, K.; Langer, F.; et al. SARS-CoV-2-related and COVID-19 vaccine-induced thromboembolic events: A comparative review. Rev. Med. Virol. 2022, 32, e2327. [Google Scholar] [CrossRef] [PubMed]
- Abrams, C.S.; Barnes, G.D. SARS-CoV-2 vaccination-induced thrombotic thrombocytopenia: A rare but serious immunological complication. Annu. Rev. Med. 2023, 74, 65–74. [Google Scholar] [CrossRef]
- Franchini, M.; Liumbruno, G.M.; Pezzo, M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur. J. Haematol. 2021, 107, 173–180. [Google Scholar] [CrossRef]
- Gabarin, N.; Arnold, D.M.; Nazy, I.; Warkentin, T.E. Treatment of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin. Hematol. 2022, 59, 89–96. [Google Scholar] [CrossRef]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Li, X.; Ostropolets, A.; Makadia, R.; Shoaibi, A.; Rao, G.; Sena, A.G.; Martinez-Hernandez, E.; Delmestri, A.; Verhamme, K.; Rijbeek, P.R.; et al. Characterising the background incidence rates of adverse events of special interest for COVID-19 vaccines in eight countries: Multinational network cohort study. Br. Med. J. 2021, 373, n1435. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, S.Y.; Ahn, J.G.; Park, S.J.; Shoenfeld, Y.; Kronbichler, A.; Koyanagi, A.; Dragioti, E.; Tizaoui, K.; Hong, S.H.; et al. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: A pharmacovigilance analysis using WHO international database. J. Med. Virol. 2021, 94, 1085–1095. [Google Scholar] [CrossRef]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936–3948.e10. [Google Scholar] [CrossRef]
- Angyal, A.; Longet, S.; Moore, S.C.; Payne, R.P.; Harding, A.; Tipton, T.; Rongkard, P.; Ali, M.; Hering, L.M.; Meardon, N.; et al. T-Cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: A multicentre prospective cohort study. Lancet Microbe 2022, 3, e21–e31. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, D.; Siracusano, G.; Mayora-Neto, M.; Pastori, C.; Fantoni, T.; Lytras, S. Analysis of Antibody Neutralisation Activity against SARS-CoV-2 Variants and Seasonal Human Coronaviruses NL63, HKU1, and 229E Induced by Three Different COVID-19 Vaccine Platforms. Vaccines 2023, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef]
