Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies
Abstract
:1. Introduction
2. The Envelope Glycoproteins as the Target of Neutralizing Antibodies
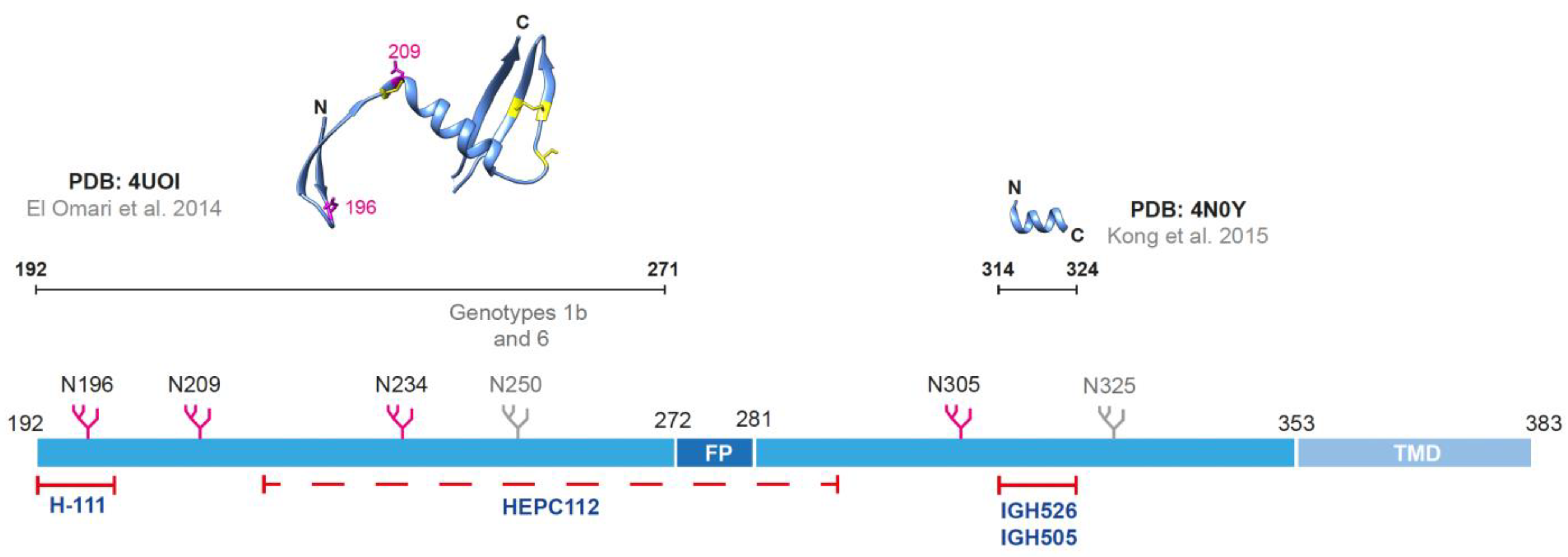
2.1. The Envelope Glycoprotein E1
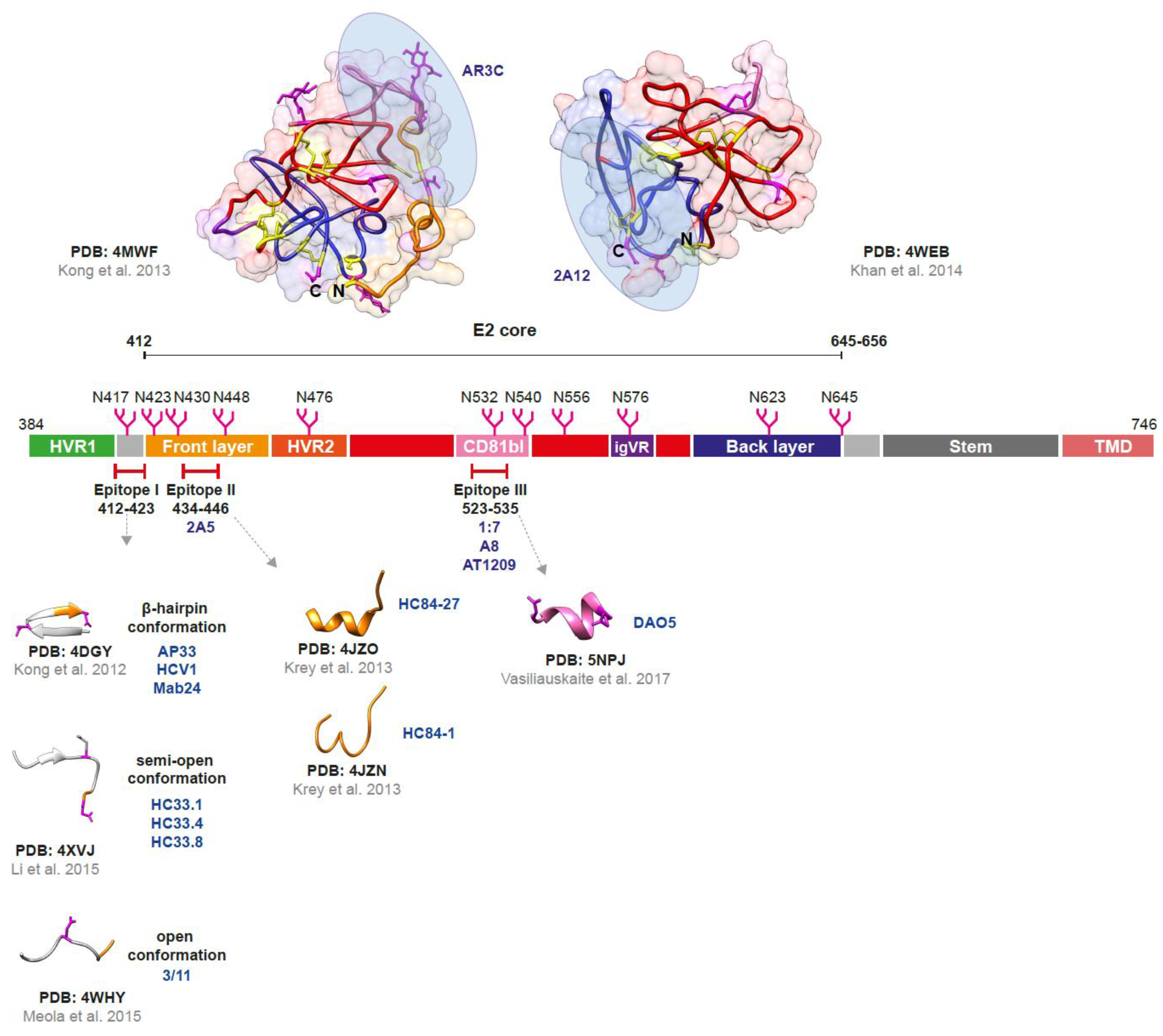
2.2. The Envelope Glycoprotein E2
2.3. The E1E2 Heterodimer
3. Analysis of Antibodies from HCV-Infected Subjects
4. B-Cell Vaccine Candidates against HCV
4.1. Vaccines Using Both HCV Envelope Glycoproteins (E1 and E2)
4.2. Vaccines Using the HCV Envelope Glycoprotein E2
4.3. Vaccines Using Peptides or Epitopes of the HCV Proteins
| Immunogen | Vaccine Candidate | Genotype | Platform | Expression System | Models Tested | Antibody Characterization | Neutralization | Cellular Responses | References |
|---|---|---|---|---|---|---|---|---|---|
| E1E2 | Recombinant E1E2/MF59 adjuvant | 1a | Recombinant membrane-bound proteins | CHO cells | Chimpanzees Healthy humans | ELISA (anti-E1E2) and competitive immunoassays (epitopes: HVR1, AR3, AR4, domains C and D) | Gt 1-7 HCVpp and HCVcc | Yes | [149,150,151,152,153,154] |
| E1E2-flag/IFA adjuvant | 1a, 1b, 2a | Recombinant soluble proteins | HEK293T cells | C57BL/6 mice | ELISA (anti-E1E2) | Gt 1a and 2a HCVcc | NA | [157] | |
| Fc-E1E2/alum adjuvant | 1a, 1b, 2a, 3a and 6a | Recombinant membrane-bound proteins | HEK293F cells | BALB/c mice | ELISA (anti-E1E2) | Gt 1a, 1b, 2a, 2b, 3a, 4c and 5a HCVcc | Yes | [158] | |
| Soluble native-like E1E2 | 1a | Recombinant soluble proteins | Expi293F cells | CD-1 mice | Competition inhibition analysis (epitopes: domains B, D and E, AR4, AR5 and E1) | Gt 1a HCVpp and HCVcc | NA | [159,160] | |
| MLV VLP E1E2 | 1a | VLP | HEK293T cells | BALB/c and C57BL/6J mice Cynomolgus macaques (Macaca fascicularis) | ELISA (anti-E1 and anti-E2) | Gt 1a, 1b, 2a, 2b and 4c HCVpp | Yes | [162] | |
| HCV VLP core, E1 and E2/alum adjuvant | 1a, 1b, 2a, 3a | VLP | Huh7 cells | BALB/c mice Landrace pigs | ELISA (HCV VLPs) | Gt 1a, 2a and 3a HCVcc | Yes | [163,164,165] | |
| Bivalent chimeric HBV/HCV vaccine/AddaVaxTM adjuvant | 1a, 3a, 4a | VLP | CHO cells | New Zealand female rabbits | ELISA (anti-E1 and anti-E2) | Gt 1a, 1b, 2a, 3a and 4a HCVpp and HCVcc | NA | [148,167,168,169,191] | |
| UV-inactivated HCVcc vaccine/K3-SPG adjuvant | 2a | Inactivated HCVcc | Huh7.5.1 cells | Chimeric liver uPA+/+-SCID mice; Marmoseth (Callithrix jacchus) | ELISA (anti-core, -E1, -E2) | Gt 1a, 1b, 2a and 3a HCVcc | Yes | [175,176] | |
| Inactivated whole HCV vaccine/AddaVaxTM adjuvant | 1a, 2a, 3a and 5a | Inactivated HCVcc | Huh7.5 cells | BALB/c mice | ELISA (anti-E2 and anti-E1E2) | Gt 1a, 1b, 2a, 2b, 3a, 4a, 5a and 6a HCVcc | NA | [84,85] | |
| E2E1-nanoparticle | 1, 2, 3, 4, 5 and 6 (cocktail and mosaic) | Recombinant soluble proteins in nanoparticles | Suspension 293F cells | New Zealand female rabbits | ELISA (anti-E2 or anti-E2E1) and competitive ELISA (bNAbs) | Gt 1-6 HCVpp | NA | [171] | |
| E2 | Soluble E2/Ferritin/Alhydrogel+MPLA adjuvants | 1a, 1b, 3a | Recombinant soluble proteins in nanoparticles | Drosophila S2 cells | BALB/c mice Rhesus macaques | ELISA (anti-E2) and competitive ELISA (AP33-like and AR3A- like bNAbs) | Gt 1-7 HCVcc | Yes | [178,179,180,181] |
| E2 Δ123 variable regions/AddaVaxTM adjuvant | 1a | Recombinant soluble proteins | FS293F cells | Albino Dunkin Hartley guinea pigs | Direct ELISA (anti-E2 and anti-E2 Δ123), capture ELISA (epitopes I, II and III) and competitive ELISA (CD81 binding) | Gt 1-7 HCVcc | NA | [182,183] | |
| Consensus core E2 ΔHVR1 ΔC-terminus | 1a (720 strains) | Recombinant soluble proteins | Drosophila S2 cells | Guinea pigs | ELISA (anti-E2) | Gt 1a HCVpp | NA | [185] | |
| E2 core nanoparticles /AddaVaxTM adjuvant | 1a, 6a | Recombinant soluble proteins in nanoparticles | HEK 293F and ExpiCHO cells | BALB/c mice | ELISA (anti-E2, and epitopes in front layer and AS412) | Gt 1a, 2a, 5a and 6a HCVpp | NA | [184] | |
| Epitopes or peptides | HBV VLPs carrying HCV E2 protein epitopes/AddaVaxTM adjuvant | 1a | VLP | Leishmania tarentolae | BALB/c mice | ELISA (anti-E2 epitopes 412–425 and 523–535) | Gt 1a, 1b, 2a, 2b, 4a and 5a HCVcc | NA | [186,187] |
| Chimeric HBV S antigen VLPs presenting HCV-neutralizing epitopes/AddaVaxTM adjuvant | 1a, 1b, 2a | VLP | HEK293T cells | BALB/c mice | ELISA (anti-HCV-neutralizing epitopes) | Gt 1a, 1b and 2a HCVcc and HCVpp | NA | [188] | |
| Multi-epitope peptide vaccine (E1, E2, NS4B, NS5A and NS5B) | 4a | Synthetic peptides | Synthesis by the 9-fluorenylmethoxy carbonylmethod | BALB/c mice | ELISA (anti-HCV peptides) | Gt 2a and 4a HCVcc | Yes | [189] | |
| Bivalent HCV peptide (HVR1) vaccine/ Freunds complete or incomplete adjuvant | 1a | Synthetic peptides | Synthesis using Fmoc chemistry | BALB/c mice | Competitive ELISA (HVR1, C-terminus) | Gt 1a, 1b, 2a, 3a, 4a, 5a and 6a HCVpp | NA | [190] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Accelerating Access to Hepatitis C Diagnostics and Treatment. Global Progress Report 2020; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. Global Hepatitis Report, 2017; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Razavi, H.; Sanchez Gonzalez, Y.; Yuen, C.; Cornberg, M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020, 40, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Cooke, G.S.; Nayagam, S.; Thursz, M.R.; Hallett, T.B. Scaling up prevention and treatment towards the elimination of hepatitis C: A global mathematical model. Lancet 2019, 393, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Nath, S.; Simmons, B. The road to elimination of hepatitis C: Analysis of cures versus new infections in 91 countries. J. Virus Erad. 2017, 3, 117–123. [Google Scholar] [CrossRef] [PubMed]
- The Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [CrossRef]
- Simmonds, P.; Alberti, A.; Alter, H.J.; Bonino, F.; Bradley, D.W.; Brechot, C.; Brouwer, J.T.; Chan, S.-W.; Chayama, K.; Chen, D.-S.; et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology 1994, 19, 1321–1324. [Google Scholar] [CrossRef]
- Smith, D.B.; Bukh, J.; Kuiken, C.; Muerhoff, A.S.; Rice, C.M.; Stapleton, J.T.; Simmonds, P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: Updated criteria and genotype assignment web resource. Hepatology 2014, 59, 318–327. [Google Scholar] [CrossRef]
- Hedskog, C.; Parhy, B.; Chang, S.; Zeuzem, S.; Moreno, C.; Shafran, S.D.; Borgia, S.M.; Asselah, T.; Alric, L.; Abergel, A.; et al. Identification of 19 novel hepatitis C virus subtypes-further expanding HCV classification. Open Forum Infect. Dis. 2019, 6, ofz076. [Google Scholar] [CrossRef]
- Borgia, S.M.; Hedskog, C.; Parhy, B.; Hyland, R.H.; Stamm, L.M.; Brainard, D.M.; Subramanian, M.G.; McHutchison, J.G.; Mo, H.; Svarovskaia, E.; et al. Identification of a novel hepatitis C virus genotype from Punjab, India: Expanding classification of hepatitis C virus into 8 genotypes. J. Infect. Dis. 2018, 218, 1722–1729. [Google Scholar] [CrossRef]
- Westbrook, R.H.; Dusheiko, G. Natural history of hepatitis C. J. Hepatol. 2014, 61, S58–S68. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef]
