Horses as Sentinels for the Circulation of Flaviviruses in Eastern–Central Germany
Abstract
:1. Introduction
2. Materials and Methods
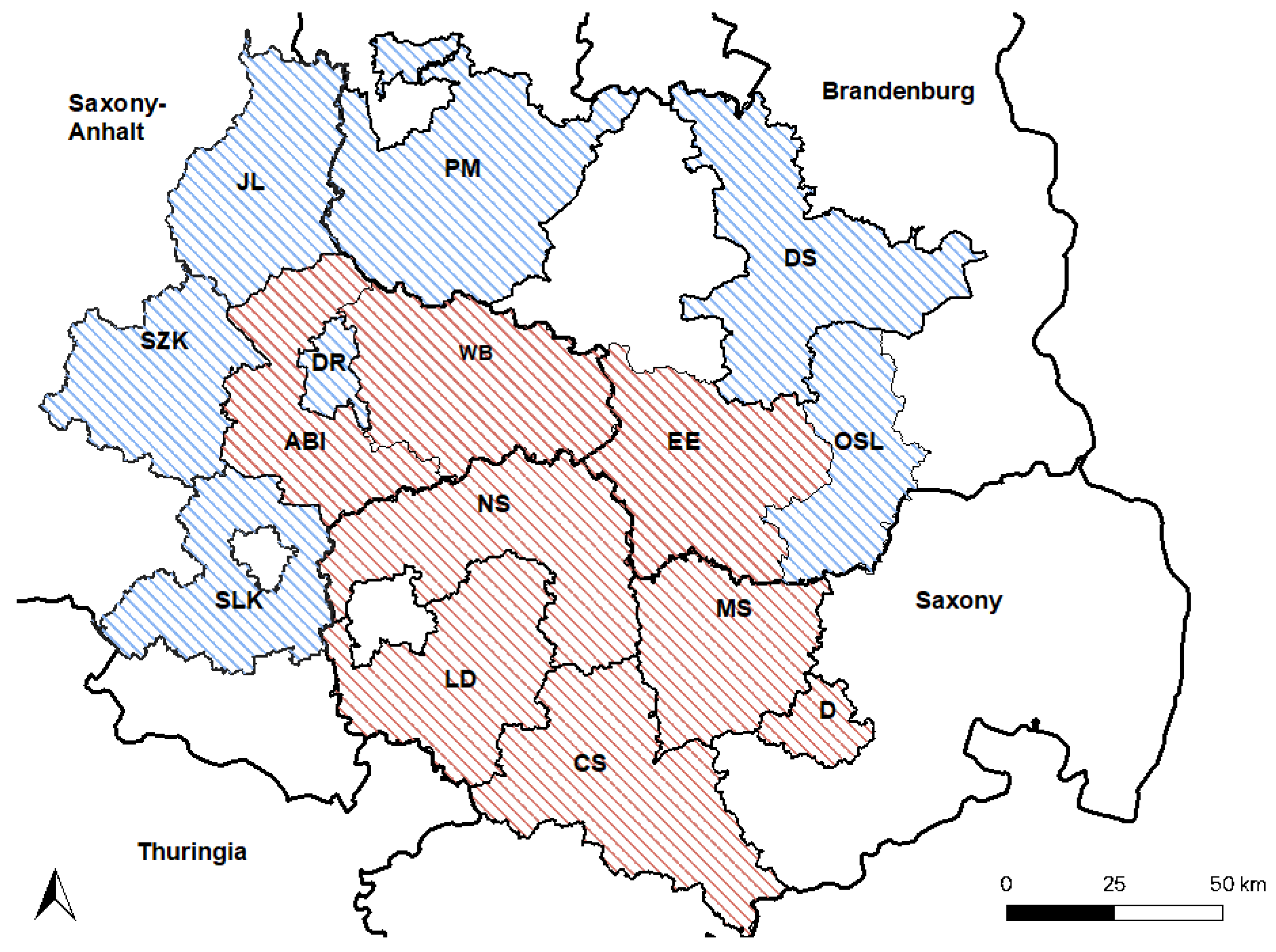
2.1. Study Area and Animals
2.2. Epidemiological Data
2.3. ELISA and VNT
2.4. Statistical Analysis
2.5. Mapping
2.6. Ethical Statement
3. Results
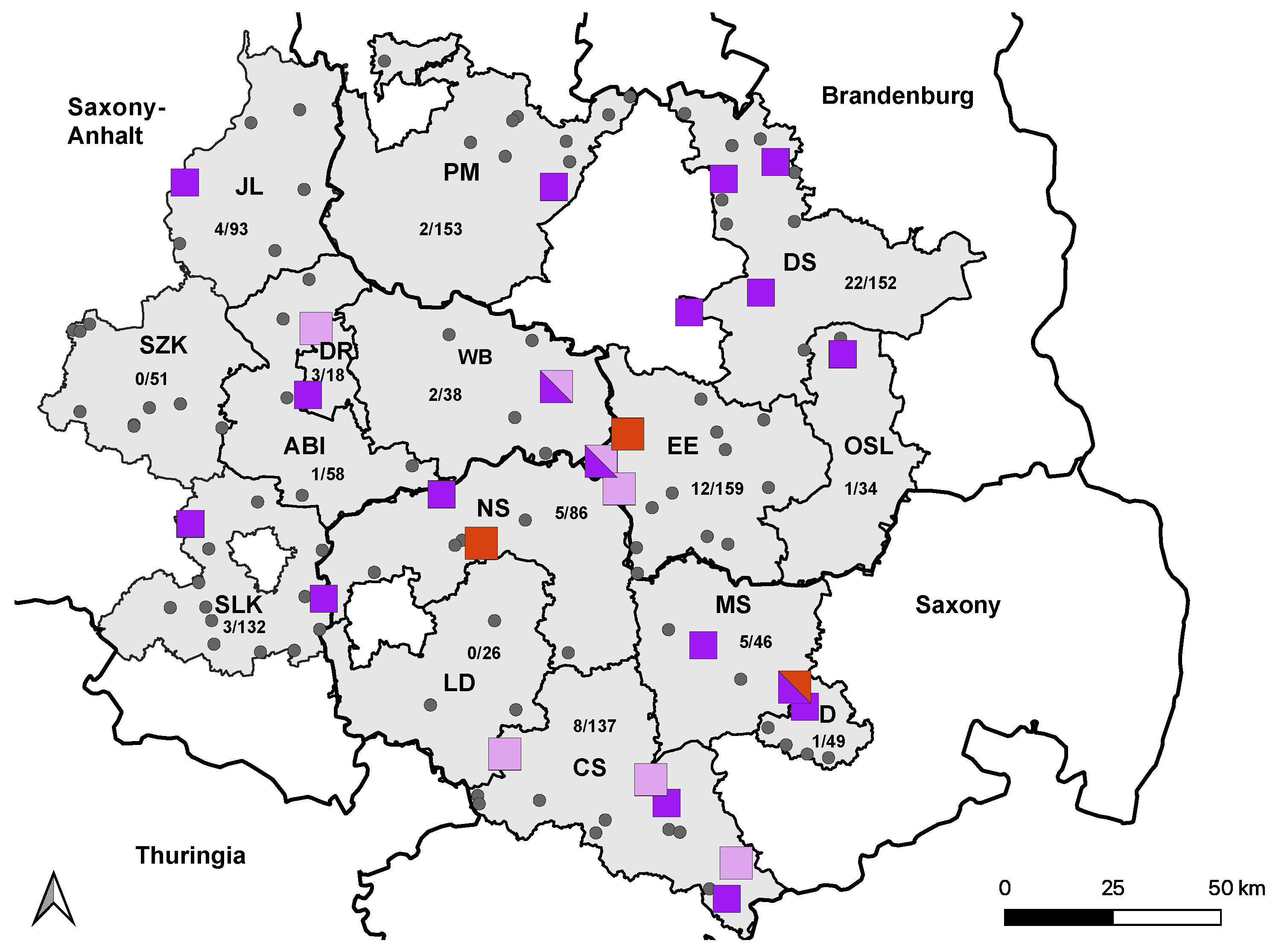
3.1. ELISA and VNT
3.2. Risk Factor Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deubel, V.; Fiette, L.; Gounon, P.; Drouet, M.T.; Khun, H.; Huerre, M.; Banet, C.; Malkinson, M.; Despres, P. Variations in biological features of West Nile viruses. Ann. N. Y. Acad. Sci. 2001, 951, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Castle, E.; Wengler, G. Nucleotide sequence of the 5’-terminal untranslated part of the genome of the flavivirus West Nile virus. Arch. Virol. 1987, 92, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Fuzik, T.; Formanova, P.; Ruzek, D.; Yoshii, K.; Niedrig, M.; Plevka, P. Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, T.; Gould, E.A.; Kolodziejek, J.; Weissenbock, H.; Nowotny, N. Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: Comparison with the South African strain SAAR-1776 and other flaviviruses. Virology 2004, 328, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. Biomed. Res. Int. 2015, 2015, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Pachler, K.; Lebl, K.; Berer, D.; Rudolf, I.; Hubalek, Z.; Nowotny, N. Putative new West Nile virus lineage in Uranotaenia unguiculata mosquitoes, Austria, 2013. Emerg. Infect. Dis. 2014, 20, 2119–2122. [Google Scholar] [CrossRef]
- Fall, G.; Di Paola, N.; Faye, M.; Dia, M.; Freire, C.C.M.; Loucoubar, C.; Zanotto, P.M.A.; Faye, O.; Sall, A.A. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLoS Negl. Trop. Dis. 2017, 11, e0006078. [Google Scholar] [CrossRef] [Green Version]
- van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch. Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Kramer, L.D.; Campbell, S.R.; Alleyne, E.O.; Dobson, A.P.; Daszak, P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005, 11, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Savage, H.M. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes (vol 61, pg 600, 1999). Am. J. Trop. Med. Hyg. 2000, 62, 162. [Google Scholar]
- Turell, M.J.; Dohm, D.J.; Sardelis, M.R.; Oguinn, M.L.; Andreadis, T.G.; Blow, J.A. An update on the potential of north American mosquitoes (Diptera: Culicidae) to transmit West Nile Virus. J. Med. Entomol. 2005, 42, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Root, J.J.; Bosco-Lauth, A.M. West Nile Virus Associations in Wild Mammals: An Update. Viruses 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunning, M.L.; Bowen, R.A.; Cropp, C.B.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; et al. Experimental infection of horses with West Nile virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Mencattelli, G.; Ndione, M.H.D.; Rosa, R.; Marini, G.; Diagne, C.T.; Diagne, M.M.; Fall, G.; Faye, O.; Diallo, M.; Faye, O.; et al. Epidemiology of West Nile virus in Africa: An underestimated threat. PLoS Negl. Trop. Dis. 2022, 16, e0010075. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, E.; Erdelyi, K.; Ursu, K.; Ferenczi, E.; Weissenbock, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- de Heus, P.; Kolodziejek, J.; Camp, J.V.; Dimmel, K.; Bago, Z.; Hubalek, Z.; van den Hoven, R.; Cavalleri, J.V.; Nowotny, N. Emergence of West Nile virus lineage 2 in Europe: Characteristics of the first seven cases of West Nile neuroinvasive disease in horses in Austria. Transbound. Emerg. Dis. 2020, 67, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, T.; Ferenczi, E.; Erdelyi, K.; Kutasi, O.; Csorgo, T.; Seidel, B.; Weissenbock, H.; Brugger, K.; Ban, E.; Nowotny, N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef]