- Tan, S.T.; Kwan, A.T.; Rodriguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, S.; Lo, N.C. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Wang, Z.; Cho, A.; Gaebler, C.; Tanfous, T.B.; DaSilva, J.; Bednarski, E.; Ramos, V.; Zong, S.; Johnson, B.; et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature 2022, 607, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Wolf, C.; Köppert, S.; Becza, N.; Kuerten, S.; Kirchenbaum, G.A.; Lehmann, P.V. Antibody levels poorly reflect on the frequency of memory B cells generated following SARS-CoV-2, Seasonal Influenza, or EBV infection. Cells 2022, 11, 3662. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.; Mateus, J.; Dan, J.M.; Rydyznski Moderbacher, C.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Microbiol. 2021, 22, 74–85. [Google Scholar] [CrossRef]
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Derqui Fernandez, N.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Yu, X.; Jiang, W.; Chen, S.; Wang, R.; Wang, M.; Jiao, S.; Yang, Y.; Wang, W.; et al. Antibody-dependent enhancement (ADE) of SARSCoV-2 pseudoviral infection requires FcγRIIB and virus-antibody complex with bivalent interaction. Nat. Commun. Biol. 2022, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Guérin, P.; Yahi, N.; Azzaz, F.; Chahinian, H.; Sabatier, J.M.; Fantini, J. Structural dynamics of the SARS-CoV-2 Spike protein: A 2-year retrospective analysis of SARS-CoV-2 variants (from Alpha to Omicron) reveals an early divergence between conserved and variable epitopes. Molecules 2022, 27, 3851. [Google Scholar] [CrossRef] [PubMed]
- Roggero, R.; Robert-Hebmann, V.; Harrington, S.; Roland, J.; Vergne, L.; Jaleco, S.; Devaux, C.; Biard-Piechaczyk, M. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol. 2001, 75, 7637–7650. [Google Scholar] [CrossRef]