- Hoofnagle, J.H. Course and outcome of hepatitis C. Hepatology 2002, 36, s21–s29. [Google Scholar] [CrossRef] [PubMed]
- Micallef, J.M.; Kaldor, J.M.; Dore, G.J. Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. J. Viral Hepat. 2006, 13, 34–41. [Google Scholar] [CrossRef]
- Pestka, J.M.; Zeisel, M.B.; Bläser, E.; Schurmann, P.; Bartosch, B.; Cosset, F.-L.; Patel, A.H.; Meisel, H.; Baumert, J.; Viazov, S.; et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc. Natl. Acad. Sci. USA 2007, 104, 6025–6030. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; Snider, A.E.; Wells, B.L.; Latanich, R.; Bailey, J.R.; Thomas, D.L.; Cox, A.L.; Ray, S.C. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 2014, 59, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kanwal, F.; Richardson, P.; Kramer, J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology 2016, 64, 130–137. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2018, 68, 25–32. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of hepatocellular cancer in HCV patients treated With Direct-Acting Antiviral agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Childs, K.; Davis, C.; Cannon, M.; Montague, S.; Filipe, A.; Tong, L.; Simmonds, P.; Smith, D.B.; Thomson, E.C.; Dusheiko, G.; et al. Suboptimal SVR rates in African patients with atypical genotype 1 subtypes: Implications for global elimination of hepatitis C. J. Hepatol. 2019, 71, 1099–1105. [Google Scholar] [CrossRef]
- Fourati, S.; Rodriguez, C.; Hézode, C.; Soulier, A.; Ruiz, I.; Poiteau, L.; Chevaliez, S.; Pawlotsky, J.-M. Frequent antiviral treatment failures in patients infected with hepatitis C virus genotype 4, subtype 4r. Hepatology 2019, 69, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Boglione, L.; Mornese Pinna, S.; De Nicolò, A.; Cusato, J.; Cariti, G.; Di Perri, G.; D’Avolio, A. Treatment with direct-acting antiviral agents of hepatitis C virus infection in injecting drug users: A prospective study. J. Viral Hepat. 2017, 24, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Midgard, H.; Bjøro, B.; Mæland, A.; Konopski, Z.; Kileng, H.; Damås, J.K.; Paulsen, J.; Heggelund, L.; Sandvei, P.K.; Ringstad, J.O.; et al. Hepatitis C reinfection after sustained virological response. J. Hepatol. 2016, 64, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Simmons, B.; Saleem, J.; Hill, A.M.; Riley, R.D.; Cooke, G.S. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: A systematic review and meta-analysis. Clin. Infect. Dis. 2016, 62, 683–694. [Google Scholar] [CrossRef]
- Thompson, W.W.; Symum, H.; Sandul, A.; Gupta, N.; Patel, P.; Nelson, N.; Mermin, J.; Wester, C. Vital Signs: Hepatitis C Treatment Among Insured Adults—United States, 2019–2020. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1011–1017. [Google Scholar] [CrossRef]
- Echevarria, D.; Gutfraind, A.; Boodram, B.; Layden, J.; Ozik, J.; Page, K.; Cotler, S.J.; Major, M.E.; Dahari, H. Modeling indicates efficient vaccine-based interventions for the elimination of hepatitis C virus among persons who inject drugs in metropolitan Chicago. Vaccine 2019, 37, 2608–2616. [Google Scholar] [CrossRef]
- Stone, J.; Martin, N.K.; Hickman, M.; Hellard, M.E.; Scott, N.; McBryde, E.; Drummer, H.E.; Vickerman, P. The Potential impact of a hepatitis C vaccine for people who inject drugs: Is a vaccine needed in the age of Direct-Acting Antivirals? PLoS ONE 2016, 11, e0156213. [Google Scholar] [CrossRef]
- Roingeard, P.; Beaumont, E. Hepatitis C Vaccine: 10 Good Reasons for Continuing. Hepatology 2020, 71, 1845–1850. [Google Scholar] [CrossRef]
- Capone, S.; Meola, A.; Ercole, B.B.; Vitelli, A.; Pezzanera, M.; Ruggeri, L.; Davies, M.E.; Tafi, R.; Santini, C.; Luzzago, A.; et al. A novel adenovirus type 6 (Ad6)-based hepatitis C virus vector that overcomes preexisting anti-Ad5 Immunity and induces potent and broad cellular immune responses in Rhesus Macaques. J. Virol. 2006, 80, 1688–1699. [Google Scholar] [CrossRef]
- Folgori, A.; Capone, S.; Ruggeri, L.; Meola, A.; Sporeno, E.; Ercole, B.B.; Pezzanera, M.; Tafi, R.; Arcuri, M.; Fattori, E.; et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 2006, 12, 190–197. [Google Scholar] [CrossRef]
- Barnes, E.; Folgori, A.; Capone, S.; Swadling, L.; Aston, S.; Kurioka, A.; Meyer, J.; Huddart, R.; Smith, K.; Townsend, R.; et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 2012, 4, 115ra1. [Google Scholar] [CrossRef] [PubMed]
- Swadling, L.; Capone, S.; Antrobus, R.D.; Brown, A.; Richardson, R.; Newell, E.W.; Halliday, J.; Kelly, C.; Bowen, D.; Fergusson, J.; et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci. Transl. Med. 2014, 6, 261ra153. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Melia, M.T.; Veenhuis, R.T.; Winter, M.; Rousseau, K.E.; Massaccesi, G.; Osburn, W.O.; Forman, M.; Thomas, E.; Thornton, K.; et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N. Engl. J. Med. 2021, 384, 541–549. [Google Scholar] [CrossRef]
- Phelps, C.C.; Walker, C.M.; Honegger, J.R. Where to Next? Research Directions after the First Hepatitis C Vaccine Efficacy Trial. Viruses 2021, 13, 1351. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F. Hepatitis C Virus Proteins: From Structure to Function; Springer: Berlin/Heidelberg, Germany, 2013; Volume 369, pp. 113–142. ISBN 978-3-642-27339-1. [Google Scholar]
- Vieyres, G.; Thomas, X.; Descamps, V.; Duverlie, G.; Patel, A.H.; Dubuisson, J. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J. Virol. 2010, 84, 10159–10168. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, C.; Combet, C.; Bukh, J.; Shin-I, T.; Deleage, G.; Mizokami, M.; Richardson, R.; Sablon, E.; Yusim, K.; Pawlotsky, J.-M.; et al. A comprehensive system for consistent numbering of HCV sequences, proteins and epitopes. Hepatology 2006, 44, 1355–1361. [Google Scholar] [CrossRef]
- Douam, F.; Dao Thi, V.L.; Maurin, G.; Fresquet, J.; Mompelat, D.; Zeisel, M.B.; Baumert, T.F.; Cosset, F.-L.; Lavillette, D. Critical interaction between E1 and E2 glycoproteins determines binding and fusion properties of hepatitis C virus during cell entry. Hepatology 2014, 59, 776–788. [Google Scholar] [CrossRef]
- Hopcraft, S.E.; Evans, M.J. Selection of a hepatitis C virus with altered entry factor requirements reveals a genetic interaction between the E1 glycoprotein and claudins. Hepatology 2015, 62, 1059–1069. [Google Scholar] [CrossRef]
- Haid, S.; Grethe, C.; Dill, M.T.; Heim, M.H.; Kaderali, L.; Pietschmann, T. Isolate-dependent use of claudins for cell entry by hepatitis C virus. Hepatology 2014, 59, 24–34. [Google Scholar] [CrossRef]
- Meertens, L.; Bertaux, C.; Cukierman, L.; Cormier, E.; Lavillette, D.; Cosset, F.-L.; Dragic, T. The tight junction proteins claudin-1, -6, and -9 are entry cofactors for hepatitis C virus. J. Virol. 2008, 82, 3555–3560. [Google Scholar] [CrossRef]