- Papa, A.; Xanthopoulou, K.; Gewehr, S.; Mourelatos, S. Detection of West Nile virus lineage 2 in mosquitoes during a human outbreak in Greece. Clin. Microbiol. Infect. 2011, 17, 1176–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirbu, A.; Ceianu, C.S.; Panculescu-Gatej, R.I.; Vazquez, A.; Tenorio, A.; Rebreanu, R.; Niedrig, M.; Nicolescu, G.; Pistol, A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011, 16, 19762. [Google Scholar] [CrossRef] [PubMed]
- Busquets, N.; Laranjo-Gonzalez, M.; Soler, M.; Nicolas, O.; Rivas, R.; Talavera, S.; Villalba, R.; San Miguel, E.; Torner, N.; Aranda, C.; et al. Detection of West Nile virus lineage 2 in North-Eastern Spain (Catalonia). Transbound. Emerg. Dis. 2019, 66, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Luhken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef]
- Wodak, E.; Richter, S.; Bago, Z.; Revilla-Fernandez, S.; Weissenbock, H.; Nowotny, N.; Winter, P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet. Microbiol. 2011, 149, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietsch, C.; Michalski, D.; Munch, J.; Petros, S.; Bergs, S.; Trawinski, H.; Lubbert, C.; Liebert, U.G. Autochthonous West Nile virus infection outbreak in humans, Leipzig, Germany, August to September 2020. Eurosurveillance 2020, 25, 2001786. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Hoper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile Virus Epidemic in Germany Triggered by Epizootic Emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, U.; Bergmann, F.; Fischer, D.; Muller, K.; Holicki, C.M.; Sadeghi, B.; Sieg, M.; Keller, M.; Schwehn, R.; Reuschel, M.; et al. Spread of West Nile Virus and Usutu Virus in the German Bird Population, 2019-2020. Microorganisms 2022, 10, 807. [Google Scholar] [CrossRef]
- Gardner, I.A.; Wong, S.J.; Ferraro, G.L.; Balasuriya, U.B.; Hullinger, P.J.; Wilson, W.D.; Shi, P.Y.; MacLachlan, N.J. Incidence and effects of West Nile virus infection in vaccinated and unvaccinated horses in California. Vet. Res. 2007, 38, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Ostlund, E.N.; Andresen, J.E.; Andresen, M. West Nile encephalitis. Vet. Clin. N. Am. Equine Pract. 2000, 16, 427–441. [Google Scholar] [CrossRef]
- Lohmann, K.L.; Sieg, M.; Landmann, M.; Ganzenberg, S.; Arnold, C.; Vahlenkamp, T.; Ulrich, R.G. West-Nil-Virus-Infektion bei 12 Pferden in Mitteldeutschland. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2022, 50, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Seino, K.K.; Long, M.T.; Gibbs, E.P.; Bowen, R.A.; Beachboard, S.E.; Humphrey, P.P.; Dixon, M.A.; Bourgeois, M.A. Comparative efficacies of three commercially available vaccines against West Nile Virus (WNV) in a short-duration challenge trial involving an equine WNV encephalitis model. Clin. Vaccine Immunol. 2007, 14, 1465–1471. [Google Scholar] [CrossRef] [Green Version]
- Minke, J.M.; Siger, L.; Cupillard, L.; Powers, B.; Bakonyi, T.; Boyum, S.; Nowotny, N.; Bowen, R. Protection provided by a recombinant ALVAC((R))-WNV vaccine expressing the prM/E genes of a lineage 1 strain of WNV against a virulent challenge with a lineage 2 strain. Vaccine 2011, 29, 4608–4612. [Google Scholar] [CrossRef]