- Ahr, B.; Robert-Hebmann, V.; Devaux, C.; Biard-Piechaczyk, M. Apoptosis of uninfected cells induced by HIV envelope glycoproteins. Retrovirology 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, D.S.; Rader, D.J. Renin-angiotensin system and atherothrombotic disease: From genes to treatment. Arch. Intern. Med. 2003, 163, 1155–1164. [Google Scholar] [CrossRef]
- Gallo, G.; Calvez, V.; Savoia, C. Hypertension and COVID-19: Current evidence and perspectives. High Blood Press. Cardiovasc. Prevent. 2022, 29, 115–123. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Kagiyama, S.; Philips, M.I. The multiple actions of angiotensin II in atherosclerosis. Regul. Pept. 2000, 93, 65–77. [Google Scholar] [CrossRef]
- Heeneman, S.; Haendeler, J.; Saito, Y.; Ishida, M.; Berk, B.C. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J. Biol. Chem. 2000, 275, 15926–15932. [Google Scholar] [CrossRef]
- Senchenkova, E.Y.; Russell, J.; Esmon, C.T.; Granger, D.N. Roles of coagulation and fibrinolysis in angiotensin II enhanced microvascular thrombosis. Microcirculation 2014, 21, 401–407. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.M.; Griendling, K.K. NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol. 2009, 302, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Raoult, D. The impact of COVID-19 on populations living at high altitude: Role of hypoxia inducible factors (HIFs) signaling pathway in SARS-CoV-2 infection and replication. Front. Physiol. 2022, 13, 960308. [Google Scholar] [CrossRef]
- Devaux, C.A.; Lagier, J.C. Unraveling the Underlying Molecular Mechanism of ‘Silent Hypoxia’ in COVID-19 Patients Suggests a Central Role for Angiotensin II Modulation of the AT1R-Hypoxia-Inducible Factor Signaling Pathway. J. Clin. Med. 2023, 12, 2445. [Google Scholar] [CrossRef] [PubMed]
- Nabah, Y.N.A.; Mateo, T.; Estellés, R.; Mata, M.; Zagorski, J.; Sarau, H.; Cortijo, J.; Morcillo, J.; Jose, P.J.; Sanz, M.-J. Angiotensin II induces neutrophil accumulation in vivo through generation and release of CXC chemokines. Circulation 2004, 110, 3581–3586. [Google Scholar] [CrossRef]