- Cheng, J.J.; Li, J.R.; Huang, M.H.; Ma, L.L.; Wu, Z.Y.; Jiang, C.C.; Li, W.J.; Li, Y.H.; Han, Y.X.; Li, H.; et al. CD36 is a co-receptor for hepatitis C virus E1 protein attachment. Sci. Rep. 2016, 6, 21808. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Modis, Y. A novel membrane fusion protein family in Flaviviridae? Trends Microbiol. 2014, 22, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.P.; Wychowski, C.; Choukhi, A.; Meunier, J.-C.; Ung, S.; Rice, C.M.; Dubuisson, J. Characterization of truncated forms of hepatitis C virus glycoproteins. J. Gen. Virol. 1997, 78, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Cocquerel, L.; Wychowski, C.; Minner, F.; Penin, F.; Dubuisson, J. Charged residues in the transmembrane domains of hepatitis C virus glycoproteins play a major role in the processing, subcellular localization, and assembly of these envelope proteins. J. Virol. 2000, 74, 3623–3633. [Google Scholar] [CrossRef]
- Patel, J.; Patel, A.H.; McLauchlan, J. The transmembrane domain of the hepatitis C virus E2 glycoprotein is required for correct folding of the E1 glycoprotein and native complex formation. Virology 2001, 279, 58–68. [Google Scholar] [CrossRef]
- El Omari, K.; Iourin, O.; Kadlec, J.; Sutton, G.; Harlos, K.; Grimes, J.M.; Stuart, D.I. Unexpected structure for the N-terminal domain of hepatitis C virus envelope glycoprotein E1. Nat. Commun. 2014, 5, 4874. [Google Scholar] [CrossRef]
- Mazumdar, B.; Banerjee, A.; Meyer, K.; Ray, R. Hepatitis C virus E1 envelope glycoprotein interacts with apolipoproteins in facilitating entry into hepatocytes. Hepatology 2011, 54, 1149–1156. [Google Scholar] [CrossRef]
- Torrents de la Peña, A.; Sliepen, K.; Eshun-Wilson, L.; Newby, M.L.; Allen, J.D.; Zon, I.; Koekkoek, S.; Chumbe, A.; Crispin, M.; Schinkel, J.; et al. Structure of the hepatitis C virus E1E2 glycoprotein complex. Science 2022, 378, 263–269. [Google Scholar] [CrossRef]
- Meunier, J.-C.; Fournillier, A.; Choukhi, A.; Cahour, A.; Cocquerel, L.; Dubuisson, J.; Wychowski, C. Analysis of the glycosylation sites of hepatitis C virus (HCV) glycoprotein E1 and the influence of E1 glycans on the formation of the HCV glycoprotein complex. J. Gen. Virol. 1999, 80, 887–896. [Google Scholar] [CrossRef]
- Falson, P.; Bartosch, B.; Alsaleh, K.; Tews, B.A.; Loquet, A.; Ciczora, Y.; Riva, L.; Montigny, C.; Montpellier, C.; Duverlie, G.; et al. Hepatitis C virus envelope glycoprotein E1 forms trimers at the surface of the virion. J. Virol. 2015, 89, 10333–10346. [Google Scholar] [CrossRef]
- Ciczora, Y.; Callens, N.; Penin, F.; Pécheur, E.-I.; Dubuisson, J. Transmembrane domains of hepatitis C virus envelope glycoproteins: Residues involved in E1E2 heterodimerization and involvement of these domains in virus entry. J. Virol. 2007, 81, 2372–2381. [Google Scholar] [CrossRef] [PubMed]
- Op De Beeck, A.; Montserret, R.; Duvet, S.; Cocquerel, L.; Cacan, R.; Barberot, B.; Le Maire, M.; Penin, F.; Dubuisson, J. The transmembrane domains of hepatitis C virus envelope glycoproteins E1 and E2 play a major role in heterodimerization. J. Biol. Chem. 2000, 275, 31428–31437. [Google Scholar] [CrossRef] [PubMed]
- Freedman, H.; Logan, M.R.; Hockman, D.; Koehler Leman, J.; Law, J.L.M.; Houghton, M. Computational prediction of the heterodimeric and higher-order structure of gpE1/gpE2 envelope glycoproteins encoded by hepatitis C virus. J. Virol. 2017, 91, e02309-16. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.; Clementi, N.; Pfaff, J.; Sautto, G.A.; Diotti, R.A.; Burioni, R.; Doranz, B.J.; Peraro, M.D.; Clementi, M.; Mancini, N. A biologically-validated HCV E1E2 heterodimer structural model. Sci. Rep. 2017, 7, 214. [Google Scholar] [CrossRef]
- Kong, L.; Kadam, R.U.; Giang, E.; Ruwona, T.B.; Nieusma, T.; Culhane, J.C.; Stanfield, R.L.; Dawson, P.E.; Wilson, I.A.; Law, M. Structure of hepatitis C virus envelope glycoprotein E1 antigenic site 314–324 in complex with antibody IGH526. J. Mol. Biol. 2015, 427, 2617–2628. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Dubuisson, J.; Hsu, H.H.; Cheung, R.; Greenberg, H.B.; Russell, D.G.; Rice, C.M. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 1994, 68, 6147–6160. [Google Scholar] [CrossRef]
- Keck, Z.-Y.; Sung, V.M.H.; Perkins, S.; Rowe, J.; Paul, S.; Liang, T.J.; Lai, M.M.C.; Foung, S.K.H. Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J. Virol. 2004, 78, 7257–7263. [Google Scholar] [CrossRef]
- Meunier, J.-C.; Russell, R.S.; Goossens, V.; Priem, S.; Walter, H.; Depla, E.; Union, A.; Faulk, K.N.; Bukh, J.; Emerson, S.U.; et al. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the E1 glycoprotein of hepatitis C virus. J. Virol. 2008, 82, 966–973. [Google Scholar] [CrossRef]
- Colbert, M.D.; Flyak, A.I.; Ogega, C.O.; Kinchen, V.J.; Massaccesi, G.; Hernandez, M.; Davidson, E.; Doranz, B.J.; Cox, A.L.; Crowe, J.E.; et al. Broadly neutralizing antibodies targeting new sites of vulnerability in hepatitis C virus E1E2. J. Virol. 2019, 93, e02070-18. [Google Scholar] [CrossRef]
- Mesalam, A.A.; Desombere, I.; Farhoudi, A.; Van Houtte, F.; Verhoye, L.; Ball, J.K.; Dubuisson, J.; Foung, S.K.H.; Patel, A.H.; Persson, M.A.A.; et al. Development and characterization of a human monoclonal antibody targeting the N-terminal region of hepatitis C virus envelope glycoprotein E1. Virology 2018, 514, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, B.; Vitelli, A.; Granier, C.; Goujon, C.; Dubuisson, J.; Pascale, S.; Scarselli, E.; Cortese, R.; Nicosia, A.; Cosset, F.-L. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 2003, 278, 41624–41630. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, E.; Ansuini, H.; Cerino, R.; Roccasecca, R.M.; Acali, S.; Filocamo, G.; Traboni, C.; Nicosia, A.; Cortese, R.; Vitelli, A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002, 21, 5017–5025. [Google Scholar] [CrossRef] [PubMed]
- Pileri, P.; Uematsu, Y.; Campagnoli, S.; Galli, G.; Falugi, F.; Petracca, R.; Weiner, A.J.; Houghton, M.; Rosa, D.; Grandi, G.; et al. Binding of hepatitis C virus to CD81. Science 1998, 282, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hossain, R.A.; Yost, S.A.; Bu, W.; Wang, Y.; Dearborn, A.D.; Grakoui, A.; Cohen, J.I.; Marcotrigiano, J. Structural insights into hepatitis C virus receptor binding and entry. Nature 2021, 598, 521–525. [Google Scholar] [CrossRef]
- Kumar, A.; Rohe, T.C.; Elrod, E.J.; Khan, A.G.; Dearborn, A.D.; Kissinger, R.; Grakoui, A.; Marcotrigiano, J. Regions of hepatitis C virus E2 required for membrane association. Nat. Commun. 2023, 14, 433. [Google Scholar] [CrossRef]
- Kong, L.; Giang, E.; Nieusma, T.; Kadam, R.U.; Cogburn, K.E.; Hua, Y.; Dai, X.; Stanfield, R.L.; Burton, D.R.; Ward, A.B.; et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science 2013, 342, 1090–1094. [Google Scholar] [CrossRef]
- Khan, A.G.; Whidby, J.; Miller, M.T.; Scarborough, H.; Zatorski, A.V.; Cygan, A.; Price, A.A.; Yost, S.A.; Bohannon, C.D.; Jacob, J.; et al. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature 2014, 509, 381–384. [Google Scholar] [CrossRef]
