Influenza A(H1N1)pdm09 Virus Aggravates Pathology of Blood Vessels in Wistar Rats with Premorbid Acute Cardiomyopathy
Abstract
:1. Introduction
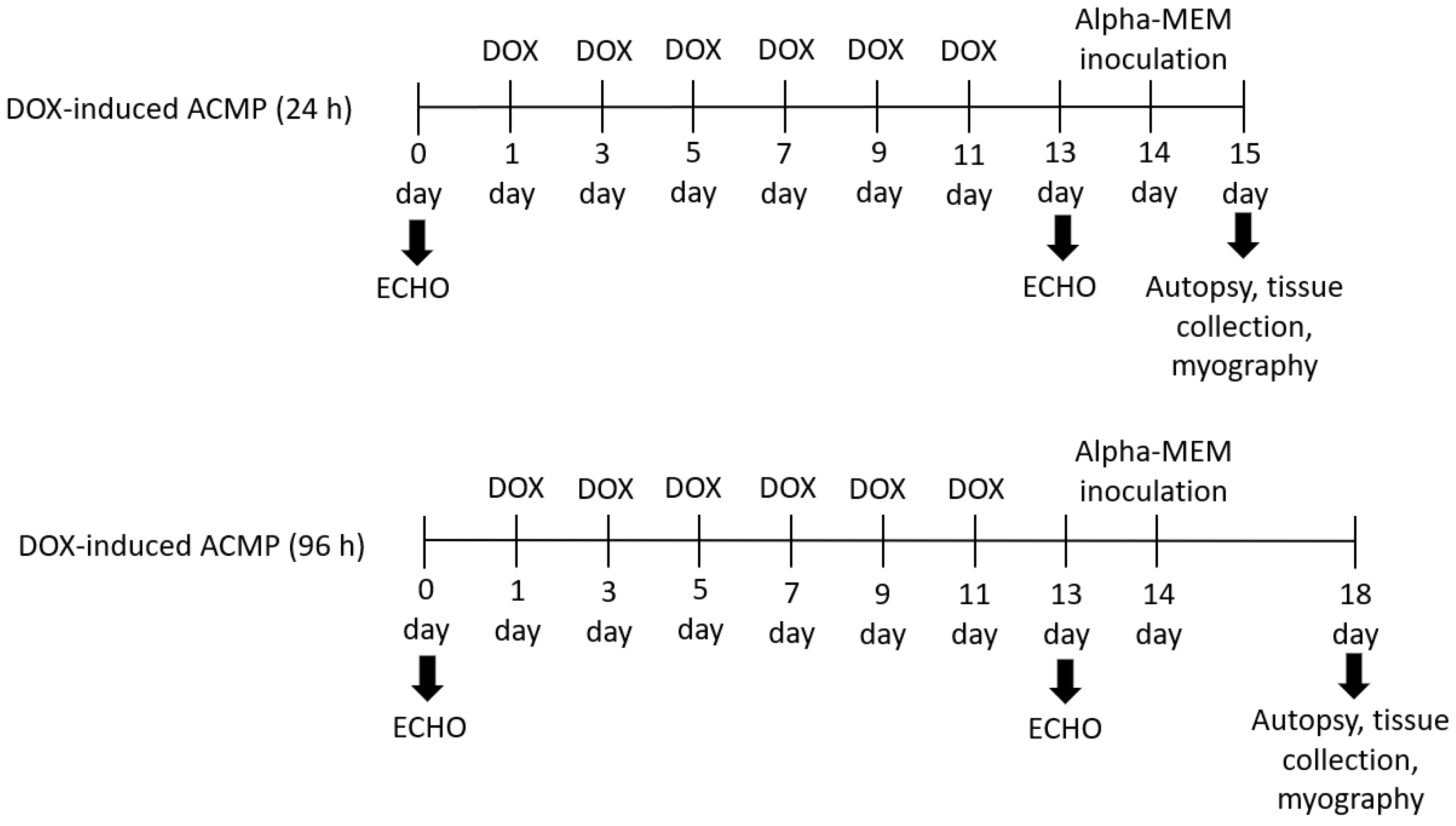
2. Materials and Methods
- (1)
- Anterior wall thickness or IVS;
- (2)
- Posterior wall thickness or LVPW;
- (3)
- Left-ventricular end-diastolic internal diameter or LVIDd;
- (4)
- Left-ventricle end-systolic internal diameter or LVIDs;
- (5)
- Fractional shortening or FS, (LVIDd − LVIDs)/LVIDd × 100.