- Han, Y.; Runge, M.S.; Brasier, A.R. Angiotensin II induces Interleukin-6 transcription in vascular smooth muscle cells through pleiotropic activation of nuclear factor-kB transcription factors. Circ. Res. 1999, 84, 695–703. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Lorenzo, O.; Suzuki, Y.; Rupérez, M.; Egido, J. Proinflammatory actions of angiotensins. Curr. Opin. Nephrol. Hypertens. 2001, 10, 321–329. [Google Scholar] [CrossRef]
- Osman, I.O.; Melenotte, C.; Brouqui, P.; Million, M.; Lagier, J.-C.; Parola, P.; Stein, A.; La Scola, B.; Meddeb, L.; Mege, J.-L.; et al. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients. Front. Immunol. 2021, 12, 625732. [Google Scholar] [CrossRef]
- Odya, C.E.; Marinkovic, D.V.; Hammon, K.J.; Stewart, T.A.; Erdos, E.G. Purification and properties of prolylcarboxypeptidase (angiotensinase C) from human kidney. J. Biol. Chem. 1978, 253, 5927–5931. [Google Scholar] [CrossRef]
- Serfozo, P.; Wysocki, J.; Gulua, G.; Schulze, A.; Ye, M.; Liu, P.; Jin, J.; Bader, M.; Myöhänen, T.; Garcia-Horsman, J.A.; et al. Ang II (Angiotensin II) conversion to angiotensin-(1- 7) in the circulation Is POP (Prolyloligopeptidase)-dependent and ACE2 (Angiotensin-converting enzyme 2)-independent. Hypertension 2020, 75, 173–182. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Trapasso, M.; Zappa, M.; Spanevello, A.; Verdecchia, P. COVID-19, vaccines and deficiency of ACE2 and other angiotensinases. Closing the loop on the “Spike effect”. Eur. J. Intern. Med. 2022, 103, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Solopov, P.A. COVID-19 vaccination and alcohol consumption: Justification of risks. Pathogens 2023, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Bidari, A.; Asgarian, S.; Pour Mohammad, A.; Naderi, D.; Anaraki, S.R.; Gholizadeh Mesgarha, M.; Naderkhani, M. Immune thrombocytopenic purpura secondary to COVID-19 vaccination: A systematic review. Eur. J. Haematol. 2023, 110, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Picod, A.; Rebibou, J.M.; Dossier, A.; Cador, B.; Ribes, D.; Vasco-Moynet, C.; Stephan, C.; Bellal, M.; Wynckel, A.; Poullin, P.; et al. Immune-mediated thrombotic thrombocytopenic purpura following COVID-19 vaccination. Blood 2022, 139, 2565–2569. [Google Scholar] [CrossRef]