- Flyak, A.I.; Ruiz, S.E.; Colbert, M.D.; Luong, T.; Crowe, J.E.; Bailey, J.R.; Bjorkman, P.J. HCV Broadly Neutralizing Antibodies Use a CDRH3 Disulfide Motif to Recognize an E2 Glycoprotein Site that Can Be Targeted for Vaccine Design. Cell Host Microbe 2018, 24, 703–716.e3. [Google Scholar] [CrossRef]
- Flyak, A.I.; Ruiz, S.E.; Salas, J.; Rho, S.; Bailey, J.R.; Bjorkman, P.J. An ultralong CDRH2 in HCV neutralizing antibody demonstrates structural plasticity of antibodies against E2 glycoprotein. eLife 2020, 9, e53169. [Google Scholar] [CrossRef]
- Tzarum, N.; Giang, E.; Kong, L.; He, L.; Prentoe, J.C.; Augestad, E.; Hua, Y.; Castillo, S.; Lauer, G.M.; Bukh, J.; et al. Genetic and structural insights into broad neutralization of hepatitis C virus by human V H 1-69 antibodies. Sci. Adv. 2019, 5, eaav1882. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Giang, E.; Nieusma, T.; Robbins, J.B.; Deller, M.C.; Stanfield, R.L.; Wilson, I.A.; Law, M. Structure of hepatitis C virus envelope glycoprotein E2 antigenic site 412 to 423 in complex with antibody AP33. J. Virol. 2012, 86, 13085–13088. [Google Scholar] [CrossRef] [PubMed]
- Bankwitz, D.; Vieyres, G.; Hueging, K.; Bitzegeio, J.; Doepke, M.; Chhatwal, P.; Haid, S.; Catanese, M.T.; Zeisel, M.B.; Nicosia, A.; et al. Role of hypervariable region 1 for the interplay of hepatitis C virus with entry factors and lipoproteins. J. Virol. 2014, 88, 12644–12655. [Google Scholar] [CrossRef] [PubMed]
- Prentoe, J.C.; Verhoye, L.; Velázquez-Moctezuma, R.; Buysschaert, C.; Farhoudi, A.; Wang, R.; Alter, H.J.; Meuleman, P.; Bukh, J. HVR1-mediated antibody evasion of highly infectious in vivo adapted HCV in humanised mice. Gut 2016, 65, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Farci, P.; Shimoda, A.; Wong, D.; Cabezon, T.; De Gioannis, D.; Strazzera, A.; Shimizu, Y.; Shapiro, M.; Alter, H.J.; Purcell, R.H. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 1996, 93, 15394–15399. [Google Scholar] [CrossRef]
- Hsu, M.; Zhang, J.; Flint, M.; Logvinoff, C.; Cheng-Mayer, C.; Rice, C.M.; McKeating, J.A. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 2003, 100, 7271–7276. [Google Scholar] [CrossRef]
- Vieyres, G.; Dubuisson, J.; Patel, A.H. Characterization of antibody-mediated neutralization directed against the hypervariable region 1 of hepatitis C virus E2 glycoprotein. J. Gen. Virol. 2011, 92, 494–506. [Google Scholar] [CrossRef]
- Keck, Z.-Y.; Girard-Blanc, C.; Wang, W.; Lau, P.; Zuiani, A.; Rey, F.A.; Krey, T.; Diamond, M.S.; Foung, S.K.H. Antibody response to hypervariable region 1 interferes with broadly neutralizing antibodies to hepatitis C virus. J. Virol. 2016, 90, 3112–3122. [Google Scholar] [CrossRef]
- Prentoe, J.C.; Velázquez-Moctezuma, R.; Foung, S.K.H.; Law, M.; Bukh, J. Hypervariable region 1 shielding of hepatitis C virus is a main contributor to genotypic differences in neutralization sensitivity. Hepatology 2016, 64, 1881–1892. [Google Scholar] [CrossRef]
- Bankwitz, D.; Steinmann, E.; Bitzegeio, J.; Ciesek, S.; Friesland, M.; Herrmann, E.; Zeisel, M.B.; Baumert, T.F.; Keck, Z.; Foung, S.K.H.; et al. Hepatitis C virus hypervariable region 1 modulates receptor interactions, conceals the CD81 binding site, and protects conserved neutralizing epitopes. J. Virol. 2010, 84, 5751–5763. [Google Scholar] [CrossRef]
- Alzua, G.P.; Pihl, A.F.; Offersgaard, A.; Duarte Hernandez, C.R.; Duan, Z.; Feng, S.; Fahnøe, U.; Sølund, C.; Weis, N.; Law, M.; et al. Inactivated genotype 1a, 2a and 3a HCV vaccine candidates induced broadly neutralising antibodies in mice. Gut 2022, 72, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Pihl, A.F.; Feng, S.; Offersgaard, A.; Alzua, G.P.; Augestad, E.H.; Mathiesen, C.K.; Jensen, T.B.; Krarup, H.; Law, M.; Prentoe, J.; et al. Inactivated whole hepatitis C virus vaccine employing a licensed adjuvant elicits cross-genotype neutralizing antibodies in mice. J. Hepatol. 2022, 76, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Meola, A.; Tarr, A.W.; England, P.; Meredith, L.W.; McClure, C.P.; Foung, S.K.H.; McKeating, J.A.; Ball, J.K.; Rey, F.A.; Krey, T. Structural flexibility of a conserved antigenic region in hepatitis C virus glycoprotein E2 recognized by broadly neutralizing antibodies. J. Virol. 2015, 89, 2170–2181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pierce, B.G.; Wang, Q.; Keck, Z.-Y.; Fuerst, T.R.; Foung, S.K.H.; Mariuzza, R.A. Structural basis for penetration of the glycan shield of hepatitis C virus E2 glycoprotein by a broadly neutralizing human antibody. J. Biol. Chem. 2015, 290, 10117–10125. [Google Scholar] [CrossRef] [PubMed]
- Krey, T.; Meola, A.; Keck, Z.-Y.; Damier-Piolle, L.; Foung, S.K.H.; Rey, F.A. Structural basis of HCV neutralization by human monoclonal antibodies resistant to viral neutralization escape. PLoS Pathog. 2013, 9, e1003364. [Google Scholar] [CrossRef] [PubMed]
- Vasiliauskaite, I.; Owsianka, A.M.; England, P.; Khan, A.G.; Cole, S.; Bankwitz, D.; Foung, S.K.H.; Pietschmann, T.; Marcotrigiano, J.; Rey, F.A.; et al. Conformational flexibility in the immunoglobulin-like domain of the hepatitis C virus glycoprotein E2. mBio 2017, 8, e00382-17. [Google Scholar] [CrossRef]
- Tarr, A.W.; Urbanowicz, R.A.; Jayaraj, D.; Brown, R.J.P.; McKeating, J.A.; Irving, W.L.; Ball, J.K. Naturally occurring antibodies that recognize linear epitopes in the Amino terminus of the hepatitis C virus E2 protein confer noninterfering, additive neutralization. J. Virol. 2012, 86, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.W.; Owsianka, A.M.; Jayaraj, D.; Brown, R.J.P.; Hickling, T.P.; Irving, W.L.; Patel, A.H.; Ball, J.K. Determination of the human antibody response to the epitope defined by the hepatitis C virus-neutralizing monoclonal antibody AP33. J. Gen. Virol. 2007, 88, 2991–3001. [Google Scholar] [CrossRef]
- Owsianka, A.M.; Clayton, R.F.; Loomis-Price, L.D.; McKeating, J.A.; Patel, A.H. Functional analysis of hepatitis C virus E2 glycoproteins and virus-like particles reveals structural dissimilarities between different forms of E2. J. Gen. Virol. 2001, 82, 1877–1883. [Google Scholar] [CrossRef]
- Potter, J.A.; Owsianka, A.M.; Jeffery, N.; Matthews, D.J.; Keck, Z.-Y.; Lau, P.; Foung, S.K.H.; Taylor, G.L.; Patel, A.H. Toward a hepatitis C virus vaccine: The structural basis of hepatitis C virus neutralization by AP33, a broadly neutralizing antibody. J. Virol. 2012, 86, 12923–12932. [Google Scholar] [CrossRef]
- Tarr, A.W.; Owsianka, A.M.; Timms, J.M.; McClure, C.P.; Brown, R.J.P.; Hickling, T.P.; Pietschmann, T.; Bartenschlager, R.; Patel, A.H.; Ball, J.K. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology 2006, 43, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Broering, T.J.; Garrity, K.A.; Boatright, N.K.; Sloan, S.E.; Sandor, F.; Thomas, W.D.; Szabo, G.; Finberg, R.W.; Ambrosino, D.M.; Babcock, G.J. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J. Virol. 2009, 83, 12473–12482. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Giang, E.; Robbins, J.B.; Stanfield, R.L.; Burton, D.R.; Wilson, I.A.; Law, M. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc. Natl. Acad. Sci. USA 2012, 109, 9499–9504. [Google Scholar] [CrossRef] [PubMed]
