PTTG1 Enhances Oncolytic Adenovirus 5 Entry into Pancreatic Adenocarcinoma Cells by Increasing CXADR Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection and Analysis of PTTG1 Expression
2.2. Immune Checkpoints’ Blockade Response
2.3. Cell Culture
2.4. Stable Cell Line Construction
2.5. Western Blot Analysis
2.6. CCK-8 Assay
2.7. Fluorescence Visualization and Quantification
2.8. Flow Cytometry Assay
2.9. Data Collection and Analysis of CXADR Expression
2.10. Statistical Analysis
3. Results
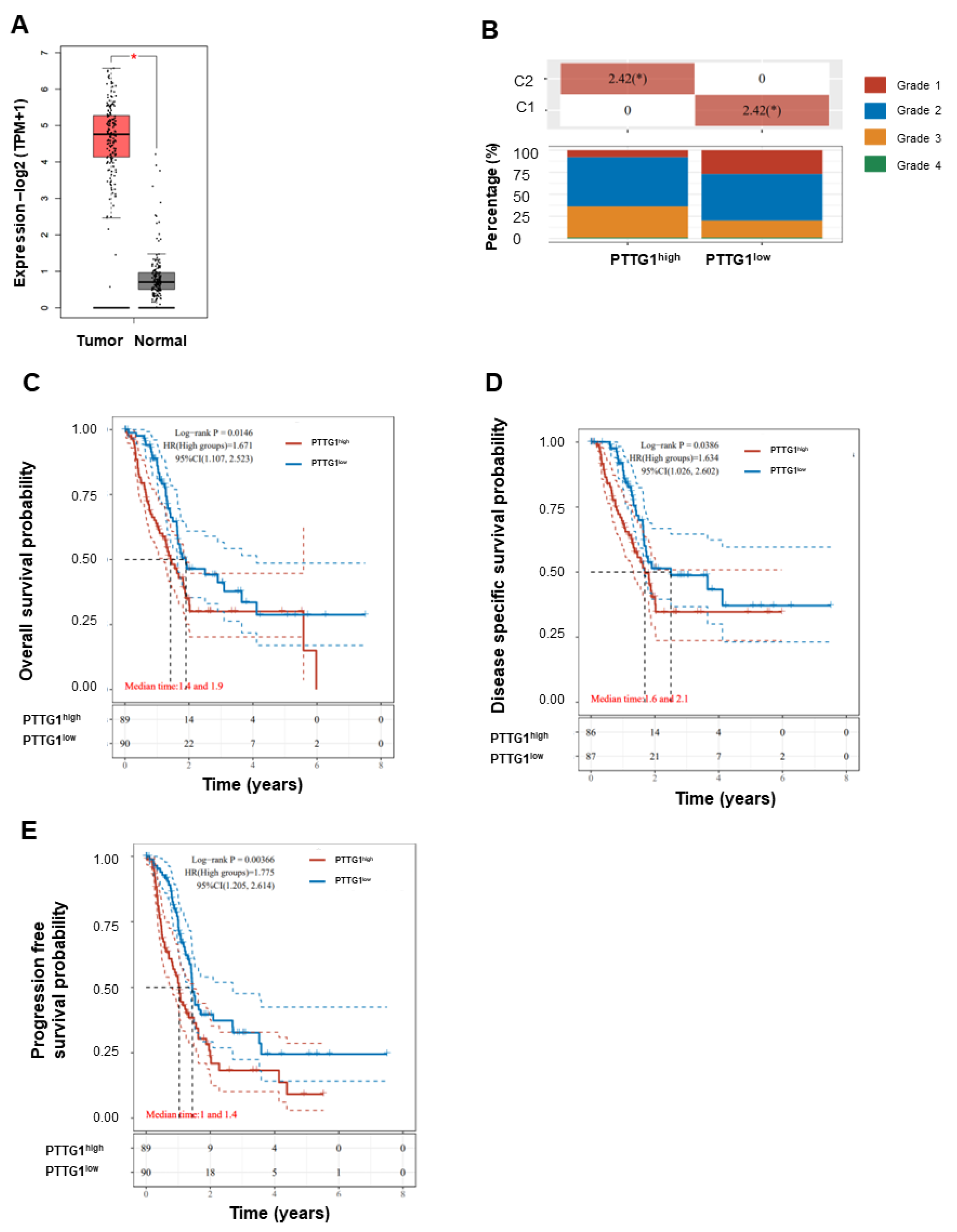
3.1. High PTTG1 Expression Correlates with High Clinical Stage and Poor Pancreatic Cancer Prognosis
3.2. Increased PTTG1 Expression Correlates with Chemotherapy and Immunotherapy Resistance
3.3. PTTG1 Enhanced Oncolytic Adenovirus Efficiency in Pancreatic Cancer Cells
3.4. PTTG1 Increased the Entry of Oncolytic Adenovirus into Pancreatic Cancer Cells
3.5. PTTG1 Increased CXADR Expression on Pancreatic Cancer Cells
3.6. The Enhancement of Oncolytic Adenovirus Entry into Cells by PTTG1 Was Dependent on CXADR
3.7. CXADR Expression Correlates with PTTG1 Expression in Normal and Malignant Pancreatic Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatcher, R.J.; Dong, J.; Liu, S.; Bian, G.; Contreras, A.; Wang, T.; Hilsenbeck, S.G.; Li, Y.; Zhang, P. Pttg1/securin is required for the branching morphogenesis of the mammary gland and suppresses mammary tumorigenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Vlotides, G.; Eigler, T.; Melmed, S. Pituitary Tumor-Transforming Gene: Physiology and Implications for Tumorigenesis. Endocr. Rev. 2007, 28, 165–186. [Google Scholar] [CrossRef]
- Huang, S.; Liao, Q.; Li, W.; Deng, G.; Jia, M.; Fang, Q.; Ji, H.; Meng, M. The lncRNA PTTG3P promotes the progression of CRPC via upregulating PTTG1. Bull. Cancer 2021, 108, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Tan, M.; Zhou, W.; Chen, C.; Xi, Y.; Gao, P.; Ma, Q.; Liang, Y.; Chen, M.; Tian, L.; et al. Bisphenol A promotes breast cancer cell proliferation by driving miR-381-3p-PTTG1-dependent cell cycle progression. Chemosphere 2021, 268, 129221. [Google Scholar] [CrossRef] [PubMed]
- Cho-Rok, J.; Yoo, J.; Jang, Y.; Kim, S.; Chu, I.; Yeom, Y.; Choi, J.; Im, D. Adenovirus-mediated transfer of siRNA against PTTG1 inhibits liver cancer cell growth in vitro and in vivo. Hepatology 2006, 43, 1042–1052. [Google Scholar]
- Qiu, M.; Li, G.; Wang, P.; Li, X.; Lai, F.; Luo, R.; Liu, B.; Lin, J. aarF domain containing kinase 5 gene promotes invasion and migration of lung cancer cells through ADCK5-SOX9-PTTG1 pathway. Exp. Cell Res. 2020, 392, 112002. [Google Scholar] [CrossRef]
- Teveroni, E.; Di Nicuolo, F.; Bianchetti, G.; Epstein, A.L.; Grande, G.; Maulucci, G.; De Spirito, M.; Pontecorvi, A.; Milardi, D.; Mancini, F. Nuclear Localization of PTTG1 Promotes Migration and Invasion of Seminoma Tumor through Activation of MMP-2. Cancers 2021, 13, 212. [Google Scholar] [CrossRef]