- Shah, H.; Kim, A.; Sukumar, S.; Mazepa, M.; Kohli, R.; Braunstein, E.M.; Brodsky, R.A.; Cataland, S.; Chaturvedi, S. SARS-CoV-2 vaccination and immune thrombotic thrombocytopenic purpura. Blood 2022, 139, 2570–2573. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Raafe Atif, A.; Sohaib Asghar, M.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Cines, D.B.; Greinacher, A. Vaccine-induced immune thrombotic thrombocytopenia. Blood 2023, 141, 1659–1665. [Google Scholar] [CrossRef]
- Law, N.; Chan, J.; Kelly, C.; Auffermann, W.F.; Dunn, D.P. Incidence of pulmonary embolism in COVID-19 infection in the ED: Ancestral, Delta, Omicron variants and vaccines. Emerg. Radiol. 2022, 29, 625–629. [Google Scholar] [CrossRef]
- Di Gennaro, C.; Galdiero, M.; Scherillo, G.; Parlamento, S.; Poggiano, M.R.; Arturo, C.; Vasta, A.; Giordano, B.; Pisano, V.; Lobasso, A.; et al. Editorial COVID-19 and Thrombosis 2023: New waves of SARS-CoV-2 infection, triage organization in emergency department and the association of VOCs/VOI with pulmonary embolism. Viruses 2022, 14, 2453. [Google Scholar] [CrossRef]
- Kyriakoulis, K.G.; Dimakakos, E.; Kyriakoulis, I.G.; Catalano, M.; Spyropoulos, A.C.; Schulman, S.; Douketis, J.; Falanga, A.; Maraveyas, A.; Olinic, D.-M.; et al. Practical recommendations for optimal thromboprophylaxis in patients with COVID-19: A consensus statement based on available clinical trials. J. Clin. Med. 2022, 11, 5997. [Google Scholar] [CrossRef] [PubMed]
- WGGTMA (Working Group on Guidelines for Thromboprophylaxis and Management of Anticoagulantion in Hospitalized Patients with COVID-19). Practice guidelines of thromboprophylaxis and management of anticoagulation in hospitalized patients with COVID-19. Zhonghua Yi Xue Za Zhi 2023, 103, 1–23. (In Chinese) [Google Scholar] [CrossRef]
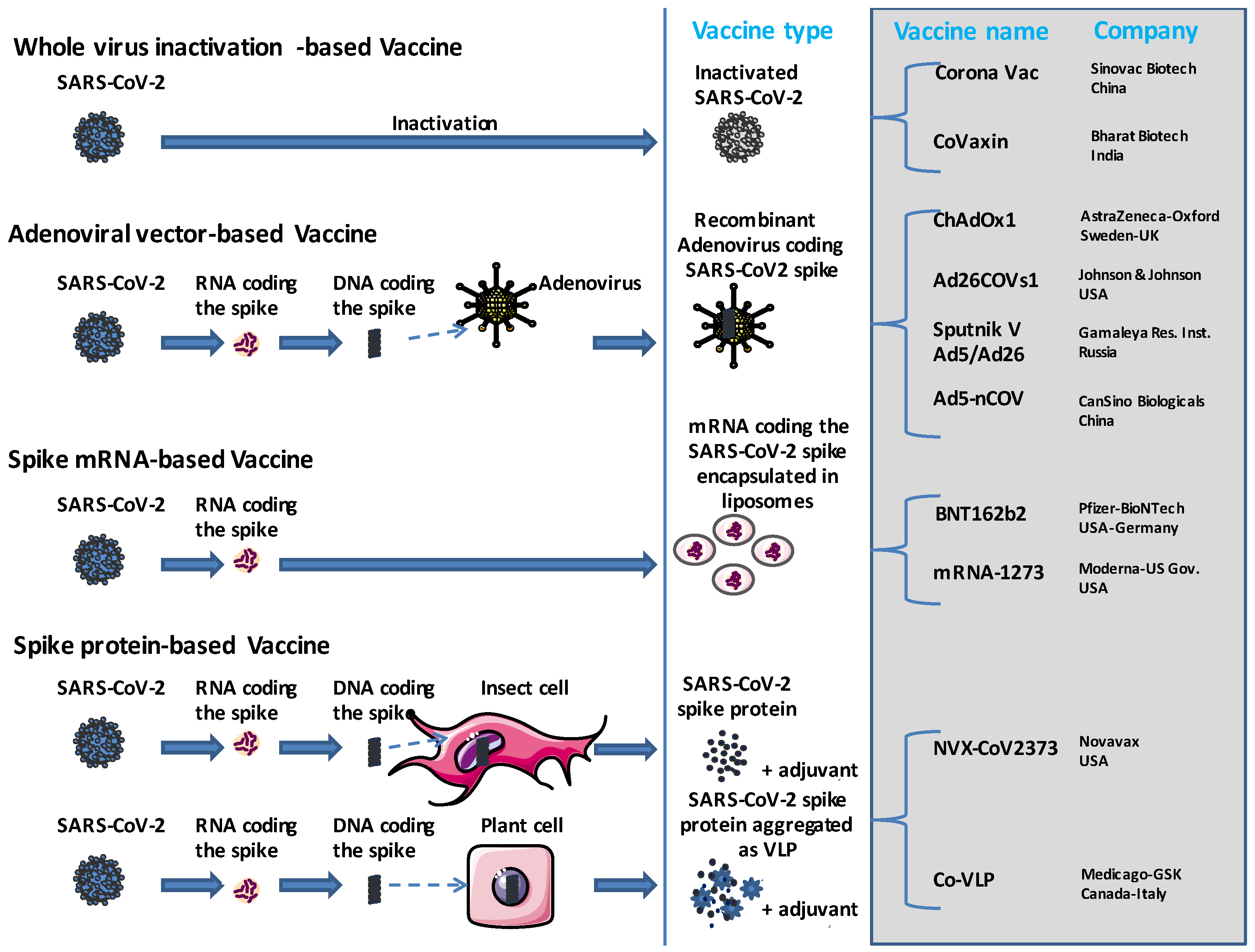

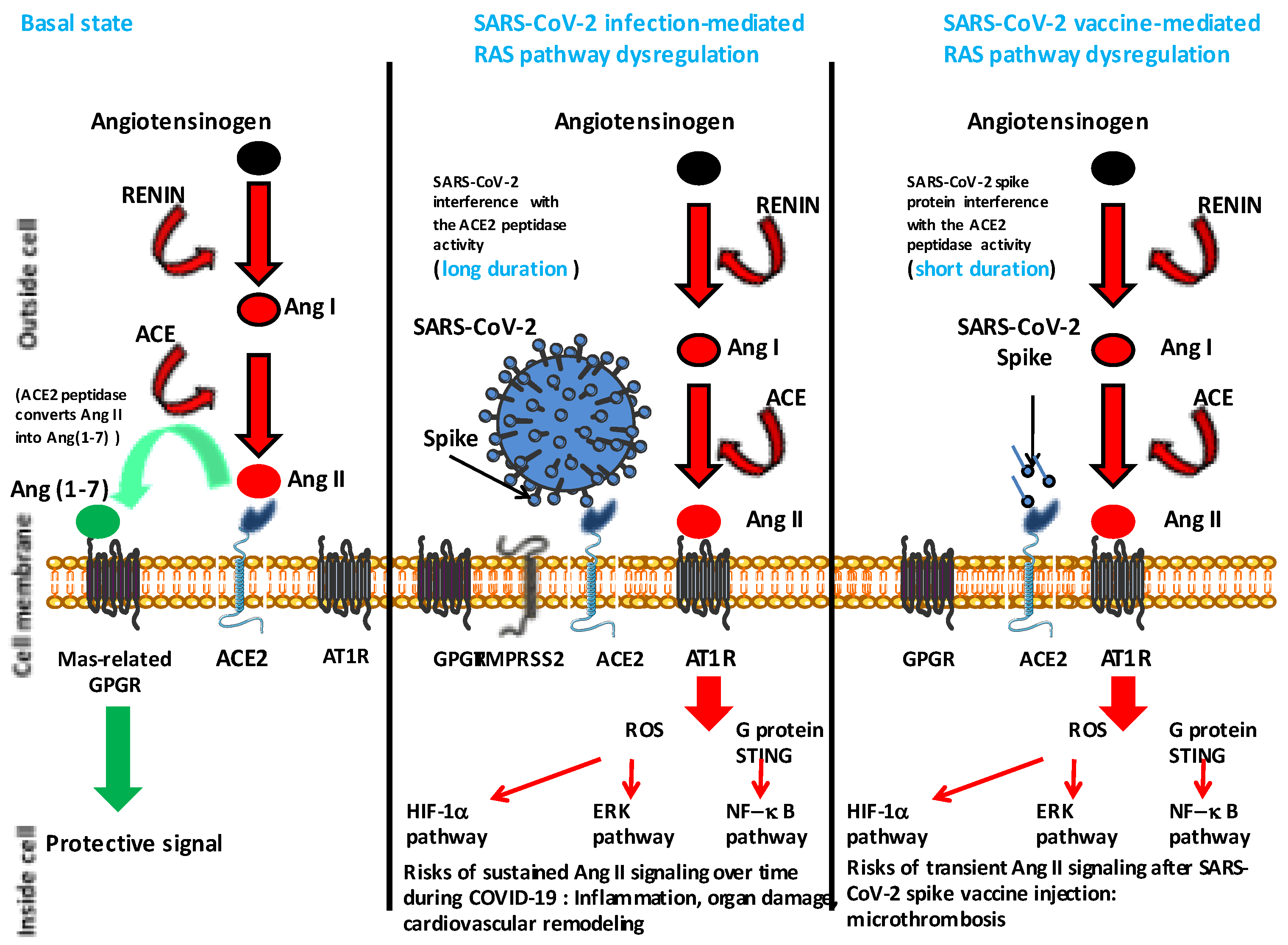
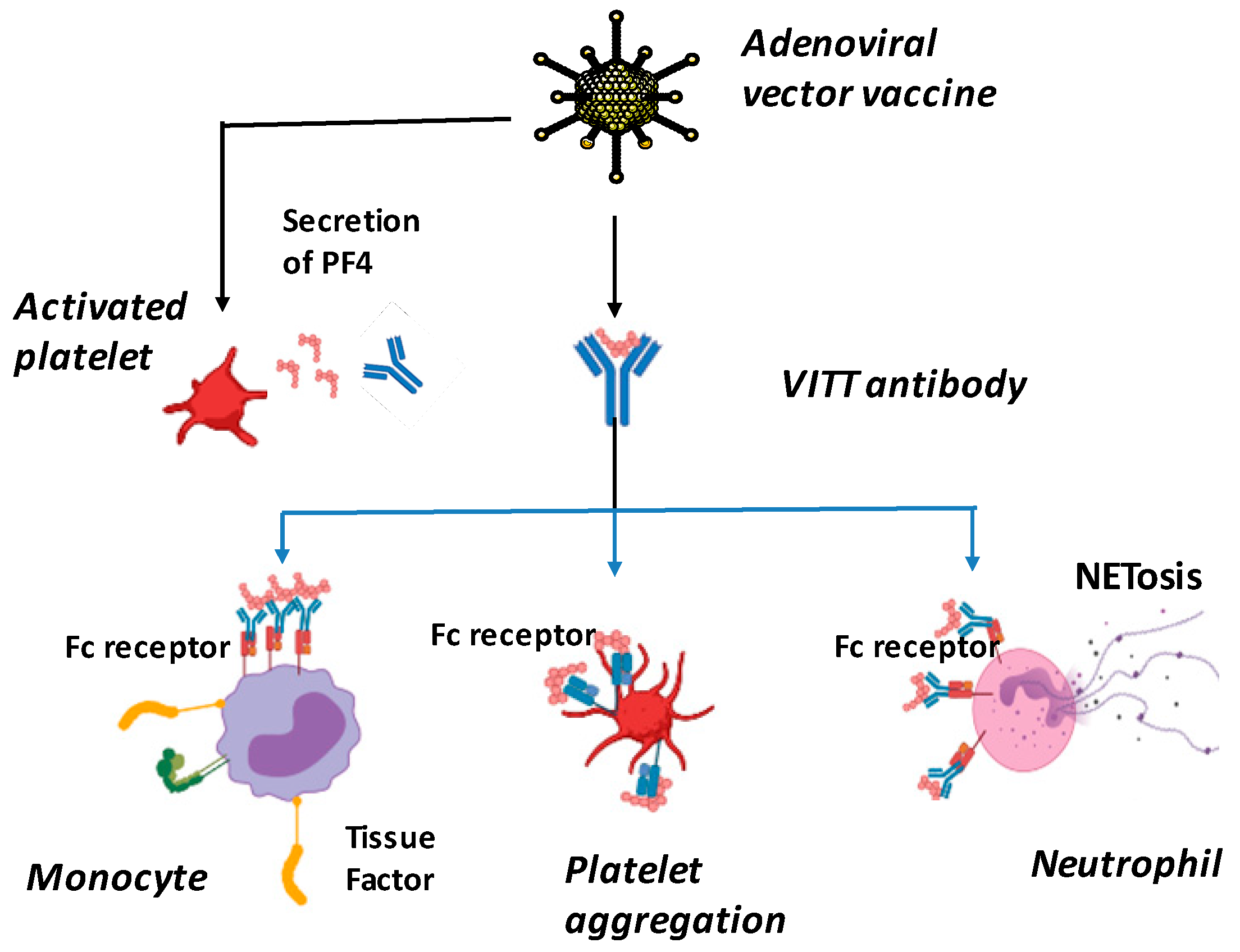
| Study | Source | Cohort (n) | Year | Vaccine | Outcome | |
|---|---|---|---|---|---|---|
| Characteristic | N, % | |||||