- Alhammad, Y.; Gu, J.; Boo, I.; Harrison, D.; McCaffrey, K.; Vietheer, P.T.; Edwards, S.; Quinn, C.; Coulibaly, F.; Poumbourios, P.; et al. Monoclonal antibodies directed toward the hepatitis C virus glycoprotein E2 detect antigenic differences modulated by the N-terminal hypervariable region 1 (HVR1), HVR2, and intergenotypic variable region. J. Virol. 2015, 89, 12245–12261. [Google Scholar] [CrossRef] [PubMed]
- Morin, T.J.; Broering, T.J.; Leav, B.A.; Blair, B.M.; Rowley, K.J.; Boucher, E.N.; Wang, Y.; Cheslock, P.S.; Knauber, M.; Olsen, D.B.; et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012, 8, e1002895. [Google Scholar] [CrossRef]
- Desombere, I.; Fafi-Kremer, S.; Van Houtte, F.; Pessaux, P.; Farhoudi, A.; Heydmann, L.; Verhoye, L.; Cole, S.; Mckeating, J.A.; Leroux-Roels, G.; et al. Monoclonal anti-envelope antibody AP33 protects humanized mice against a patient-derived hepatitis C virus challenge. Hepatology 2016, 63, 1120–1134. [Google Scholar] [CrossRef]
- Gu, J.; Hardy, J.; Boo, I.; Vietheer, P.T.; McCaffrey, K.; Alhammad, Y.; Chopra, A.; Gaudieri, S.; Poumbourios, P.; Coulibaly, F.; et al. Escape of hepatitis C virus from epitope I neutralization increases sensitivity of other neutralization epitopes. J. Virol. 2018, 92, e02066-17. [Google Scholar] [CrossRef]
- Pantua, H.; Diao, J.; Ultsch, M.; Hazen, M.; Mathieu, M.; McCutcheon, K.; Takeda, K.; Date, S.; Cheung, T.K.; Phung, Q.; et al. Glycan shifting on hepatitis C virus (HCV) E2 glycoprotein is a mechanism for escape from broadly neutralizing antibodies. J. Mol. Biol. 2013, 425, 1899–1914. [Google Scholar] [CrossRef]
- Keck, Z.-Y.; Wang, W.; Wang, Y.; Lau, P.; Carlsen, T.; Prentoe, J.C.; Xia, J.; Patel, A.H.; Bukh, J.; Foung, S.K.H. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J. Virol. 2013, 87, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.-Y.; Angus, A.G.N.; Wang, W.; Lau, P.; Wang, Y.; Gatherer, D.; Patel, A.H.; Foung, S.K.H. Non-random escape pathways from a broadly neutralizing human monoclonal antibody map to a highly conserved region on the hepatitis C virus E2 glycoprotein encompassing amino acids 412–423. PLoS Pathog. 2014, 10, e1004297. [Google Scholar] [CrossRef]
- Flint, M.; Maidens, C.; Loomis-Price, L.D.; Shotton, C.; Dubuisson, J.; Monk, P.; Higginbottom, A.; Levy, S.; McKeating, J.A. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 1999, 73, 6235–6244. [Google Scholar] [CrossRef] [PubMed]
- Helle, F.; Vieyres, G.; Elkrief, L.; Popescu, C.-I.; Wychowski, C.; Descamps, V.; Castelain, S.; Roingeard, P.; Duverlie, G.; Dubuisson, J. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J. Virol. 2010, 84, 11905–11915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, C.G.; Mihalik, K.; Virata-Theimer, M.L.; Yu, M.Y.W.; Alter, H.J.; Feinstone, S.M. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc. Natl. Acad. Sci. USA 2007, 104, 8449–8454. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.-Y.; Xia, J.; Wang, Y.; Wang, W.; Krey, T.; Prentoe, J.C.; Carlsen, T.; Li, A.Y.J.; Patel, A.H.; Lemon, S.M.; et al. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog. 2012, 8, e1002653. [Google Scholar] [CrossRef]
- Desombere, I.; Mesalam, A.A.; Urbanowicz, R.A.; Van Houtte, F.; Verhoye, L.; Keck, Z.-Y.; Farhoudi, A.; Vercauteren, K.; Weening, K.E.; Baumert, T.F.; et al. A novel neutralizing human monoclonal antibody broadly abrogates hepatitis C virus infection in vitro and in vivo. Antivir. Res. 2017, 148, 53–64. [Google Scholar] [CrossRef]
- Merat, S.J.; Molenkamp, R.; Wagner, K.; Koekkoek, S.M.; van de Berg, D.; Yasuda, E.; Böhne, M.; Claassen, Y.B.; Grady, B.P.; Prins, M.; et al. Hepatitis C virus broadly neutralizing monoclonal antibodies isolated 25 years after spontaneous clearance. PLoS ONE 2016, 11, e0165047. [Google Scholar] [CrossRef]
- Johansson, D.X.; Voisset, C.; Tarr, A.W.; Aung, M.; Ball, J.K.; Dubuisson, J.; Persson, M.A.A. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc. Natl. Acad. Sci. USA 2007, 104, 16269–16274. [Google Scholar] [CrossRef]
- Kong, L.; Lee, D.E.; Kadam, R.U.; Liu, T.; Giang, E.; Nieusma, T.; Garces, F.; Tzarum, N.; Woods, V.L.; Ward, A.B.; et al. Structural flexibility at a major conserved antibody target on Hepatitis C virus E2 antigen. Proc. Natl. Acad. Sci. USA 2016, 113, 12768–12773. [Google Scholar] [CrossRef]
- Gopal, R.; Jackson, K.; Tzarum, N.; Kong, L.; Ettenger, A.; Guest, J.D.; Pfaff, J.M.; Barnes, T.; Honda, A.; Giang, E.; et al. Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis. PLOS Pathog. 2017, 13, e1006735. [Google Scholar] [CrossRef]
- Owsianka, A.M.; Timms, J.M.; Tarr, A.W.; Brown, R.J.P.; Hickling, T.P.; Szwejk, A.; Bienkowska-Szewczyk, K.; Thomson, B.J.; Patel, A.H.; Ball, J.K. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J. Virol. 2006, 80, 8695–8704. [Google Scholar] [CrossRef]
- Giang, E.; Dorner, M.; Prentoe, J.C.; Dreux, M.; Evans, M.J.; Bukh, J.; Rice, C.M.; Ploss, A.; Burton, D.R.; Law, M. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc. Natl. Acad. Sci. USA 2012, 109, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Maruyama, T.; Lewis, J.; Giang, E.; Tarr, A.W.; Stamataki, Z.; Gastaminza, P.; Chisari, F.V.; Jones, I.M.; Fox, R.I.; et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat. Med. 2008, 14, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.-Y.; Li, T.-K.; Xia, J.; Gal-Tanamy, M.; Olson, O.; Li, S.H.; Patel, A.H.; Ball, J.K.; Lemon, S.M.; Foung, S.K.H. Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J. Virol. 2008, 82, 6061–6066. [Google Scholar] [CrossRef] [PubMed]
- Keck, Z.-Y.; Saha, A.; Xia, J.; Wang, Y.; Lau, P.; Krey, T.; Rey, F.A.; Foung, S.K.H. Mapping a region of hepatitis C virus E2 that Is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J. Virol. 2011, 85, 10451–10463. [Google Scholar] [CrossRef]
- Pierce, B.G.; Keck, Z.-Y.; Lau, P.; Fauvelle, C.; Gowthaman, R.; Baumert, T.F.; Fuerst, T.R.; Mariuzza, R.A.; Foung, S.K.H. Global mapping of antibody recognition of the hepatitis C virus E2 glycoprotein: Implications for vaccine design. Proc. Natl. Acad. Sci. USA 2016, 113, E6946–E6954. [Google Scholar] [CrossRef]
- Tzarum, N.; Wilson, I.A.; Law, M. The neutralizing face of hepatitis C virus E2 envelope glycoprotein. Front. Immunol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Velázquez-Moctezuma, R.; Galli, A.; Law, M.; Bukh, J.; Prentoe, J.C. Hepatitis C virus escape studies of human antibody AR3A reveal a high barrier to resistance and novel insights on viral antibody evasion mechanisms. J. Virol. 2018, 93, e01909-18. [Google Scholar] [CrossRef]
- Keck, M.-L.; Wrensch, F.; Pierce, B.G.; Baumert, T.F.; Foung, S.K.H. Mapping Determinants of Virus Neutralization and Viral Escape for Rational Design of a Hepatitis C Virus Vaccine. Front. Immunol. 2018, 9, 1194. [Google Scholar] [CrossRef]
- Cao, L.; Yu, B.; Kong, D.; Cong, Q.; Yu, T.; Chen, Z.; Hu, Z.; Chang, H.; Zhong, J.; Baker, D.; et al. Functional expression and characterization of the envelope glycoprotein E1E2 heterodimer of hepatitis C virus. PLoS Pathog. 2019, 15, e1007759. [Google Scholar] [CrossRef]