- Long, M.T.; Gibbs, E.P.J.; Mellencamp, M.W.; Bowen, R.A.; Seino, K.K.; Zhang, S.; Beachboard, S.E.; Humphrey, P.P. Efficacy, duration, and onset of immunogenicity of a West Nile virus vaccine, live Flavivirus chimera, in horses with a clinical disease challenge model. Equine Vet. J. 2007, 39, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Gibbs, E.P.J.; Mellencamp, M.W.; Zhang, S.; Barnett, D.C.; Seino, K.K.; Beachboard, S.E.; Humphrey, P.P. Safety of an attenuated West Nile virus vaccine, live Flavivirus chimera in horses. Equine Vet. J. 2007, 39, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, F.; Trachsel, D.S.; Stoeckle, S.D.; Bernis Sierra, J.; Lübke, S.; Groschup, M.H.; Gehlen, H.; Ziegler, U. Seroepidemiological Survey of West Nile Virus Infections in Horses from Berlin/Brandenburg and North Rhine-Westphalia, Germany. Viruses 2022, 14, 243. [Google Scholar] [CrossRef]
- Ziegler, U.; Angenvoort, J.; Klaus, C.; Nagel-Kohl, U.; Sauerwald, C.; Thalheim, S.; Horner, S.; Braun, B.; Kenklies, S.; Tyczka, J.; et al. Use of competition ELISA for monitoring of West Nile virus infections in horses in Germany. Int. J. Environ. Res. Public Health 2013, 10, 3112–3120. [Google Scholar] [CrossRef] [Green Version]
- Ganzenberg, S.; Sieg, M.; Ziegler, U.; Pfeffer, M.; Vahlenkamp, T.W.; Hörügel, U.; Groschup, M.H.; Lohmann, K.L. Seroprevalence and Risk Factors for Equine West Nile Virus Infections in Eastern Germany, 2020. Viruses 2022, 14, 1191. [Google Scholar] [CrossRef]
- Williams, M.C.; Knight, E.M.; Haddow, A.J.; Simpson, D.I.H. Isolation of West Nile Virus from Man + of Usutu Virus from Bird-Biting Mosquito Mansonia Aurites ( Theobald ) in Entebbe Area of Uganda. Ann. Trop. Med. Parasit. 1964, 58, 367–374. [Google Scholar] [CrossRef]
- Weissenbock, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne Flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; Mrzljak, A.; Ilic, M.; Bogdanic, M.; et al. Epidemiology of Usutu Virus: The European Scenario. Pathogens 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Weissenbock, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chvala, S.; Bakonyi, T.; Bukovsky, C.; Meister, T.; Brugger, K.; Rubel, F.; Nowotny, N.; Weissenbock, H. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet. Microbiol. 2007, 122, 237–245. [Google Scholar] [CrossRef]
- Weissenbock, H.; Hubalek, Z.; Halouzka, J.; Pichlmair, A.; Maderner, A.; Fragner, K.; Kolodziejek, J.; Loupal, G.; Kolbl, S.; Nowotny, N. Screening for West Nile virus infections of susceptible animal species in Austria. Epidemiol. Infect. 2003, 131, 1023–1027. [Google Scholar] [CrossRef]
- Cook, C.L.; Huang, Y.S.; Lyons, A.C.; Alto, B.W.; Unlu, I.; Higgs, S.; Vanlandingham, D.L. North American Culex pipiens and Culex quinquefasciatus are competent vectors for Usutu virus. PLoS Negl. Trop. Dis. 2018, 12, e0006732. [Google Scholar] [CrossRef] [Green Version]
- Holicki, C.M.; Scheuch, D.E.; Ziegler, U.; Lettow, J.; Kampen, H.; Werner, D.; Groschup, M.H. German Culex pipiens biotype molestus and Culex torrentium are vector-competent for Usutu virus. Parasit. Vectors 2020, 13, 625. [Google Scholar] [CrossRef]
- Zannoli, S.; Sambri, V. West Nile Virus and Usutu Virus Co-Circulation in Europe: Epidemiology and Implications. Microorganisms 2019, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Constant, O.; Gil, P.; Barthelemy, J.; Bollore, K.; Foulongne, V.; Desmetz, C.; Leblond, A.; Desjardins, I.; Pradier, S.; Joulie, A.; et al. One Health surveillance of West Nile and Usutu viruses: A repeated cross-sectional study exploring seroprevalence and endemicity in Southern France, 2016 to 2020. Eurosurveillance 2022, 27, 2200068. [Google Scholar] [CrossRef]