- Meng, C.; Zou, Y.; Hong, W.; Bao, C.; Jia, X. Estrogen-regulated PTTG1 promotes breast cancer progression by regulating cyclin kinase expression. Mol. Med. 2020, 26, 33. [Google Scholar] [CrossRef]
- Cui, L.; Ren, T.; Zhao, H.; Chen, S.; Zheng, M.; Gao, X.; Feng, D.; Yang, L.; Jin, X.; Zhuo, R. Suppression of PTTG1 inhibits cell angiogenesis, migration and invasion in glioma cells. Med. Oncol. 2020, 37, 73. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiog, Y.; Wang, G.; Li, W.; Tang, T.; Sun, J.; Li, J. miR-374c-5p regulates PTTG1 and inhibits cell growth and me-tastasis in hepatocellular carcinoma by regulating epithelial-mesenchymal transition. Mol. Med. Rep. 2022, 25, 148. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef] [PubMed]
- Fesler, A.; Ju, J. Development of microRNA-based therapy for pancreatic cancer. J. Pancreatol. 2019, 2, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.; Dyhl-Polk, A.; Chen, I.; Nielsen, D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat. Rev. 2019, 78, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Renouf, D.J.; Loree, J.M.; Knox, J.J.; Topham, J.T.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 5020. [Google Scholar] [CrossRef]
- Akhuba, L.; Tigai, Z.; Shek, D. Where Do We Stand with Immunotherapy for Advanced Pancreatic Ductal Adenocarcinoma: A Synopsis of Clinical Outcomes. Biomedicines 2022, 10, 3196. [Google Scholar] [CrossRef]
- Ma, R.; Li, Z.; Chiocca, E.A.; Caligiuri, M.A.; Yu, J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer 2022, 9, 122–139. [Google Scholar] [CrossRef]
- Duan, S.; Wang, S.; Qiao, L.; Yu, X.; Wang, N.; Chen, L.; Zhang, X.; Zhao, X.; Liu, H.; Wang, T.; et al. Oncolytic Virus-Driven Biotherapies from Bench to Bedside. Small 2023, e2206948. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Arenas, S.S.; Sirima, S.B.; Dzomo, G.R.T.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines 2019, 18, 597–613. [Google Scholar]
- Wold, W.S.M.; Toth, K. Adenovirus Vectors for Gene Therapy, Vaccination and Cancer Gene Therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Gastman, B.; Conley, A.P.; Reid, C.; Caroen, S.; Reid, T. Oncolytic Adenoviruses: The Cold War against Cancer Finally Turns Hot. Cancers 2022, 14, 4701. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, K.; Hou, Y.; Lu, F.; Li, L.; Wang, L.; Xia, Y.; Zhang, L.; Chen, H.; Li, R.; et al. PTTG1 knockdown en-hances radiation-induced antitumour immunity in lung adenocarcinoma. Life Sci. 2021, 277, 119594. [Google Scholar] [CrossRef]
- Tian, X.; Xu, W.H.; Xu, F.J.; Li, H.; Anwaier, A.; Wang, H.K.; Wan, F.N.; Zhu, Y.; Cao, D.L.; Zhu, Y.P.; et al. Identification of prognostic biomarkers in papillary renal cell carcinoma and PTTG1 may serve as a bi-omarker for predicting immunotherapy response. Ann. Med. 2022, 54, 211–226. [Google Scholar] [CrossRef]