- Pfaff-Kilgore, J.M.; Davidson, E.; Kadash-Edmondson, K.; Hernandez, M.; Rosenberg, E.; Chambers, R.; Castelli, M.; Clementi, N.; Mancini, N.; Bailey, J.R.; et al. Sites of vulnerability in HCV E1E2 identified by comprehensive functional screening. Cell Rep. 2022, 39, 110859. [Google Scholar] [CrossRef]
- Deleersnyder, V.; Pillez, A.; Wychowski, C.; Blight, K.J.; Xu, J.; Hahn, Y.S.; Rice, C.M.; Dubuisson, J. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 1997, 71, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guan, M.; Liu, Y.; Xu, Q.; Peng, H.; Liu, X.; Tang, Z.; Zhu, Y.; Wu, D.; Ren, H.; et al. Alanine scanning mutagenesis of hepatitis C virus E2 cysteine residues: Insights into E2 biogenesis and antigenicity. Virology 2014, 448, 229–237. [Google Scholar] [CrossRef]
- Glynn, S.A.; Wright, D.J.; Kleinman, S.H.; Hirschkorn, D.; Tu, Y.; Heldebrant, C.; Smith, R.; Giachetti, C.; Gallarda, J.; Busch, M.P. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005, 45, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Major, M.E.; Dahari, H.; Mihalik, K.; Puig, M.; Rice, C.M.; Neumann, A.U.; Feinstone, S.M. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology 2004, 39, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Boisvert, M.; Upadhyay, A.A.; Bédard, N.; Nelson, S.A.; Bruneau, J.; Derdeyn, C.A.; Marcotrigiano, J.; Evans, M.J.; Bosinger, S.E.; et al. Early T follicular helper cell activity accelerates hepatitis C virus-specific B cell expansion. J. Clin. Investig. 2021, 131, e140590. [Google Scholar] [CrossRef] [PubMed]
- Osburn, W.O.; Fisher, B.E.; Dowd, K.A.; Urban, G.; Liu, L.; Ray, S.C.; Thomas, D.L.; Cox, A.L. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 2010, 138, 315–324. [Google Scholar] [CrossRef]
- Bailey, J.R.; Flyak, A.I.; Cohen, V.J.; Li, H.; Wasilewski, L.N.; Snider, A.E.; Wang, S.; Learn, G.H.; Kose, N.; Loerinc, L.; et al. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight 2017, 2, e92872. [Google Scholar] [CrossRef]
- Kinchen, V.J.; Massaccesi, G.; Flyak, A.I.; Mankowski, M.C.; Colbert, M.D.; Osburn, W.O.; Ray, S.C.; Cox, A.L.; Crowe, J.E.; Bailey, J.R. Plasma deconvolution identifies broadly neutralizing antibodies associated with hepatitis C virus clearance. J. Clin. Investig. 2019, 129, 4786–4796. [Google Scholar] [CrossRef]
- Keck, Z.-Y.; Wang, Y.; Lau, P.; Lund, G.; Rangarajan, S.; Fauvelle, C.; Liao, G.C.; Holtsberg, F.W.; Warfield, K.L.; Aman, M.J.; et al. Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute hepatitis C virus infection in mice. Hepatology 2016, 64, 1922–1933. [Google Scholar] [CrossRef]
- De Jong, Y.P.; Dorner, M.; Mommersteeg, M.C.; Xiao, J.W.; Balazs, A.B.; Robbins, J.B.; Winer, B.Y.; Gerges, S.; Vega, K.; Labitt, R.N.; et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci. Transl. Med. 2014, 6, 254ra129. [Google Scholar] [CrossRef]
- Brasher, N.A.; Eltahla, A.A.; Underwood, A.; Boo, I.; Rizzetto, S.; Walker, M.R.; Rodrigo, C.; Luciani, F.; Maher, L.; Drummer, H.E.; et al. B cell immunodominance in primary hepatitis C virus infection. J. Hepatol. 2020, 72, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Potthoff, J.; Bizu, S.; Labuhn, M.; Dold, L.; Schoofs, T.; Horning, M.; Ercanoglu, M.S.; Kreer, C.; Gieselmann, L.; et al. Analysis of antibodies from HCV elite neutralizers identifies genetic determinants of broad neutralization. Immunity 2022, 55, 341–354.e7. [Google Scholar] [CrossRef]
- Chen, F.; Nagy, K.; Chavez, D.; Willis, S.; McBride, R.; Giang, E.; Honda, A.; Bukh, J.; Ordoukhanian, P.; Zhu, J.; et al. Antibody responses to immunization with HCV envelope glycoproteins as a baseline for B-cell–based vaccine development. Gastroenterology 2020, 158, 1058–1071.e6. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Venturi, M.; Majeed, S.; Moore, M.J.; Phogat, S.; Zhang, M.Y.; Dimitrov, D.S.; Hendrickson, W.A.; Robinson, J.; Sodroski, J.; et al. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc. Natl. Acad. Sci. USA 2004, 101, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Breden, F.; Lepik, C.; Longo, N.S.; Montero, M.; Lipsky, P.E.; Scott, J.K. Comparison of antibody repertoires produced by HIV-1 infection, other chronic and acute infections, and systemic autoimmune disease. PLoS ONE 2011, 6, e16857. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.M.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef]
- Throsby, M.; van den Brink, E.; Jongeneelen, M.; Poon, L.L.M.; Alard, P.; Cornelissen, L.; Bakker, A.; Cox, F.; van Deventer, E.; Guan, Y.; et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS ONE 2008, 3, e3942. [Google Scholar] [CrossRef]
- Bailey, J.R.; Wasilewski, L.N.; Snider, A.E.; El-Diwany, R.; Osburn, W.O.; Keck, Z.-Y.; Foung, S.K.H.; Ray, S.C. Naturally selected hepatitis C virus polymorphisms confer broad neutralizing antibody resistance. J. Clin. Investig. 2015, 125, 437–447. [Google Scholar] [CrossRef]
- Wasilewski, L.N.; El-Diwany, R.; Munshaw, S.; Snider, A.E.; Brady, J.K.; Osburn, W.O.; Ray, S.C.; Bailey, J.R. A hepatitis C virus envelope polymorphism confers resistance to neutralization by polyclonal sera and broadly neutralizing monoclonal antibodies. J. Virol. 2016, 90, 3773–3782. [Google Scholar] [CrossRef]
- Rodrigo, C.; Walker, M.R.; Leung, P.; Eltahla, A.A.; Grebely, J.; Dore, G.J.; Applegate, T.; Page, K.; Dwivedi, S.; Bruneau, J.; et al. Limited naturally occurring escape in broadly neutralizing antibody epitopes in hepatitis C glycoprotein E2 and constrained sequence usage in acute infection. Infect. Genet. Evol. 2017, 49, 88–96. [Google Scholar] [CrossRef]
- Prentoe, J.C.; Velázquez-Moctezuma, R.; Augestad, E.; Galli, A.; Wang, R.; Law, M.; Alter, H.J.; Bukh, J. Hypervariable region 1 and N-linked glycans of hepatitis C regulate virion neutralization by modulating envelope conformations. Proc. Natl. Acad. Sci. USA 2019, 116, 10039–10047. [Google Scholar] [CrossRef] [PubMed]
- Khera, T.; Behrendt, P.; Bankwitz, D.; Brown, R.J.P.; Todt, D.; Doepke, M.; Khan, A.G.; Schulze, K.; Law, J.; Logan, M.; et al. Functional and immunogenic characterization of diverse HCV glycoprotein E2 variants. J. Hepatol. 2019, 70, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Bankwitz, D.; Doepke, M.; Hueging, K.; Weller, R.; Bruening, J.; Behrendt, P.; Lee, J.-Y.; Vondran, F.W.R.; Manns, M.P.; Bartenschlager, R.; et al. Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity. J. Hepatol. 2017, 67, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Fauvelle, C.; Felmlee, D.J.; Crouchet, E.; Lee, J.-Y.; Heydmann, L.; Lefèvre, M.; Magri, A.; Hiet, M.-S.; Fofana, I.; Habersetzer, F.; et al. Apolipoprotein E mediates evasion from hepatitis C virus neutralizing antibodies. Gastroenterology 2016, 150, 206–217.e4. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, E.; Burlaud-Gaillard, J.; Visdeloup, C.; Silva, A.R.E.; Coutant, P.; Roingeard, P.; Beaumont, E. Incorporation of apolipoprotein E into HBV–HCV subviral envelope particles to improve the hepatitis vaccine strategy. Sci. Rep. 2021, 11, 21856. [Google Scholar] [CrossRef]