- (1)
- Measured area—the area of binaries inside the region of interest (ROI).
- (2)
- Sum density—the summed individual optical densities (OD) of each pixel in the ROI; OD was evaluated according to the following formula:
- (3)
- Sum intensity—the summed intensity of all pixels of the object under study in the field of view. The intensity was determined in the signal registration range.
3. Results
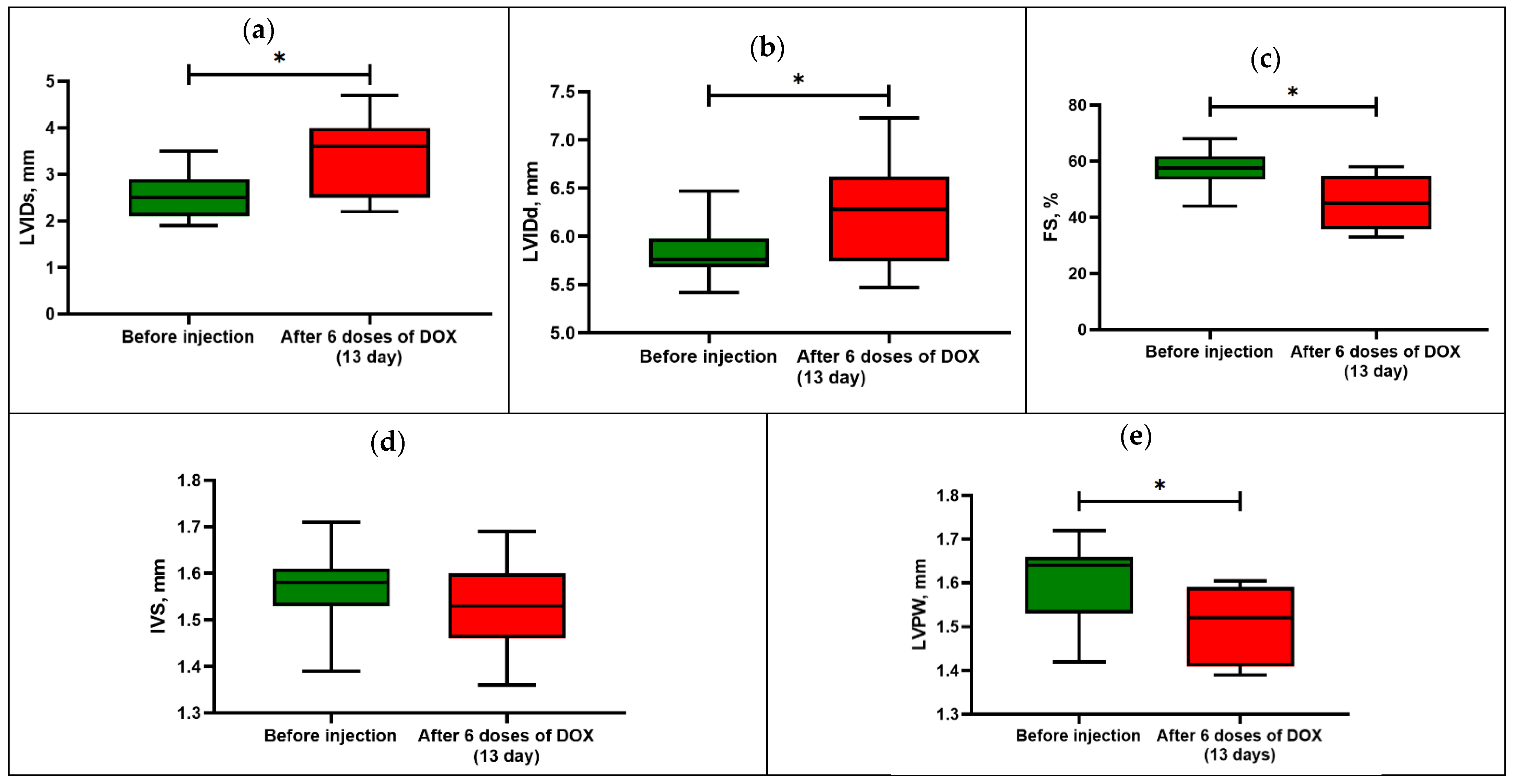
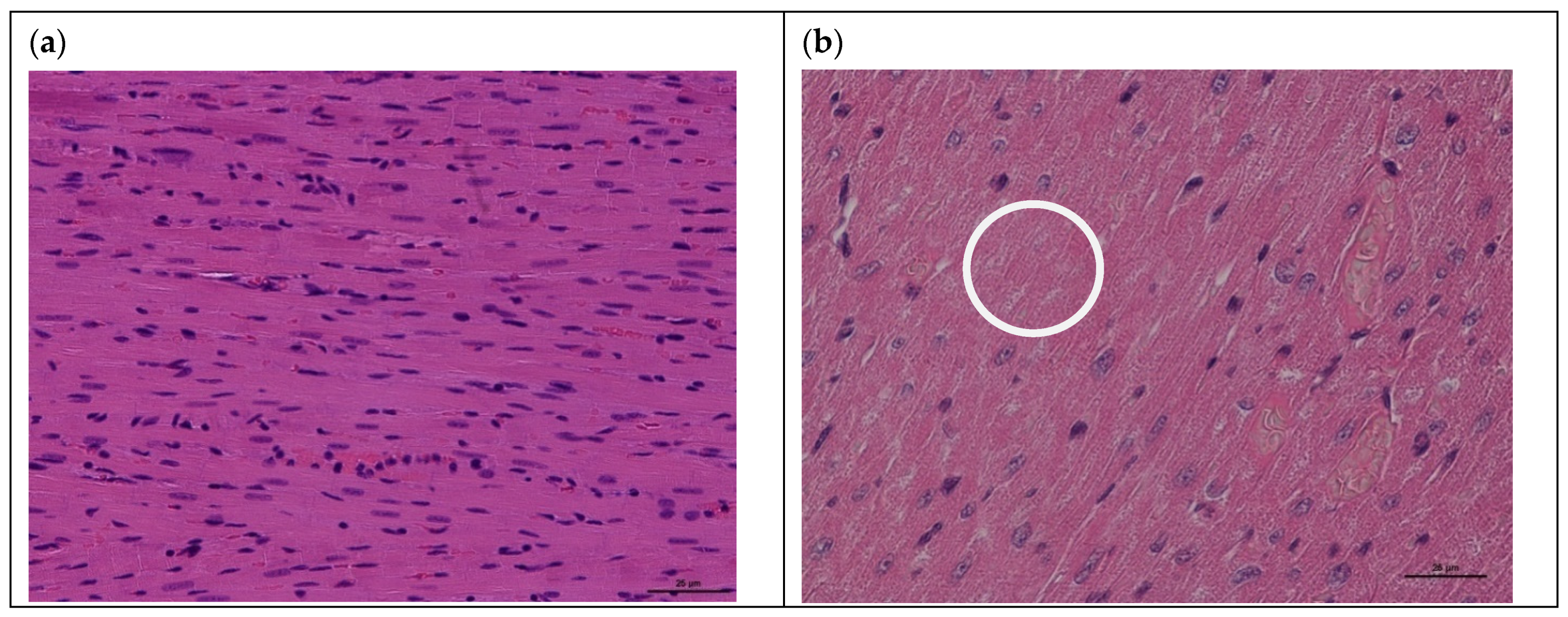
3.1. Model of Acute Cardiomyopathy
3.2. Influenza Virus Infectivity Titer in Pulmonary and Mesenteric Tissues
3.3. Vasomotor Activity of Mesenteric Arteries of Rats with Acute Cardiomyopathy