- Cavalleri, J.M.V.; Korbacska-Kutasi, O.; Leblond, A.; Paillot, R.; Pusterla, N.; Steinmann, E.; Tomlinson, J. European College of Equine Internal Medicine consensus statement on equine flaviviridae infections in Europe. J. Vet. Intern. Med. 2022, 36, 1858–1871. [Google Scholar] [CrossRef]
- Santini, M.; Vilibic-Cavlek, T.; Barsic, B.; Barbic, L.; Savic, V.; Stevanovic, V.; Listes, E.; Di Gennaro, A.; Savini, G. First cases of human Usutu virus neuroinvasive infection in Croatia, August-September 2013: Clinical and laboratory features. J. Neurovirol. 2015, 21, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.M.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.L.; Lelli, R.; et al. First human case of usutu virus neuroinvasive infection, italy, august-september 2009. Eurosurveillance 2009, 14, 15–16. [Google Scholar] [CrossRef]
- Holbrook, M.R.; Shope, R.E.; Barrett, A.D.T. Use of Recombinant E Protein Domain III-Based Enzyme-Linked Immunosorbent Assays for Differentiation of Tick-Borne Encephalitis Serocomplex Flaviviruses from Mosquito-Borne Flaviviruses. J. Clin. Microbiol. 2004, 42, 4101–4110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wondim, M.A.; Czupryna, P.; Pancewicz, S.; Kruszewska, E.; Groth, M.; Moniuszko-Malinowska, A. Epidemiological Trends of Trans-Boundary Tick-Borne Encephalitis in Europe, 2000–2019. Pathogens 2022, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Tonteri, E.; Kipar, A.; Voutilainen, L.; Vene, S.; Vaheri, A.; Vapalahti, O.; Lundkvist, A. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the bank vole (Myodes glareolus). PLoS ONE 2013, 8, e81214. [Google Scholar] [CrossRef]
- Imhoff, M.; Hagedorn, P.; Schulze, Y.; Hellenbrand, W.; Pfeffer, M.; Niedrig, M. Review: Sentinels of tick-borne encephalitis risk. Ticks Tick. Borne Dis. 2015, 6, 592–600. [Google Scholar] [CrossRef]
- Brandenburg, P.J.; Obiegala, A.; Schmuck, H.M.; Dobler, G.; Chitimia-Dobler, L.; Pfeffer, M. Seroprevalence of Tick-Borne Encephalitis (TBE) Virus Antibodies in Wild Rodents from Two Natural TBE Foci in Bavaria, Germany. Pathogens 2023, 12, 185. [Google Scholar] [CrossRef]
- Estrada-Pena, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Deviatkin, A.A.; Kholodilov, I.S.; Vakulenko, Y.A.; Karganova, G.G.; Lukashev, A.N. Tick-Borne Encephalitis Virus: An Emerging Ancient Zoonosis? Viruses 2020, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Dobler, G. Zoonotic tick-borne flaviviruses. Vet. Microbiol. 2010, 140, 221–228. [Google Scholar] [CrossRef]
- Kaiser, R. Tick-borne encephalitis. Nervenarzt 2016, 87, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Horugel, U.; Hoffmann, B.; Beer, M. Tick-borne encephalitis virus (TBEV) infection in horses: Clinical and laboratory findings and epidemiological investigations. Vet. Microbiol. 2013, 163, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Pautienius, A.; Armonaite, A.; Simkute, E.; Zagrabskaite, R.; Buitkuviene, J.; Alpizar-Jara, R.; Grigas, J.; Zakiene, I.; Zienius, D.; Salomskas, A.; et al. Cross-Sectional Study on the Prevalence and Factors Influencing Occurrence of Tick-Borne Encephalitis in Horses in Lithuania. Pathogens 2021, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Magouras, I.; Schoster, A.; Fouche, N.; Gerber, V.; Groschup, M.H.; Ziegler, U.; Fricker, R.; Griot, C.; Vogtlin, A. Neurological disease suspected to be caused by tick-borne encephalitis virus infection in 6 horses in Switzerland. J. Vet. Intern. Med. 2022, 36, 2254–2262. [Google Scholar] [CrossRef]