- Dai, L.; Song, Z.-X.; Wei, D.-P.; Zhang, J.-D.; Liang, J.-Q.; Wang, B.-B.; Ma, W.-T.; Li, L.-Y.; Dang, Y.-L.; Zhao, L.; et al. CDC20 and PTTG1 are Important Biomarkers and Potential Therapeutic Targets for Metastatic Prostate Cancer. Adv. Ther. 2021, 38, 2973–2989. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Ho, M.; Renouf, D.J.; Lim, H.J.; Gill, S.; Ruan, J.Y.; Cheung, W.Y. Eligibility of Metastatic Pancreatic Cancer Pa-tients for First-Line Palliative Intent nab-Paclitaxel Plus Gemcitabine Versus FOLFIRINOX. Am. J. Clin. Oncol. 2017, 40, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Nevala-Plagemann, C.; Garrido-Laguna, I. Updates on adjuvant and neoadjuvant treatment strategies for surgi-cally resectable and borderline resectable pancreatic ductal adenocarcinoma. Ther. Adv. Med. Oncol. 2021, 13, 17588359211045861. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Ozaka, M.; Ishii, H.; Sato, T.; Ueno, M.; Ikeda, M.; Uesugi, K.; Sata, N.; Miyashita, K.; Mizuno, N.; Tsuji, K.; et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2018, 81, 1017–1023. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Zhang, F.; Deng, T.; Zhang, L.; Feng, F.; Wang, F.-H.; Wang, W.; Wang, D.-S.; Luo, H.-Y.; Xu, R.-H.; et al. The efficacy and safety of modified FOLFIRINOX as first-line chemotherapy for Chinese patients with metastatic pancreatic cancer. Cancer Commun. 2019, 39, 26–28. [Google Scholar] [CrossRef]
- Yu, J.X.; Hubbard-Lucey, V.M.; Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019, 18, 899–900. [Google Scholar]
- Ono, R.; Takayama, K.; Sakurai, F.; Mizuguchi, H. Efficient antitumor effects of a novel oncolytic adenovirus fully composed of species B adenovirus serotype 35. Mol. Ther. Oncolytics 2021, 20, 399–409. [Google Scholar] [CrossRef]
- Evgin, L.; Huff, A.L.; Wongthida, P.; Thompson, J.; Kottke, T.; Tonne, J.; Schuelke, M.; Ayasoufi, K.; Driscoll, C.B.; Shim, K.G.; et al. Oncolytic virus-derived type I interferon restricts CAR T cell therapy. Nat. Commun. 2020, 11, 3187. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Rep-lication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nagasato, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Nattress, C.B.; Halldén, G. Advances in oncolytic adenovirus therapy for pancreatic cancer. Cancer Lett. 2018, 434, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Harada, J.N.; Berk, A.J. p53-Independent and -Dependent Requirements for E1B-55K in Adenovirus Type 5 Replication. J. Virol. 1999, 73, 5333–5344. [Google Scholar] [CrossRef]
- O’Shea, C.C.; Johnson, L.; Bagus, B.; Choi, S.; Nicholas, C.; Shen, A.; Boyle, L.; Pandey, K.; Soria, C.; Kunich, J.; et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004, 6, 611–623. [Google Scholar] [CrossRef]