3.4. Expression of eNOS in Mesenteric Blood Vessels of Infected Rats with Premorbid Acute Cardiomyopathy
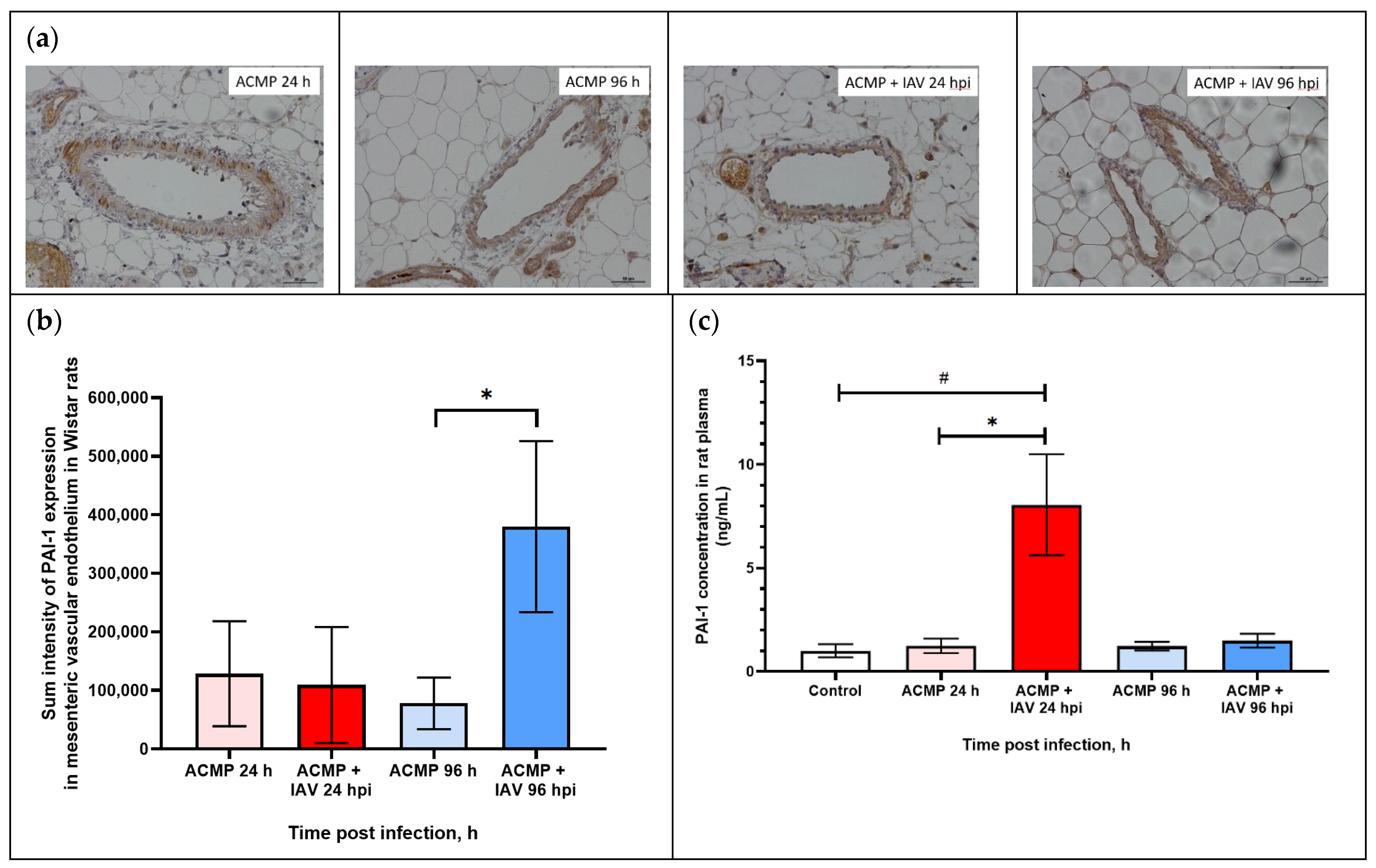
3.5. Expression of PAI-1 in Mesenteric Blood Vessels of Infected Rats with Premorbid Acute Cardiomyopathy
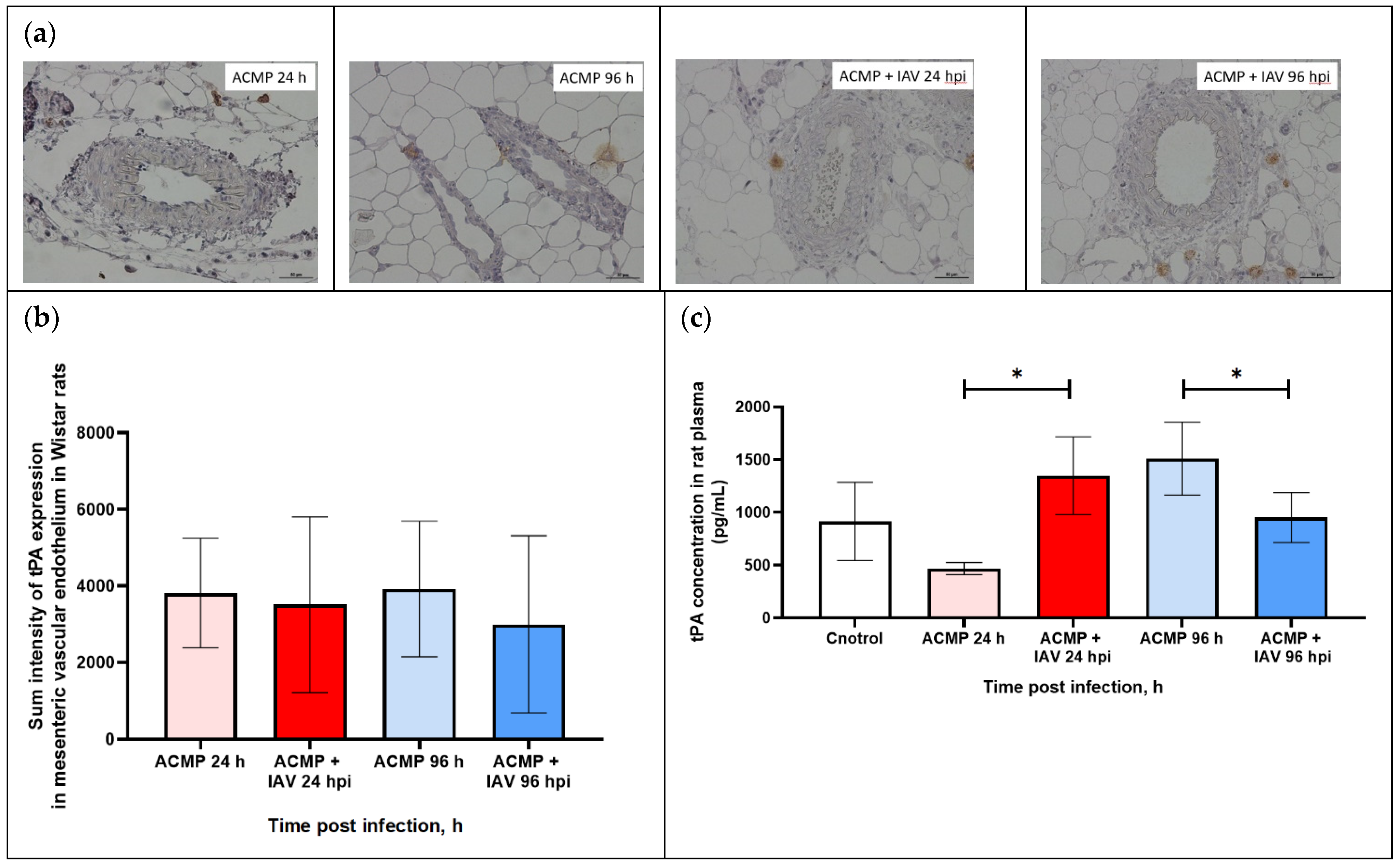
3.6. Expression of tPA in Mesenteric Blood Vessels of Infected Rats with Premorbid Acute Cardiomyopathy
3.7. PAI-1 and tPA Expression in Blood Plasma of Rats with Premorbid Acute Cardiomyopathy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Programmes and Projects. Immunization, Vaccines and Biologicals: Influenza. 2008. Available online: http://www.who.int/immunization/topics/influenza/en/index.html (accessed on 13 August 2012).