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile virus circulation in Emilia-Romagna, Italy: The integrated surveillance system 2009. Eurosurveillance 2010, 15, 11–15. [Google Scholar] [CrossRef]
- Paternoster, G.; Martins, S.B.; Mattivi, A.; Cagarelli, R.; Angelini, P.; Bellini, R.; Santi, A.; Galletti, G.; Pupella, S.; Marano, G.; et al. Economics of One Health: Costs and benefits of integrated West Nile virus surveillance in Emilia-Romagna. PLoS ONE 2017, 12, e0188156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leblond, A.; Hendrikx, P.; Sabatier, P. West Nile virus outbreak detection using syndromic monitoring in horses. Vector-Borne Zoonotic Dis. 2007, 7, 403–410. [Google Scholar] [CrossRef]
- Klaus, C.; Beer, M.; Saier, R.; Schubert, H.; Bischoff, S.; Suss, J. Evaluation of serological tests for detecting tick-borne encephalitis virus (TBEV) antibodies in animals. Berl. Und Munch. Tierarztl. Wochenschr. 2011, 124, 443–449. [Google Scholar] [CrossRef]
- Seidowski, D.; Ziegler, U.; von Ronn, J.A.; Muller, K.; Huppop, K.; Muller, T.; Freuling, C.; Muhle, R.U.; Nowotny, N.; Ulrich, R.G.; et al. West Nile virus monitoring of migratory and resident birds in Germany. Vector Borne Zoonotic Dis. 2010, 10, 639–647. [Google Scholar] [CrossRef]
- de Heus, P.; Kolodziejek, J.; Hubalek, Z.; Dimmel, K.; Racher, V.; Nowotny, N.; Cavalleri, J.M.V. West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria. Viruses 2021, 13, 1873. [Google Scholar] [CrossRef]
- Barbic, L.; Listes, E.; Katic, S.; Stevanovic, V.; Madic, J.; Staresina, V.; Labrovic, A.; Di Gennaro, A.; Savini, G. Spreading of West Nile virus infection in Croatia. Vet. Microbiol. 2012, 159, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Mangana-Vougiouka, O.; Boutsini, S.; Ntousi, D.; Patakakis, M.; Orfanou, E.; Zafiropoulou, K.; Dilaveris, D.; Panagiotatos, D.; Nomikou, K. Epizootiological investigation of the most important infectious equine diseases in Greece. Rev. Sci. Tech. Oie 2013, 32, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napp, S.; Llorente, F.; Beck, C.; Jose-Cunilleras, E.; Soler, M.; Pailler-Garcia, L.; Amaral, R.; Aguilera-Sepulveda, P.; Pifarre, M.; Molina-Lopez, R.; et al. Widespread Circulation of Flaviviruses in Horses and Birds in Northeastern Spain (Catalonia) between 2010 and 2019. Viruses 2021, 13, 2404. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Clavero, M.A.; Llorente, F.; Sotelo, E.; Soriguer, R.; Gomez-Tejedor, C.; Figuerola, J. West Nile virus serosurveillance in horses in Donana, Spain, 2005 to 2008. Vet. Rec. 2010, 167, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bocanegra, I.; Arenas-Montes, A.; Napp, S.; Jaen-Tellez, J.A.; Fernandez-Morente, M.; Fernandez-Molera, V.; Arenas, A. Seroprevalence and risk factors associated to West Nile virus in horses from Andalusia, Southern Spain. Vet. Microbiol. 2012, 160, 341–346. [Google Scholar] [CrossRef]
- Rexhepi, A.; Sherifi, K.; Berxholi, K.; Xhekaj, B.; Muja-Bajraktari, N.; Ozkul, A.; von Possel, R.; Emmerich, P. First Serological Evidence of West Nile Virus Among Equines and Birds in Kosovo, 2018–2019. Vector-Borne Zoonotic Dis. 2021, 21, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Skrypnyk, A.; Keller, M.; Staubach, C.; Bezymennyi, M.; Damiani, A.M.; Osterrieder, N.; Groschup, M.H. West nile virus antibody prevalence in horses of Ukraine. Viruses 2013, 5, 2469–2482. [Google Scholar] [CrossRef] [Green Version]