- Choo, Q.-L.; Kuo, G.; Ralston, R.; Weiner, A.J.; Chien, D.Y.; Van Nest, G.; Han, J.H.; Berger, K.; Thudium, K.; Kuo, C.; et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 1994, 91, 1294–1298. [Google Scholar] [CrossRef]
- Meunier, J.-C.; Gottwein, J.M.; Houghton, M.; Russell, R.S.; Emerson, S.U.; Bukh, J.; Purcell, R.H. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J. Infect. Dis. 2011, 204, 1186–1190. [Google Scholar] [CrossRef]
- Frey, S.E.; Houghton, M.; Coates, S.; Abrignani, S.; Chien, D.Y.; Rosa, D.; Pileri, P.; Ray, R.; Di Bisceglie, A.M.; Rinella, P.; et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367–6373. [Google Scholar] [CrossRef]
- Law, J.L.M.; Chen, C.; Wong, J.; Hockman, D.; Santer, D.M.; Frey, S.E.; Belshe, R.B.; Wakita, T.; Bukh, J.; Jones, C.T.; et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS ONE 2013, 8, e59776. [Google Scholar] [CrossRef]
- Ray, R.; Meyer, K.; Banerjee, A.; Basu, A.; Coates, S.; Abrignani, S.; Houghton, M.; Frey, S.E.; Belshe, R.B. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J. Infect. Dis. 2010, 202, 862–866. [Google Scholar] [CrossRef]
- Stamataki, Z.; Coates, S.; Abrignani, S.; Houghton, M.; McKeating, J.A. Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J. Infect. Dis. 2011, 204, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Law, J.L.M.; Logan, M.R.; Wong, J.; Kundu, J.; Hockman, D.; Landi, A.; Chen, C.; Crawford, K.; Wininger, M.; Johnson, J.; et al. Role of the E2 Hypervariable Region (HVR1) in the immunogenicity of a recombinant hepatitis C virus vaccine. J. Virol. 2018, 92, e02141-17. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Freedman, H.; Logan, M.; Wong, J.A.J.-X.; Hockman, D.; Chen, C.; He, J.; Beard, M.R.; Eyre, N.S.; Baumert, T.F.; et al. A recombinant hepatitis C virus genotype 1a E1/E2 envelope glycoprotein vaccine elicits antibodies that differentially neutralize closely related 2a strains through interactions of the N-terminal hypervariable region 1 of E2 with scavenger receptor B1. J. Virol. 2019, 93, e00810-19. [Google Scholar] [CrossRef] [PubMed]
- Krapchev, V.B.; Rychłowska, M.; Chmielewska, A.; Zimmer, K.; Patel, A.H.; Bieńkowska-Szewczyk, K. Recombinant Flag-tagged E1E2 glycoproteins from three hepatitis C virus genotypes are biologically functional and elicit cross-reactive neutralizing antibodies in mice. Virology 2018, 519, 33–41. [Google Scholar] [CrossRef]
- Lin, T.; Chi, X.; Liu, X.; Pan, S.; Chen, W.; Duan, H.; Zhang, X.; Yang, W. Recombinant full-length hepatitis C virus E1E2 dimer elicits pangenotypic neutralizing antibodies. Front. Immunol. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Guest, J.D.; Wang, R.; Elkholy, K.H.; Chagas, A.; Chao, K.L.; Cleveland, T.E.; Kim, Y.C.; Keck, Z.-Y.; Marin, A.; Yunus, A.S.; et al. Design of a native-like secreted form of the hepatitis C virus E1E2 heterodimer. Proc. Natl. Acad. Sci. USA 2021, 118, e2015149118. [Google Scholar] [CrossRef]
- Wang, R.; Suzuki, S.; Guest, J.D.; Heller, B.; Almeda, M.; Andrianov, A.K.; Marin, A.; Mariuzza, R.A.; Keck, Z.Y.; Foung, S.K.H.; et al. Induction of broadly neutralizing antibodies using a secreted form of the hepatitis C virus E1E2 heterodimer as a vaccine candidate. Proc. Natl. Acad. Sci. USA 2022, 119, e2112008119. [Google Scholar] [CrossRef]
- Zabel, F.; Kündig, T.M.; Bachmann, M.F. Virus-induced humoral immunity: On how B cell responses are initiated. Curr. Opin. Virol. 2013, 3, 357–362. [Google Scholar] [CrossRef]
- Garrone, P.; Fluckiger, A.C.; Mangeot, P.E.; Gauthier, E.; Dupeyrot-Lacas, P.; Mancip, J.; Cangialosi, A.; Du Chéné, I.; LeGrand, R.; Mangeot, I.; et al. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci. Transl. Med. 2011, 3, 94ra71. [Google Scholar] [CrossRef]
- Christiansen, D.; Earnest-Silveira, L.; Grubor-Bauk, B.; Wijesundara, D.K.; Boo, I.; Ramsland, P.A.; Vincan, E.; Drummer, H.E.; Gowans, E.J.; Torresi, J. Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery. Sci. Rep. 2019, 9, 9251. [Google Scholar] [CrossRef]
- Christiansen, D.; Earnest-Silveira, L.; Chua, B.; Meuleman, P.; Boo, I.; Grubor-Bauk, B.; Jackson, D.C.; Keck, Z.-Y.; Foung, S.K.H.; Drummer, H.E.; et al. Immunological responses following administration of a genotype 1a/1b/2/3a quadrivalent HCV VLP vaccine. Sci. Rep. 2018, 8, 6483. [Google Scholar] [CrossRef] [PubMed]
- Earnest-Silveira, L.; Chua, B.; Chin, R.; Christiansen, D.; Johnson, D.; Herrmann, S.; Ralph, S.A.; Vercauteren, K.; Mesalam, A.A.; Meuleman, P.; et al. Characterization of a hepatitis C virus-like particle vaccine produced in a human hepatocyte-derived cell line. J. Gen. Virol. 2016, 97, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Patient, R.; Hourioux, C.; Vaudin, P.; Pagès, J.-C.; Roingeard, P. Chimeric hepatitis B and C viruses envelope proteins can form subviral particles: Implications for the design of new vaccine strategies. New Biotechnol. 2009, 25, 226–234. [Google Scholar] [CrossRef]
- Beaumont, E.; Patient, R.; Hourioux, C.; Dimier-Poisson, I.; Roingeard, P. Chimeric hepatitis B virus/hepatitis C virus envelope proteins elicit broadly neutralizing antibodies and constitute a potential bivalent prophylactic vaccine. Hepatology 2013, 57, 1303–1313. [Google Scholar] [CrossRef]
- Beaumont, E.; Roch, E.; Chopin, L.; Roingeard, P. Hepatitis C virus E1 and E2 proteins used as separate immunogens induce neutralizing antibodies with additive properties. PLoS ONE 2016, 11, e0151626. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, E.; Joël Clément, B.; Guérin, V.; Chopin, L.; Roch, E.; Gomez-Escobar, E.; Roingeard, P. Mixing particles from various HCV genotypes increases the HBV-HCV vaccine ability to elicit broadly cross-neutralizing antibodies. Liver Int. 2020, 40, 1865–1871. [Google Scholar] [CrossRef]
- Butkovich, N.; Li, E.; Ramirez, A.; Burkhardt, A.M.; Wang, S. Advancements in protein nanoparticle vaccine platforms to combat infectious disease. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1681. [Google Scholar] [CrossRef]
- Sliepen, K.; Radić, L.; Capella-Pujol, J.; Watanabe, Y.; Zon, I.; Chumbe, A.; Lee, W.-H.; de Gast, M.; Koopsen, J.; Koekkoek, S.; et al. Induction of cross-neutralizing antibodies by a permuted hepatitis C virus glycoprotein nanoparticle vaccine candidate. Nat. Commun. 2022, 13, 7271. [Google Scholar] [CrossRef]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Wakita, T.; Pietschmann, T.; Kato, T.; Date, T.; Miyamoto, M.; Zhao, Z.; Murthy, K.K.; Habermann, A.; Kräusslich, H.-G.; Mizokami, M.; et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005, 11, 791–796. [Google Scholar] [CrossRef]