- Zeng, H.; Goldsmith, C.S.; Maines, T.R.; Belser, J.A.; Gustin, K.M.; Pekosz, A.; Zaki, S.R.; Katz, J.M.; Tumpey, T.M. Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures. J. Virol. 2013, 87, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Smith, C.W.; Katkin, J.P.; Thurmon, L.M.; Xu, X.; Mendoza, L.H.; Ballantyne, C.M. Endothelial alpha 2,6-linked sialic acid inhibits VCAM-1-dependent adhesion under flow conditions. J. Immunol. 1999, 163, 2867–2876. [Google Scholar] [CrossRef]
- Kenney, A.D.; Aron, S.L.; Gilbert, C.; Kumar, N.; Chen, P.; Eddy, A.; Zhang, L.; Zani, A.; Vargas-Maldonado, N.; Speaks, S.; et al. Influenza virus replication in cardiomyocytes drives heart dysfunction and fibrosis. Sci. Adv. 2022, 8, eabm5371. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Fraser, D.; Neyses, L. Cardiovascular manifestations associated with influenza virus infection. Int. J. Cardiol. 2008, 130, 304–309. [Google Scholar] [CrossRef]
- Estabragh, Z.R.; Mamas, M.A. The cardiovascular manifestations of influenza: A systematic review. Int. J. Cardiol. 2013, 167, 2397–2403. [Google Scholar] [CrossRef]
- Radovanovic, M.; Petrovic, M.; Barsoum, M.K.; Nordstrom, C.W.; Calvin, A.D.; Dumic, I.; Jevtic, D.; Hanna, R.D. Influenza Myopericarditis and Pericarditis: A Literature Review. J. Clin. Med. 2022, 11, 4123. [Google Scholar] [CrossRef] [PubMed]
- Sin, P.; Siddiqui, M.; Wozniak, R.; Bare, I.; Minion, J.; Sanche, S.; Udell, J.; Lavoie, A.; Dehghani, P. Heart Failure after Laboratory Confirmed Influenza Infection (FLU-HF). Glob. Heart 2022, 17, 43. [Google Scholar] [CrossRef]
- Pan, H.Y.; Sun, H.M.; Xue, L.J.; Pan, M.; Wang, Y.P.; Kido, H.; Zhu, J.H. Ectopic trypsin in the myocardium promotes dilated cardiomyopathy after influenza A virus infection. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H922–H932. [Google Scholar] [CrossRef]
- Costantino, C.; Vitale, F. Influenza vaccination in high-risk groups: A revision of existing guidelines and rationale for an evidence-based preventive strategy. J. Prev. Med. Hyg. 2016, 57, E13–E18. [Google Scholar]
- Cioffi, D.L.; Pandey, S.; Alvarez, D.F.; Cioffi, E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L1067–L1077. [Google Scholar] [CrossRef]
- Sumikoshi, M.; Hashimoto, K.; Kawasaki, Y.; Sakuma, H.; Suzutani, T.; Suzuki, H.; Hosoya, M. Human influenza virus infection and apoptosis induction in human vascular endothelial cells. J. Med. Virol. 2008, 80, 1072–1078. [Google Scholar] [CrossRef]
- Marchenko, V.A.; Barashkova, S.V.; Zelinskaya, I.A.; Toropova, Y.G.; Ramsay, E.S.; Zhilinskaya, I.N. Modulation of endothelial factors activity in human endothelial cells in influenza A(H1N1)pdm09 virus infection. Probl. Virol. 2021, 66, 198–210. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Pleschka, S.; Planz, O.; Wolff, T. Ringing the alarm bells: Signalling and apoptosis in influenza virus infected cells. Cell. Microbiol. 2006, 8, 375–386. [Google Scholar] [CrossRef]
- Marchenko, V.; Zelinskaya, I.; Toropova, Y.; Shmakova, T.; Podyacheva, E.; Lioznov, D.; Zhilinskaya, I.N. Influenza A Virus Causes Histopathological Changes and Impairment in Functional Activity of Blood Vessels in Different Vascular Beds. Viruses 2022, 14, 396. [Google Scholar] [CrossRef]
- Marchenko, V.; Mukhametdinova, D.; Amosova, I.; Lioznov, D.; Zhilinskaya, I. Influenza A(H1N1)pdm09 Virus Alters Expression of Endothelial Factors in Pulmonary Vascular Endothelium in Rats. Viruses 2022, 14, 2518. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health. Risk. Manag. 2005, 1, 183–198. [Google Scholar]