- Bazanow, B.; Jansen van Vuren, P.; Szymanski, P.; Stygar, D.; Fracka, A.; Twardon, J.; Kozdrowski, R.; Paweska, J.T. A Survey on West Nile and Usutu Viruses in Horses and Birds in Poland. Viruses 2018, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Maquart, M.; Dahmani, M.; Marie, J.L.; Gravier, P.; Leparc-Goffart, I.; Davoust, B. First Serological Evidence of West Nile Virus in Horses and Dogs from Corsica Island, France. Vector Borne Zoonotic Dis. 2017, 17, 275–277. [Google Scholar] [CrossRef]
- Lupulovic, D.; Martin-Acebes, M.A.; Lazic, S.; Alonso-Padilla, J.; Blazquez, A.B.; Escribano-Romero, E.; Petrovic, T.; Saiz, J.C. First serological evidence of West Nile virus activity in horses in Serbia. Vector Borne Zoonotic Dis. 2011, 11, 1303–1305. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Carvajal, F.; Bravo-Barriga, D.; Martin-Cuervo, M.; Aguilera-Sepulveda, P.; Ferraguti, M.; Jimenez-Clavero, M.A.; Llorente, F.; Alonso, J.M.; Frontera, E. Serological evidence of co-circulation of West Nile and Usutu viruses in equids from western Spain. Transbound. Emerg. Dis. 2021, 68, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Berxholi, K.; Ziegler, U.; Rexhepi, A.; Schmidt, K.; Mertens, M.; Korro, K.; Cuko, A.; Angenvoort, J.; Groschup, M.H. Indigenous West Nile Virus Infections in Horses in Albania. Transbound. Emerg. Dis. 2013, 60, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Folly, A.J.; Waller, E.S.L.; McCracken, F.; McElhinney, L.M.; Roberts, H.; Johnson, N. Equine seroprevalence of West Nile virus antibodies in the UK in 2019. Parasit. Vectors 2020, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Lecollinet, S.; Pancewicz, S.A.; Kozdrun, W.; Czekaj, H. Occurrence of West Nile Virus Antibodies in Wild Birds, Horses, and Humans in Poland. Biomed. Res. Int. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madic, J.; Savini, G.; Di Gennaro, A.; Monaco, F.; Jukic, B.; Kovac, S.; Rudan, N.; Listes, E. Serological evidence for West Nile virus infection in horses in Croatia. Vet. Rec. 2007, 160, 772–773. [Google Scholar] [CrossRef]
- Angenvoort, J.; Brault, A.C.; Bowen, R.A.; Groschup, M.H. West Nile viral infection of equids. Vet. Microbiol. 2013, 167, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, C.F.; Reisen, W.K.; Armijos, M.V.; MacLachlan, N.J.; Scott, T.W. High subclinical West Nile virus incidence among nonvaccinated horses in Northern California associated with low vector abundance and infection. Am. J. Trop. Med. Hyg. 2008, 78, 45–52. [Google Scholar] [CrossRef]
- Beck, C.; Jimenez-Clavero, M.A.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex Circulation Patterns and Their Consequences for the Diagnosis and Control of West Nile Disease. Int. J. Environ. Res. Public Health 2013, 10, 6049–6083. [Google Scholar] [CrossRef] [Green Version]
- Vinogradova, E.B. Culex Pipiens Pipiens Mosquitoes: Taxonomy, Distribution, Ecology, Physiology, Genetics, Applied Importance and Control; Pensoft: Moscow and Sofia, Russia, 2000; p. 250. [Google Scholar]
- Camino, E.; Schmid, S.; Weber, F.; Pozo, P.; de Juan, L.; Konig, M.; Cruz-Lopez, F. Detection of antibodies against tick-borne encephalitis flaviviruses in breeding and sport horses from Spain. Ticks Tick. Borne Dis. 2020, 11, 101487. [Google Scholar] [CrossRef]