| Bouhanick et al. [42] | Pharmacovigilance database | 91,761 | 2021 | BNT162b2, ChAdOx1nCoV-19, Ad26.COV2.S | Abnormal BP * | 1776, 1.9% |
| Kaur et al. [44] | Pharmacovigilance database | 30,523 | 2021 | BNT162b2 ChAdOx1nCoV-19mRNA-1273 BNT 62b2 | Abnormal BP * Stage III hypertension or hypertensive emergency | 283, 5.82% 36, 0.11% |
| Lehmann et al. [43] | Pharmacovigilance database | 212,053 | 2021 | ChAdOx1nCoV-19, Ad26.COV2.S mRNA-1273 | Abnormal BP * Stage III hypertension or hypertensive emergency | 6130, 2.9% 551, 0.25% |
| Tran et al. [49] | Cross-sectional online survey | 1028 | 2021 | ChAdOx1nCoV-19 | Self-reported hypertension | 52, 5% |
| Zappa et al. [40] | Cross-sectional online survey | 113 | 2021 | BNT162b2 | Raise in home BP > 110 mmHg Stage III hypertension or hypertensive emergency | 6, 5.3% 2, 1.7% |
| Bouhanick et al. [50] | Patients and healthcare workers | 21,909 | 2022 | BNT162b2 | Persistent BP (≥140/90, 15 min after vaccination) Stage III hypertension or hypertensive emergency | 5197, 23.7% 709, 3.23% |
| Mean of Incidence Rate/100,000 Person-Years | Acute Myocardial Infarction | Deep Vein Thrombosis | Pulmonary Embolism | Myocarditis, Pericarditis | ||||
|---|---|---|---|---|---|---|---|---|
| Years | Men | Women | Men | Women | Men | Women | Men | Women |
| 18–34 | 16 | 6 | 80 | 140 | 20 | 38 | 37 | 16 |
| 35–54 | 172 | 54 | 272 | 306 | 81 | 80 | 37 | 22 |
| 55–64 | 171 | 467 | 499 | 428 | 171 | 125 | 45 | 31 |
| 65–74 | 653 | 312 | 695 | 683 | 256 | 217 | 49 | 35 |
| 75–84 | 934 | 617 | 831 | 975 | 349 | 358 | 54 | 39 |
| >85 | 1514 | 1144 | 1003 | 1206 | 398 | 427 | 41 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devaux, C.A.; Camoin-Jau, L. Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection. Viruses 2023, 15, 1045. https://doi.org/10.3390/v15051045
Devaux CA, Camoin-Jau L. Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection. Viruses. 2023; 15(5):1045. https://doi.org/10.3390/v15051045
Chicago/Turabian StyleDevaux, Christian A., and Laurence Camoin-Jau. 2023. "Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection" Viruses 15, no. 5: 1045. https://doi.org/10.3390/v15051045