- Madjid, M.; Aboshady, I.; Awan, I.; Litovsky, S.; Casscells, S.W. Influenza and cardiovascular disease: Is there a causal relationship? Tex. Heart Inst. J. 2004, 31, 4–13. [Google Scholar]
- Azambuja, M.I. Spanish flu and early 20th-century expansion of a coronary heart disease-prone subpopulation. Tex. Heart Inst. J. 2004, 31, 14–21. [Google Scholar] [PubMed]
- Tsinzerling, V.A.; Vorob’ev, S.L.; Zarubaev, V.V.; Dedov, V.A.; Belyaevskaya, S.V.; Esaulenko, E.V.; Dedov, V.A.; Grudinin, M.P.; Buzitskaia, Z.V.; Pisareva, M.M.; et al. Pathogenic aspects of influenza during the epidemics caused by H1N1v virus in 2009–2010. Arkhiv. Patol. 2011, 73, 21–25. (In Russian) [Google Scholar]
- Marchenko, V.A.; Barashkova, S.V.; Zelinskaya, I.A.; Toropova, Y.G.; Sorokin, E.V.; Zhilinskaya, I.N. Modeling influenza virus infection in mature Wistar rats. Probl. Virol. 2020, 65, 159–166. (In Russian) [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Taylor, C.R.; Levenson, R.M. Quantification of immunohistochemistry-issues concerning methods, utility and semiquantitative assessment II. Histopathology 2006, 49, 411–424. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View from the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.P.; Teng, T.; Yan, L.; Tang, Q.Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kaikita, K.; Yamamoto, E.; Jinnouchi, H.; Tsujita, K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc. Interv. Ther. 2021, 36, 39–51. [Google Scholar] [CrossRef]
- Vita, J.A.; Treasure, C.B.; Nabel, E.G.; McLenachan, J.M.; Fish, R.D.; Yeung, A.C.; Vekshtein, V.I.; Selwyn, A.P.; Ganz, P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation 1990, 81, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Tse, L.V.; Marcano, V.C.; Huang, W.; Pocwierz, M.S.; Whittaker, G.R. Plasmin-mediated activation of pandemic H1N1 influenza virus hemagglutinin is independent of the viral neuraminidase. J. Virol. 2013, 87, 5161–5169. [Google Scholar] [CrossRef]
- Lin, L.; Wu, C.; Hu, K. Tissue plasminogen activator activates NF-κB through a pathway involving annexin A2/CD11b and integrin-linked kinase. J. Am. Soc. Nephrol. 2012, 23, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Hekman, C.M.; Loskutoff, D.J. Kinetic analysis of the interactions between plasminogen activator inhibitor 1 and both urokinase and tissue plasminogen activator. Arch. Biochem. Biophys. 1988, 262, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Boudier, C.; Gils, A.; Declerck, P.J.; Bieth, J.G. The conversion of active to latent plasminogen activator inhibitor-1 is an energetically silent event. Biophys. J. 2005, 88, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen activator inhibitor-1 (PAI-1): A key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E. PAI-1 and atherothrombosis. J. Thromb. Haemost. 2005, 3, 1879–1883. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, H. Aberrant coagulation causes a hyper-inflammatory response in severe influenza pneumonia. Cell. Mol. Immunol. 2016, 13, 432–442. [Google Scholar] [CrossRef]
- Zhilinskaya, I.N.; Marchenko, V.A.; Kharchenko, E.P. Comparison of Fragments in Human Hemostatic Proteins That Mimics Fragments in Proteins of A/H1N1 Viruses and Coronaviruses. Mol. Gen. Microbiol. Virol. 2022, 37, 209–225. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Yang, W.; Ito, K.; Matte, T.D.; Shaman, J.; Kinney, P.L. Seasonal Influenza Infections and Cardiovascular Disease Mortality. JAMA Cardiol. 2016, 1, 274–281. [Google Scholar] [CrossRef]



| Hours Post Infection | Virus Titer (log EID50/mL) | Type of Tissue | ||
|---|---|---|---|---|