- Müller, K. Untersuchung zum Vorkommen von Antikörpern gegen das “Tick Borne Encephalitis Virus” (TBEV) beim Pferd im Endemiegebiet Marburg-Biedenkopf. Ph.D. Thesis, VVB Laufersweiler. Fachbereich Veterinärmedizin der Justus-Liebig-Universität Giessen, Giessen, Germany, 2006. [Google Scholar]
- Rushton, J.O.; Lecollinet, S.; Hubalek, Z.; Svobodova, P.; Lussy, H.; Nowotny, N. Tick-borne Encephalitis Virus in Horses, Austria, 2011. Emerg. Infect. Dis. 2013, 19, 635–637. [Google Scholar] [CrossRef]
- Janitza-Futterer, D. Serologische Untersuchungen zur endemischen Situation der Infektion mit dem FSME-Virus in einer südbadischen Pferde- und Hundepopulation. Ph.D. Thesis, Tierärztliche Fakultät der Ludwig-Maximillians-Universität, München, Germany, 2003. [Google Scholar]
- Sikutova, S.; Hornok, S.; Hubalek, Z.; Dolezalkova, I.; Juricova, Z.; Rudolf, I. Serological survey of domestic animals for tick-borne encephalitis and Bhanja viruses in northeastern Hungary. Vet. Microbiol. 2009, 135, 267–271. [Google Scholar] [CrossRef]
- Mburu, M.M.; Zembere, K.; Mzilahowa, T.; Terlouw, A.D.; Malenga, T.; van den Berg, H.; Takken, W.; McCann, R.S. Impact of cattle on the abundance of indoor and outdoor resting malaria vectors in southern Malawi. Malar. J. 2021, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.I.; Kay, S.C.; Davis, S.; Tufts, D.M.; Gaffett, K.; Tefft, B.; Diuk-Wasser, M.A. High burdens of Ixodes scapularis larval ticks on white-tailed deer may limit Lyme disease risk in a low biodiversity setting. Ticks Tick-Borne Dis. 2019, 10, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Beer, M.; Saier, R.; Schau, U.; Moog, U.; Hoffmann, B.; Diller, R.; Suss, J. Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus—Epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks Tick-Borne Dis. 2012, 3, 27–37. [Google Scholar] [CrossRef] [PubMed]
- RKI. Karte der FSME-Risikogebiete. Available online: https://www.rki.de/DE/Content/InfAZ/F/FSME/Karte_Tab.html (accessed on 17 January 2023).


| Federal State | County | Registered Equids # | Eligible Equids ⊕ (in %) | Tested Equids ◊ (in %) | WNV-Seropositive Equids ∇ (in %) | TBEV-Seropositive Equids ᶲ (in %) | USUV-Seropositive Equids ● (in %) |
|---|---|---|---|---|---|---|---|
| Saxony- Anhalt | Saalekreis (SLK) | 2507 | 1444/2507 (58%) | 132/1444 (9%) | 11/132 (8%) | 3/132 (2%) | 0/132 (0%) |
| Salzlandkreis (SZK) | 2347 | 1044/2347 (45%) | 51/1044 (5%) | 2/51 (4%) | 0/51 (0%) | 0/51 (0%) | |
| Jerichower Land (JL) | 2245 | 1262/2245 (56%) | 93/1262 (7%) | 1/93 (1%) | 4/93 (4%) | 1/93 (1%) | |
| Dessau-Rosslau (DR) | 466 | 285/466 (61%) | 18/285 (6%) | 0/18 (0%) | 3/18 (17%) | 0/18 (0%) | |
| Wittenberg (WB) | 1762 | 857/1762 (49%) | 38/857 (4%) | 1/38 (3%) | 2/38 (*) (5%) | 1 */38 (3%) | |
| Anhalt- Bitterfeld (ABI) | 2380 | 1293/2380 (54%) | 58/1293 (5%) | 0/58 (0%) | 1 */58 (2%) | 0/58 (0%) | |
| Saxony | Central Saxony (CS) | 4495 | 1881/4495 (42%) | 137/1881 (7%) | 1/137 (1%) | 8/137 (6 *) (6%) | 0/137 (0%) |
| Northern Saxony (NS) | 3233 | 1304/3233 (44%) | 86/1304 (7%) | 2/86 (2%) | 5/86 (6%) | 0/86 (0%) | |
| Dresden City (D) | 948 | 428/948 (45%) | 49/428 (12%) | 2/49 (4%) | 1/49 (2%) | 2 */49 (4%) | |
| Meissen (MS) | 2749 | 1189/2749 (43%) | 46/1189 (4%) | 2/46 (4%) | 5/46 (1 *) (11%) | 0/46 (0%) | |
| Leipzig district (LD) | 3435 | 1255/3435 (37%) | 26/1255 (2%) | 1/26 (4%) | 0/26 (0%) | 0/26 (0%) | |
| Brandenburg | Elbe-Elster (EE) | 2349 | 1216/2349 (52%) | 159/1216 (13%) | 8/159 (5%) | 12/159 (8 *) (8%) | 1 */159 (1%) |
| Dahme-Spreewald (DS) | 3478 | 2418/3478 (70%) | 152/2418 (6%) | 4/152 (3%) | 22/152 (15%) | 0/152 (0%) | |