- Murayama, A.; Date, T.; Morikawa, K.; Akazawa, D.; Miyamoto, M.; Kaga, M.; Ishii, K.; Suzuki, T.; Kato, T.; Mizokami, M.; et al. The NS3 Helicase and NS5B-to-3′X Regions Are Important for Efficient Hepatitis C Virus Strain JFH-1 Replication in Huh7 Cells. J. Virol. 2007, 81, 8030–8040. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, D.; Moriyama, M.; Yokokawa, H.; Omi, N.; Watanabe, N.; Date, T.; Morikawa, K.; Aizaki, H.; Ishii, K.; Kato, T.; et al. Neutralizing antibodies induced by cell culture-derived hepatitis C virus protect against infection in mice. Gastroenterology 2013, 145, 447–455.e4. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, H.; Higashino, A.; Suzuki, S.; Moriyama, M.; Nakamura, N.; Suzuki, T.; Suzuki, R.; Ishii, K.; Kobiyama, K.; Ishii, K.J.; et al. Induction of humoural and cellular immunity by immunisation with HCV particle vaccine in a non-human primate model. Gut 2018, 67, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, C.K.; Jensen, T.B.; Prentoe, J.C.; Krarup, H.; Nicosia, A.; Law, M.; Bukh, J.; Gottwein, J.M. Production and characterization of high-titer serum-free cell culture grown hepatitis C virus particles of genotype 1-6. Virology 2014, 458–459, 190–208. [Google Scholar] [CrossRef]
- Li, D.; von Schaewen, M.; Wang, X.; Tao, W.; Zhang, Y.; Li, L.; Heller, B.; Hrebikova, G.; Deng, Q.; Ploss, A.; et al. Altered glycosylation patterns increase immunogenicity of a subunit hepatitis C virus vaccine, inducing neutralizing antibodies which confer protection in mice. J. Virol. 2016, 90, 10486–10498. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; von Schaewen, M.; Tao, W.; Zhang, Y.; Heller, B.; Hrebikova, G.; Deng, Q.; Sun, Q.; Ploss, A.; et al. Immunization with a subunit hepatitis C virus vaccine elicits pan-genotypic neutralizing antibodies and intrahepatic T-cell responses in nonhuman primates. J. Infect. Dis. 2017, 215, 1824–1831. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Gan, T.; Yang, X.; Li, D.; Zhou, D.; Sun, Q.; Huang, Z.; Zhong, J. A trivalent HCV vaccine elicits broad and synergistic polyclonal antibody response in mice and rhesus monkey. Gut 2019, 68, 140–149. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Lou, P.; Hu, Z.; Qu, P.; Li, D.; Li, Q.; Xu, Y.; Niu, J.; He, Y.; et al. A nanoparticle-based hepatitis c virus vaccine with enhanced potency. J. Infect. Dis. 2020, 221, 1304–1314. [Google Scholar] [CrossRef]
- Vietheer, P.T.; Boo, I.; Gu, J.; McCaffrey, K.; Edwards, S.; Owczarek, C.; Hardy, M.P.; Fabri, L.; Center, R.J.; Poumbourios, P.; et al. The core domain of hepatitis C virus glycoprotein E2 generates potent cross-neutralizing antibodies in guinea pigs. Hepatology 2017, 65, 1117–1131. [Google Scholar] [CrossRef]
- Center, R.J.; Boo, I.; Phu, L.; McGregor, J.; Poumbourios, P.; Drummer, H.E. Enhancing the antigenicity and immunogenicity of monomeric forms of hepatitis C virus E2 for use as a preventive vaccine. J. Biol. Chem. 2020, 295, 7179–7192. [Google Scholar] [CrossRef]
- He, L.; Tzarum, N.; Lin, X.; Shapero, B.; Sou, C.; Mann, C.J.; Stano, A.; Zhang, L.; Nagy, K.; Giang, E.; et al. Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. Sci. Adv. 2020, 6, eaaz6225. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.W.; Backx, M.; Hamed, M.R.; Urbanowicz, R.A.; McClure, C.P.; Brown, R.J.P.; Ball, J.K. Immunization with a synthetic consensus hepatitis C virus E2 glycoprotein ectodomain elicits virus-neutralizing antibodies. Antivir. Res. 2018, 160, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, A.; Tyborowska, J.; Peszyńska-Sularz, G.; Gromadzka, B.; Bieńkowska-Szewczyk, K.; Grzyb, K. Immunogenicity of Leishmania-derived hepatitis B small surface antigen particles exposing highly conserved E2 epitope of hepatitis C virus. Microb. Cell Fact. 2016, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, A.; Offersgaard, A.F.; Pihl, A.F.; Prentoe, J.C.; Bukh, J.; Gottwein, J.M.; Bieńkowska-Szewczyk, K.; Grzyb, K. Specific antibodies induced by immunization with hepatitis B virus-like particles carrying hepatitis C virus envelope glycoprotein 2 epitopes show differential neutralization efficiency. Vaccines 2020, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Lei, Y.; Yang, J.; Wang, X.; Shu, F.; Wei, X.; Lin, F.; Li, B.; Cui, Y.; Zhang, H.; et al. Neutralization effects of antibody elicited by chimeric HBV S antigen viral-like particles presenting HCV neutralization epitopes. Vaccine 2018, 36, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Dawood, R.M.; Moustafa, R.I.; Abdelhafez, T.H.; El-Shenawy, R.; El-Abd, Y.; Bader El Din, N.G.; Dubuisson, J.; El Awady, M.K. A multiepitope peptide vaccine against HCV stimulates neutralizing humoral and persistent cellular responses in mice. BMC Infect. Dis. 2019, 19, 932. [Google Scholar] [CrossRef]
- Mosa, A.I.; Urbanowicz, R.A.; AbouHaidar, M.G.; Tavis, J.E.; Ball, J.K.; Feld, J.J. A bivalent HCV peptide vaccine elicits pan-genotypic neutralizing antibodies in mice. Vaccine 2020, 38, 6864–6867. [Google Scholar] [CrossRef]
- Beaumont, E.; Roingeard, P. Chimeric hepatitis B virus (HBV)/hepatitis C virus (HCV) subviral envelope particles induce efficient anti-HCV antibody production in animals pre-immunized with HBV vaccine. Vaccine 2015, 33, 973–976. [Google Scholar] [CrossRef]
- Bankwitz, D.; Bahai, A.; Labuhn, M.; Doepke, M.; Ginkel, C.; Khera, T.; Todt, D.; Ströh, L.J.; Dold, L.; Klein, F.; et al. Hepatitis C reference viruses highlight potent antibody responses and diverse viral functional interactions with neutralising antibodies. Gut 2021, 70, 1734–1745. [Google Scholar] [CrossRef]
- Salas, J.H.; Urbanowicz, R.A.; Guest, J.D.; Frumento, N.; Figueroa, A.; Clark, K.E.; Keck, Z.; Cowton, V.M.; Cole, S.J.; Patel, A.H.; et al. An antigenically diverse, representative panel of envelope glycoproteins for hepatitis C virus vaccine development. Gastroenterology 2022, 162, 562–574. [Google Scholar] [CrossRef]
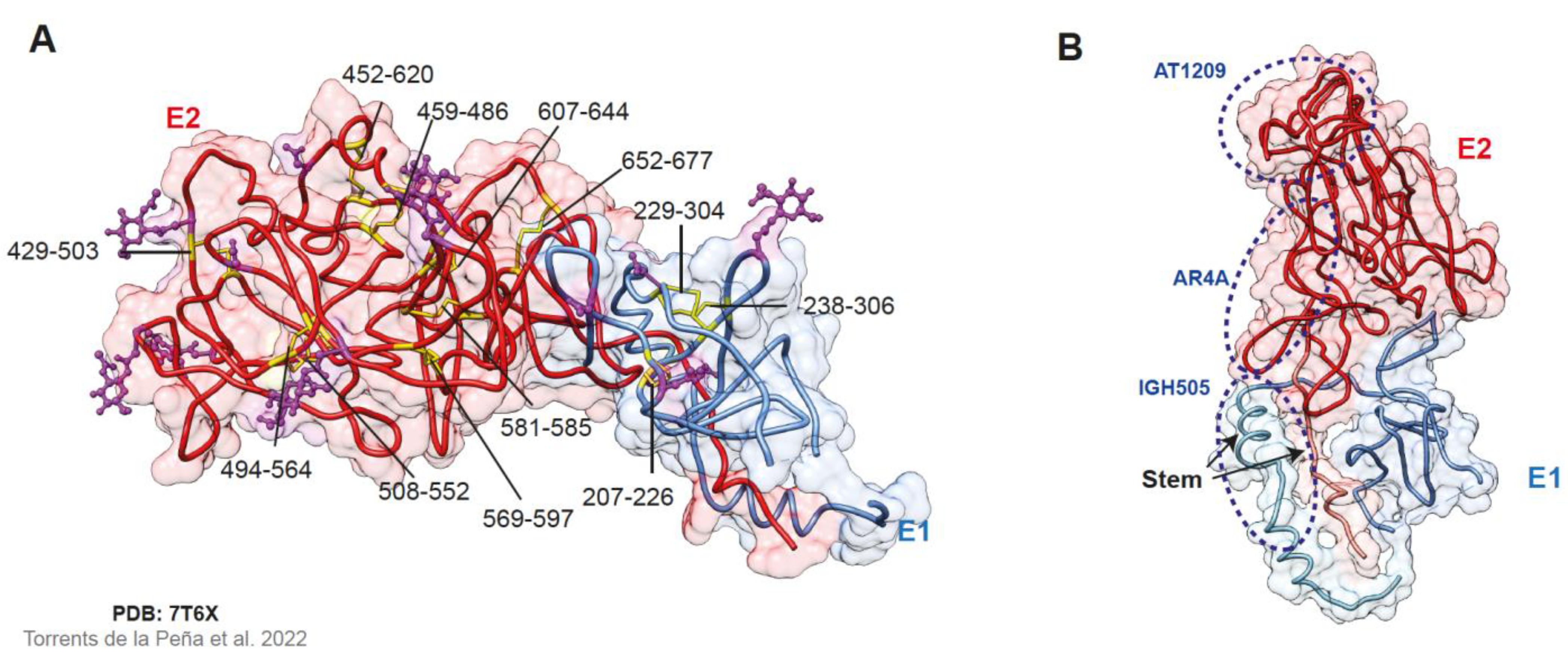
| Antigenic Region | Contact Residues | Overlapping Domain | Characteristics |
|---|---|---|---|
| 1 | 495, 519, 544, 545, 547, 548, 549 and 632 | Some residues of domain C | Non-neutralizing region |
| 2 | 597–645 | A | Back layer and poor neutralizing region |
| 3 | 396–424, 436–447, and 523–540 | B, D, and E | Neutralizing region inducing bNAbs |
| 4 | 201–206, 279, 487, 540, 547, 657, 658, 692, 698 *, 700 | E1 antigenic site aa 192–207 | Region comprising residues in E1 and E2 proteins, induction of bNAbs |
| 5 | 201–206, 639 *, 657, 658, 665, 692 | E1 antigenic site aa 192–207, and some residues of domain A | Region comprising residues in E1 and E2 proteins, induction of bNAbs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Escobar, E.; Roingeard, P.; Beaumont, E. Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies. Viruses 2023, 15, 1151. https://doi.org/10.3390/v15051151
Gomez-Escobar E, Roingeard P, Beaumont E. Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies. Viruses. 2023; 15(5):1151. https://doi.org/10.3390/v15051151
Chicago/Turabian StyleGomez-Escobar, Elsa, Philippe Roingeard, and Elodie Beaumont. 2023. "Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies" Viruses 15, no. 5: 1151. https://doi.org/10.3390/v15051151
APA StyleGomez-Escobar, E., Roingeard, P., & Beaumont, E. (2023). Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies. Viruses, 15(5), 1151. https://doi.org/10.3390/v15051151