- Wang, Y.; Hallden, G.; Hill, R.; Anand, A.; Liu, T.-C.; Francis, J.; Brooks, G.; Lemoine, N.; Kirn, D. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat. Biotechnol. 2003, 21, 1328–1335. [Google Scholar] [CrossRef]
- Heise, C.; Hermiston, T.; Johnson, L.; A Brooks, G.; Sampson-Johannes, A.; Williams, A.; Hawkins, L.; Kirn, D.H. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000, 6, 1134–1139. [Google Scholar] [CrossRef]
- Oberg, D.; Yanover, E.; Adam, V.; Sweeney, K.; Costas, C.; Lemoine, N.R.; Halldén, G. Improved potency and selectivity of an oncolytic E1ACR2 and E1B19K deleted adenoviral mutant in prostate and pancreatic cancers. Clin. Cancer Res. 2010, 16, 541–553. [Google Scholar] [CrossRef]
- Man, Y.K.S.; Davies, J.A.; Coughlan, L.; Pantelidou, C.; Blazquez-Moreno, A.; Marshall, J.F.; Parker, A.L.; Hallden, G. The Novel Oncolytic Adenoviral Mutant Ad5-3Delta-A20T Retargeted to alphavbeta6 Integrins Efficiently Eliminates Pancreatic Cancer Cells. Mol. Cancer Ther. 2018, 17, 575–587. [Google Scholar] [CrossRef]
- Mach, N.; Gao, J.; Schaffarczyk, L.; Janz, S.; Ehrke-Schulz, E.; Dittmar, T.; Ehrhardt, A.; Zhang, W. Spectrum-Wide Exploration of Human Adenoviruses for Breast Cancer Therapy. Cancers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Nakachi, I.; Helfrich, B.; Spillman, M.; Mickler, E.; Olson, C.; Rice, J.; Coldren, C.; Heasley, L.; Geraci, M.; Stearman, R. PTTG1 Levels Are Predictive of Saracatinib Sensitivity in Ovarian Cancer Cell Lines. Clin. Transl. Sci. 2016, 9, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.; Nemeth, K.; Meszaros, K.; Krokker, L.; Liko, I.; Saskoi, K.; Nemeth, K.; Szabo, P.T.; Szucs, N.; Czirjak, S.; et al. Aspirin Mediates Its Antitumoral Effect Through Inhibiting PTTG1 in Pituitary Adenoma. J. Clin. Endocrinol. Metab. 2022, 107, 3066–3079. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, L.; Gao, J.; Zhang, R. PTTG1 Enhances Oncolytic Adenovirus 5 Entry into Pancreatic Adenocarcinoma Cells by Increasing CXADR Expression. Viruses 2023, 15, 1153. https://doi.org/10.3390/v15051153
Long L, Gao J, Zhang R. PTTG1 Enhances Oncolytic Adenovirus 5 Entry into Pancreatic Adenocarcinoma Cells by Increasing CXADR Expression. Viruses. 2023; 15(5):1153. https://doi.org/10.3390/v15051153
Chicago/Turabian StyleLong, Lu, Jian Gao, and Ruiyang Zhang. 2023. "PTTG1 Enhances Oncolytic Adenovirus 5 Entry into Pancreatic Adenocarcinoma Cells by Increasing CXADR Expression" Viruses 15, no. 5: 1153. https://doi.org/10.3390/v15051153
APA StyleLong, L., Gao, J., & Zhang, R. (2023). PTTG1 Enhances Oncolytic Adenovirus 5 Entry into Pancreatic Adenocarcinoma Cells by Increasing CXADR Expression. Viruses, 15(5), 1153. https://doi.org/10.3390/v15051153