| Control No. 1 (Healthy Rats) | Control No. 2 (Uninfected Rats with Premorbid Acute Cardiomyopathy) | Rats with Acute Cardiomyopathy Infected with Influenza A(H1N1)pdm09 Virus | ||
| 24 | 0.0 | 0.0 | 7.2 ± 0.3 *,# | Lungs |
| 96 | 0.0 | 0.0 | 2.6 ± 0.5 *,# | |
| 24 | 0.0 | 0.0 | 0.0 | Mesentery |
| 96 | 0.0 | 0.0 | 0.0 | |
| Agonist | Parameter | Control (Healthy Rats) (n = 10) | ACMP 24 h (n = 5) | ACMP 96 h (n = 5) | ACMP + IAV 24 hpi (n = 5) | ACMP + IAV 96 hpi (n = 5) |
|---|---|---|---|---|---|---|
| PE | log EC50, M | −6.00 ± 0.04 | −5.29 ± 0.05 **** | −2.86 ± 0.56 ** | −5.82 ± 0.18 | −5.39 ± 0.18 * |
| Emax, % | 84.66 ± 1.68 | 89.54 ± 8.00 | 10.38 ± 2.21 **** | 94.02 ± 5.87 | 48.20 ± 10.59 * | |
| ACh | log EC50, M | −6.71 ± 0.18 | −6.35 ± 0.07 | −6.50 ± 0.86 | −5.62 ± 0.10 *** | −5.46 ± 0.10 *** |
| Emax, % | 100.98 ± 0.73 | 86.02 ± 6.18 | −50.52 ± 32.59 * | 86.50 ± 10.79 | 78.03 ± 5.41 * |
| Signal Parameter | ACMP | ACMP + IAV | ||
|---|---|---|---|---|
| Time after Inoculation with α-MEM | Time after Infection | |||
| 24 h (n = 5) | 96 h (n = 5) | 24 h (n = 5) | 96 h (n = 5) | |
| Measured area | 2387.0 ± 423.5 | 23,848.8 ± 6548.6 | 40,641.0 ± 14,438.2 * | 6406.5 ± 3091.7 * |
| Sum density | 1544.5 ± 274.0 | 15,431.4± 4237.3 | 26,297.1 ± 9342.4 * | 5120.8 ± 2000.5 * |
| Sum intensity | 2948.7 ± 523.2 | 29,460.4 ± 8089.5 | 50,203.6 ± 17,835.5 * | 7914.0 ± 3819.2 * |
| Signal Parameter | ACMP | ACMP + IAV | ||
|---|---|---|---|---|
| Time after Inoculation with α-MEM | Time after Infection | |||
| 24 h (n = 5) | 96 h (n = 5) | 24 h (n = 5) | 96 h (n = 5) | |
| Measured area | 111,592.6 ± 72,523.2 | 60,786.0 ± 35,494.1 | 84,595.3 ± 80,153.2 | 300,052.6 ± 118,314.1 * |
| Sum density | 80,282.4 ± 46,926.8 | 47,120.9 ± 22,966.7 | 53,541.3 ± 51,863.8 | 223,919.8 ± 76,556.2 * |
| Sum intensity | 128,267.4 ± 89,587.6 | 77,930.8 ± 43,845.6 | 109,155.3 ± 99,012.8 | 379,813.5 ± 146,152.8 * |
| Signal Parameter | ACMP | ACMP + IAV | ||
|---|---|---|---|---|
| Time after Inoculation with α-MEM | Time after Infection | |||
| 24 h (n = 5) | 96 h (n = 5) | 24 h (n = 5) | 96 h (n = 5) | |
| Measured area | 3202.1 ± 1158.6 | 3409.9 ± 1432.2 | 3053.2 ± 1863.7 | 2514.2 ± 1874.6 |
| Sum density | 2354.4 ± 749.7 | 2418.3 ± 926.7 | 2008.6 ± 1205.9 | 3670.7 ± 1213.0 |
| Sum intensity | 3812.1 ± 1431.3 | 3919.5 ± 1769.3 | 3509.5 ± 2302.3 | 2993.1 ± 2315.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, V.; Zelinskaya, I.; Toropova, Y.; Podyacheva, E.; Martynov, M.; Mukhametdinova, D.; Lioznov, D.; Zhilinskaya, I.N. Influenza A(H1N1)pdm09 Virus Aggravates Pathology of Blood Vessels in Wistar Rats with Premorbid Acute Cardiomyopathy. Viruses 2023, 15, 1114. https://doi.org/10.3390/v15051114
Marchenko V, Zelinskaya I, Toropova Y, Podyacheva E, Martynov M, Mukhametdinova D, Lioznov D, Zhilinskaya IN. Influenza A(H1N1)pdm09 Virus Aggravates Pathology of Blood Vessels in Wistar Rats with Premorbid Acute Cardiomyopathy. Viruses. 2023; 15(5):1114. https://doi.org/10.3390/v15051114
Chicago/Turabian StyleMarchenko, Vladimir, Irina Zelinskaya, Yana Toropova, Ekaterina Podyacheva, Mikhail Martynov, Daria Mukhametdinova, Dmitry Lioznov, and Irina N. Zhilinskaya. 2023. "Influenza A(H1N1)pdm09 Virus Aggravates Pathology of Blood Vessels in Wistar Rats with Premorbid Acute Cardiomyopathy" Viruses 15, no. 5: 1114. https://doi.org/10.3390/v15051114
APA StyleMarchenko, V., Zelinskaya, I., Toropova, Y., Podyacheva, E., Martynov, M., Mukhametdinova, D., Lioznov, D., & Zhilinskaya, I. N. (2023). Influenza A(H1N1)pdm09 Virus Aggravates Pathology of Blood Vessels in Wistar Rats with Premorbid Acute Cardiomyopathy. Viruses, 15(5), 1114. https://doi.org/10.3390/v15051114