| Oberspreewald- Lausitz (OSL) | 1147 | 620/1147 (54%) | 34/620 (6%) | 3/34 (9%) | 1/34 (3%) | 0/34 (0%) | |
| Potsdam- Mittelmark (PM) | 6226 | 2857/6226 (46%) | 153/2857 (5%) | 2/153 (1%) | 2/153 (1%) | 0/153 (0%) | |
| Total | 39,767 | 19,353/39,767 (49%) | 1232/19,353 (6%) | 40/1232 (3.3%) | 69/1232 (17 *) (6%) | 5/1232 (4 *) (0.4%) |
| Federal State | County | Registered Holdings # | Eligible Holdings ⊕ (in %) | Tested Holdings ◊ (in %) | Holdings with ≥1 WNV-Seropositive Horse (in %) | Holdings with ≥1 TBEV-Seropositive Horse (in %) | Holdings with ≥1 USUV-Seropositive Horse (in %) |
|---|---|---|---|---|---|---|---|
| Saxony- Anhalt | Saalekreis (SLK) | 660 | 106/660 (16%) | 15/106 (14%) | 5/15 (33%) | 2/15 (13%) | 0/15 (0%) |
| Salzlandkreis (SZK) | 760 | 105/760 (13.8%) | 9/105 (9%) | 2/9 (22%) | 0/9 (0%) | 0/9 (0%) | |
| Jerichower Land (JL) | 575 | 112/575 (20%) | 8/112 (7%) | 1/8 (13%) | 1/8 (13%) | 1/8 (13%) | |
| Dessau-Rosslau (DS) | 105 | 19/105 (18%) | 1/19 (5%) | 0/1 (0%) | 1/1 (100%) | 0/1 (0%) | |
| Wittenberg (WB) | 525 | 80/525 (15%) | 4/80 (5%) | 1/4 (25%) | 1 */4 (25%) | 1 */4 (25%) | |
| Anhalt- Bitterfeld (ABI) | 640 | 109/640 (17%) | 6/109 (6%) | 0/6 (0%) | 1 */6 (17%) | 0/6 (0%) | |
| Saxony | Central Saxony (CS) | 1662 | 208/1662 (13%) | 14/208 (7%) | 1/14 (7%) | 5/14 (3*) (36%) | 0/14 (0%) |
| Northern Saxony (NS) | 1256 | 120/1256 (10%) | 7/120 (6%) | 2/7 (29%) | 2/7 (29%) | 0/7 (0%) | |
| Dresden City (D) | 375 | 42/375 (11%) | 5/42 (12%) | 1/5 (20%) | 2/5 (40%) | 1 */5 (20%) | |
| Meissen (MS) | 1053 | 105/1053 (10%) | 4/105 (4%) | 1/4 (25%) | 1 */4 (25%) | 0/4 (0%) | |
| Leipzig district (LD) | 1326 | 132/1326 (10%) | 3/132 (2%) | 1/3 (33%) | 0/3 (0%) | 0/3 (0%) | |
| Brandenburg | Elbe-Elster (EE) | 663 | 103/663 (16%) | 13/103 (13%) | 4/13 (31%) | 3/13 (2 *) (23%) | 1 */13 (8%) |
| Dahme-Spreewald (DS) | 630 | 160/630 (25%) | 12/160 (8%) | 3/12 (25%) | 4/12 (33%) | 0/12 (0%) | |
| Oberspreewald- Lausitz (OSL) | 300 | 47/300 (16%) | 3/47 (6%) | 1/3 (33%) | 1/3 (33%) | 0/3 (0%) | |
| Potsdam- Mittelmark (PM) | 942 | 165/942 (18%) | 10/165 (6%) | 1/10 (10%) | 1/10 (10%) | 0/10 (0%) | |
| Total | 11,472 | 1613/11,472 (14%) | 114/1613 (7%) | 24/114 (21%) | 25/114 (8 *) (22%) | 5/114 (4 *) (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gothe, L.M.R.; Ganzenberg, S.; Ziegler, U.; Obiegala, A.; Lohmann, K.L.; Sieg, M.; Vahlenkamp, T.W.; Groschup, M.H.; Hörügel, U.; Pfeffer, M. Horses as Sentinels for the Circulation of Flaviviruses in Eastern–Central Germany. Viruses 2023, 15, 1108. https://doi.org/10.3390/v15051108
Gothe LMR, Ganzenberg S, Ziegler U, Obiegala A, Lohmann KL, Sieg M, Vahlenkamp TW, Groschup MH, Hörügel U, Pfeffer M. Horses as Sentinels for the Circulation of Flaviviruses in Eastern–Central Germany. Viruses. 2023; 15(5):1108. https://doi.org/10.3390/v15051108
Chicago/Turabian StyleGothe, Leonard M. R., Stefanie Ganzenberg, Ute Ziegler, Anna Obiegala, Katharina L. Lohmann, Michael Sieg, Thomas W. Vahlenkamp, Martin H. Groschup, Uwe Hörügel, and Martin Pfeffer. 2023. "Horses as Sentinels for the Circulation of Flaviviruses in Eastern–Central Germany" Viruses 15, no. 5: 1108. https://doi.org/10.3390/v15051108
APA StyleGothe, L. M. R., Ganzenberg, S., Ziegler, U., Obiegala, A., Lohmann, K. L., Sieg, M., Vahlenkamp, T. W., Groschup, M. H., Hörügel, U., & Pfeffer, M. (2023). Horses as Sentinels for the Circulation of Flaviviruses in Eastern–Central Germany. Viruses, 15(5), 1108. https://doi.org/10.3390/v15051108






